Correlations between Kidney and Heart Function Bioindicators and the Expressions of Toll-Like, ACE2, and NRP-1 Receptors in COVID-19
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Patients
2.3. Inclusion and Exclusion Criteria
2.4. The Demographic Data
2.5. Blood Samples
2.6. Laboratory Assays
2.6.1. Detection of Serum Biomarkers of Kidney and Heart Functions
2.6.2. RNA Isolation and qRT-PCR
2.7. Statistical Analysis
3. Results
3.1. Demographic Data and Laboratory Findings
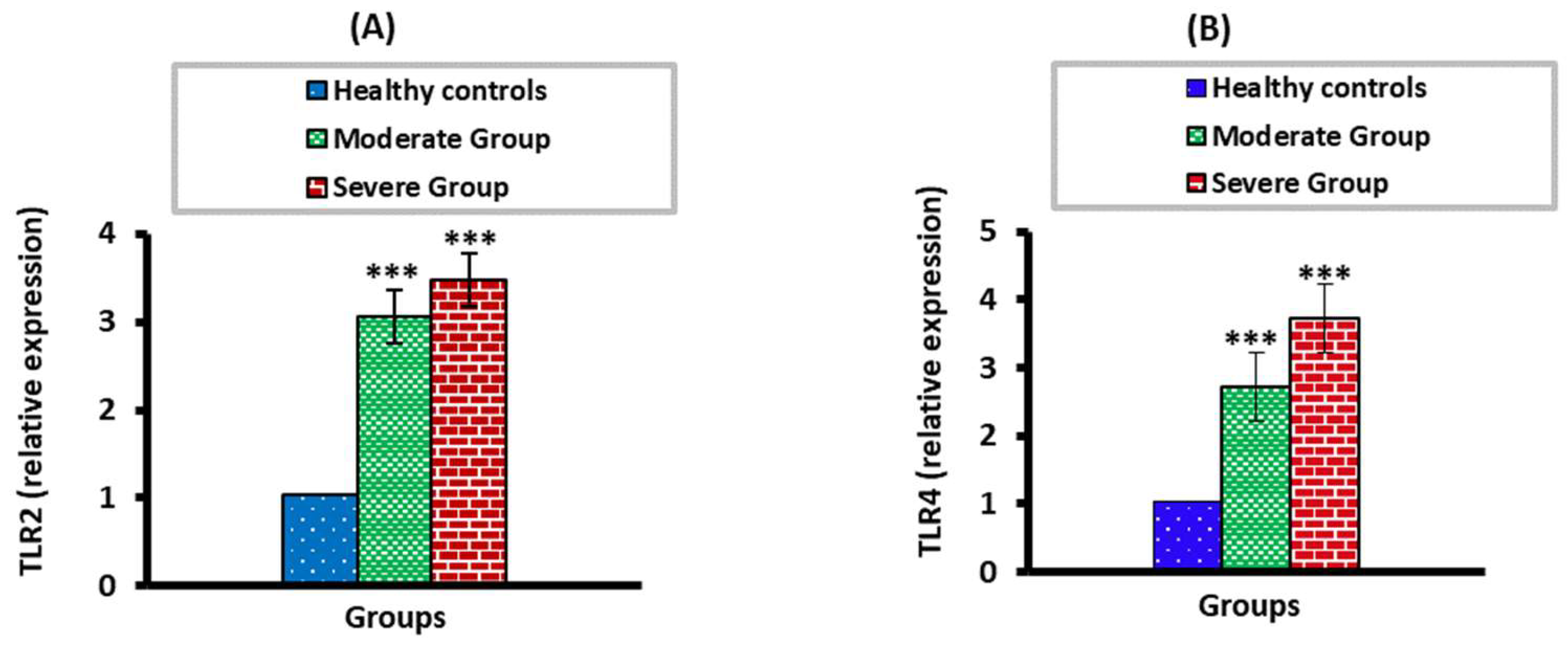
3.2. The mRNA Expression Levels of TLR2 and TLR4 in Moderate and Severe COVID-19 Patients
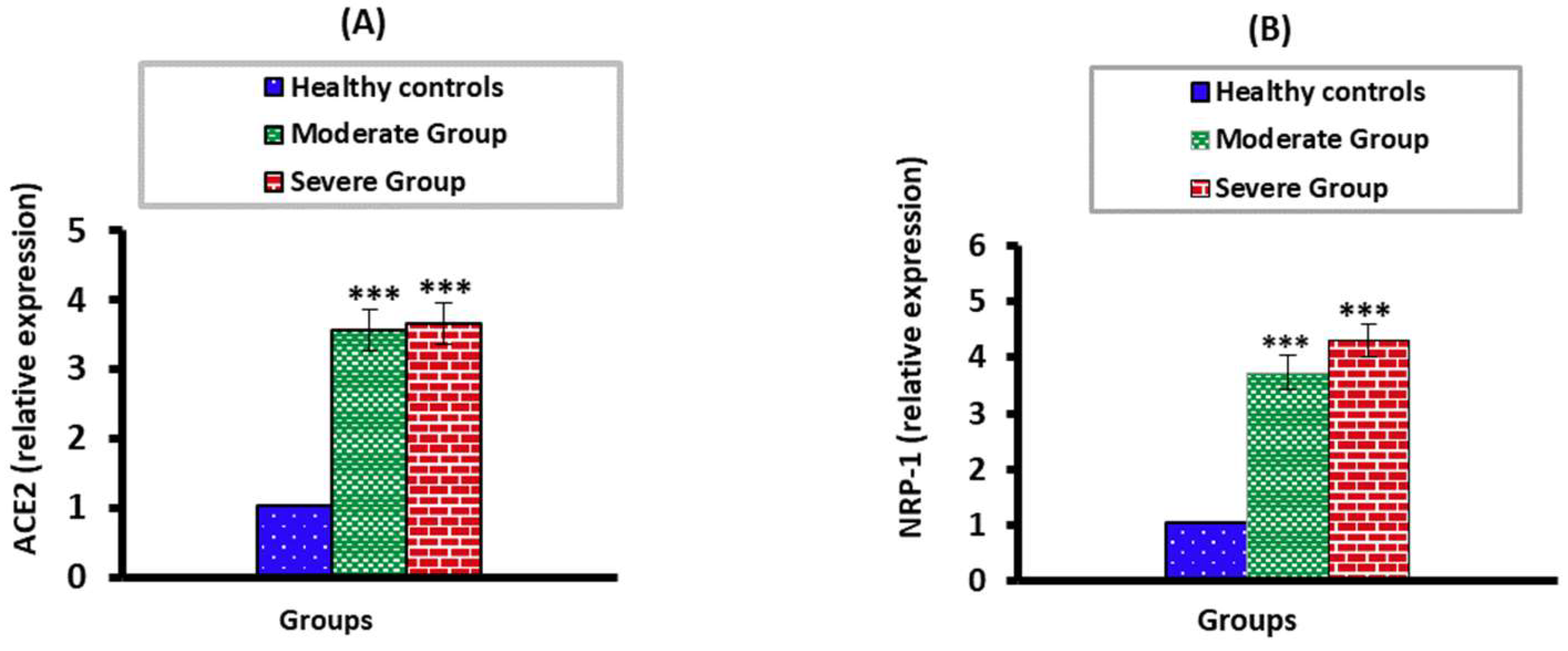
3.3. The mRNA Expression Levels of ACE2 and NPP-2 in Moderate and Severe COVID-19 Patients
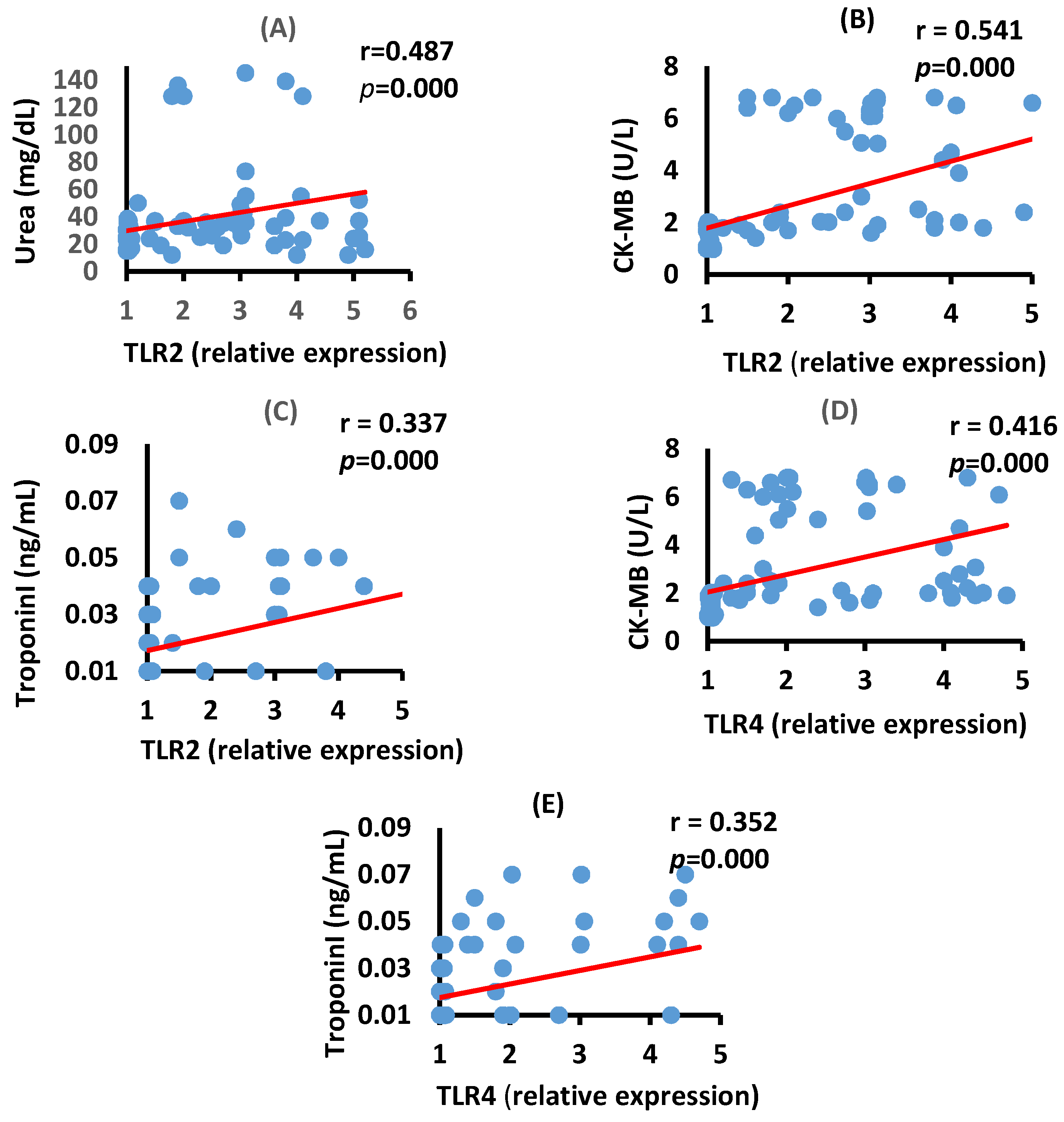
3.4. Correlation between TLR2, TLR4, Renal, and Cardiac Biomarkers in the Moderate COVID-19 Patients
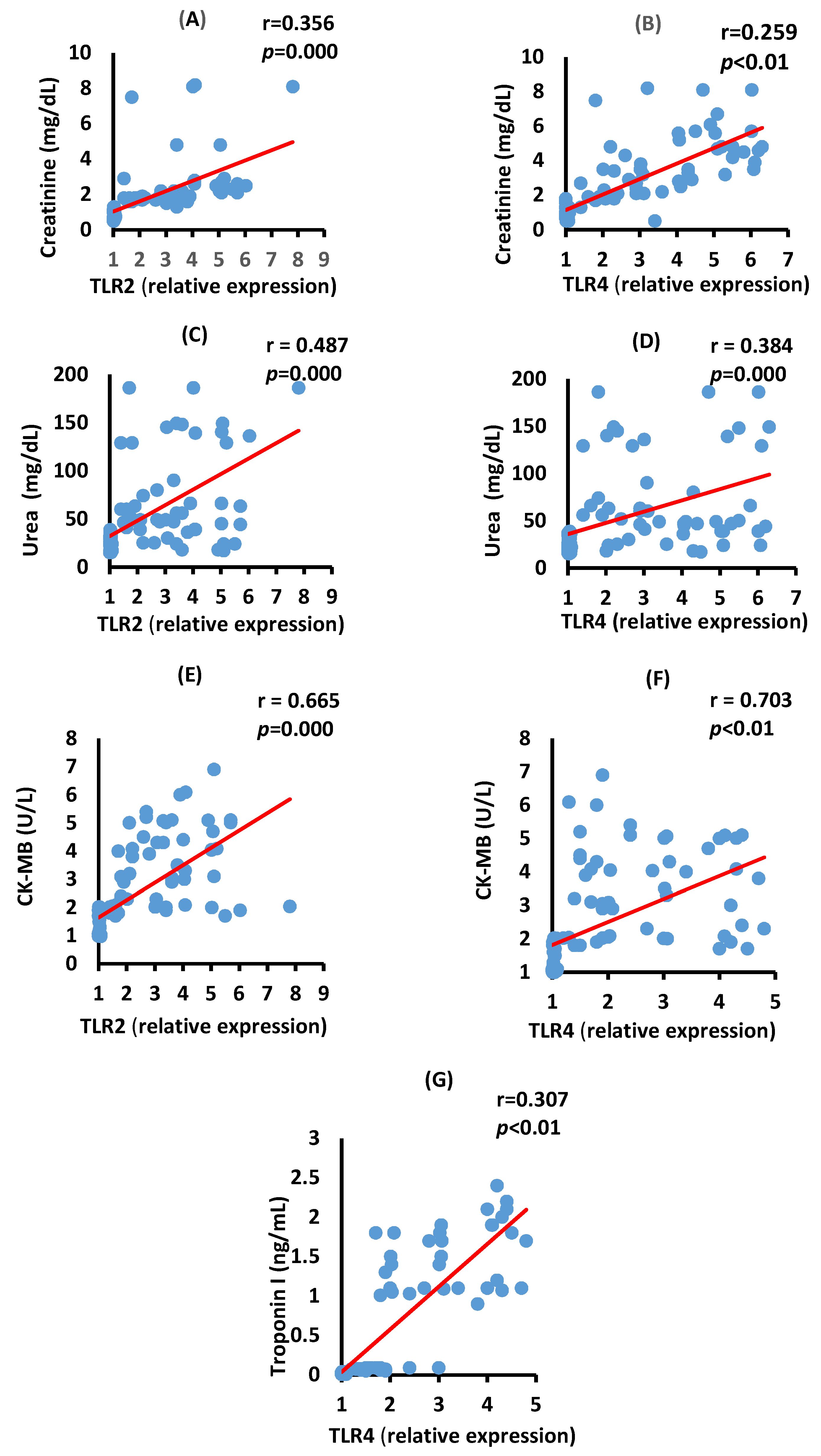
3.5. Correlation between TLR2, TLR4, Renal, and Cardiac Biomarkers in the Severe COVID-19 Patients
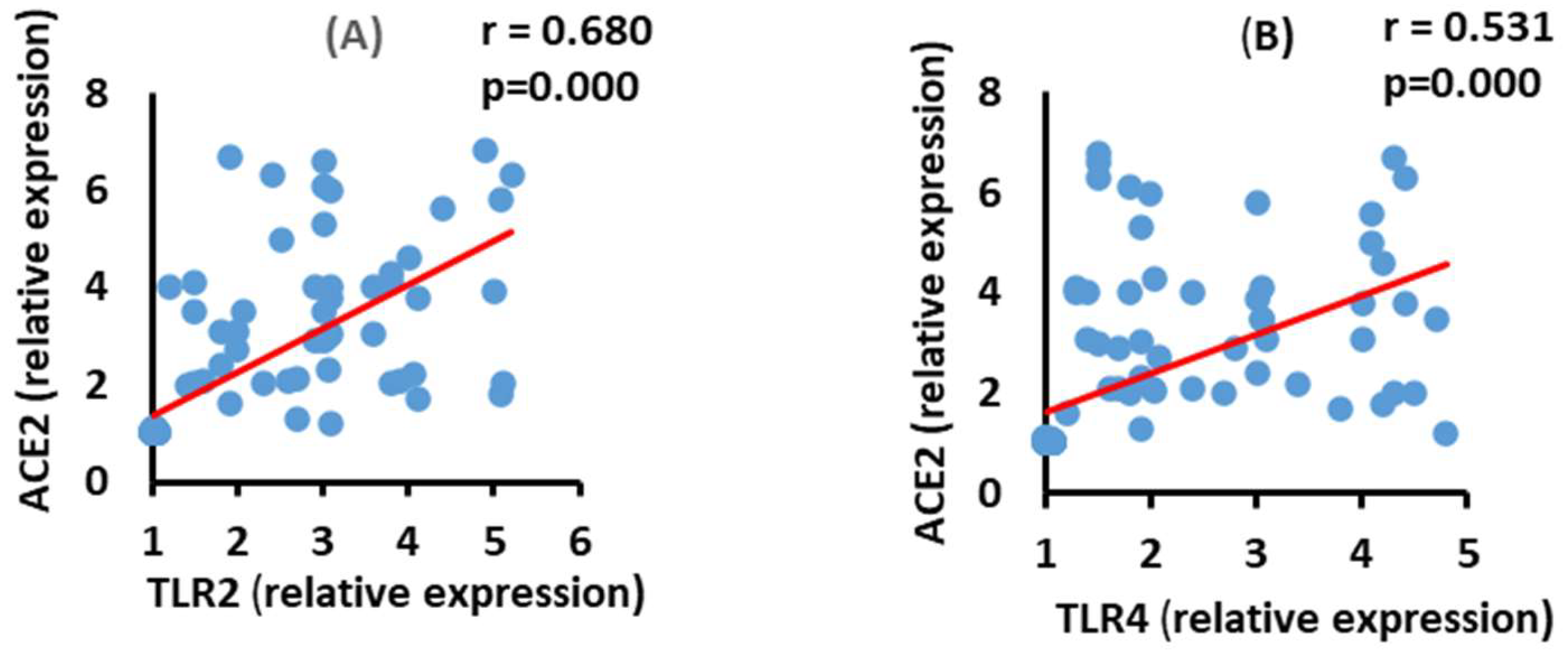
3.6. Correlation between ACE2 and TLR2 and TLR4 mRNA Expression in the Moderate COVID-19 Patients
3.7. Correlation between ACE2 and TLR2 and TLR4 mRNA Expression in the Severe COVID-19 Patients
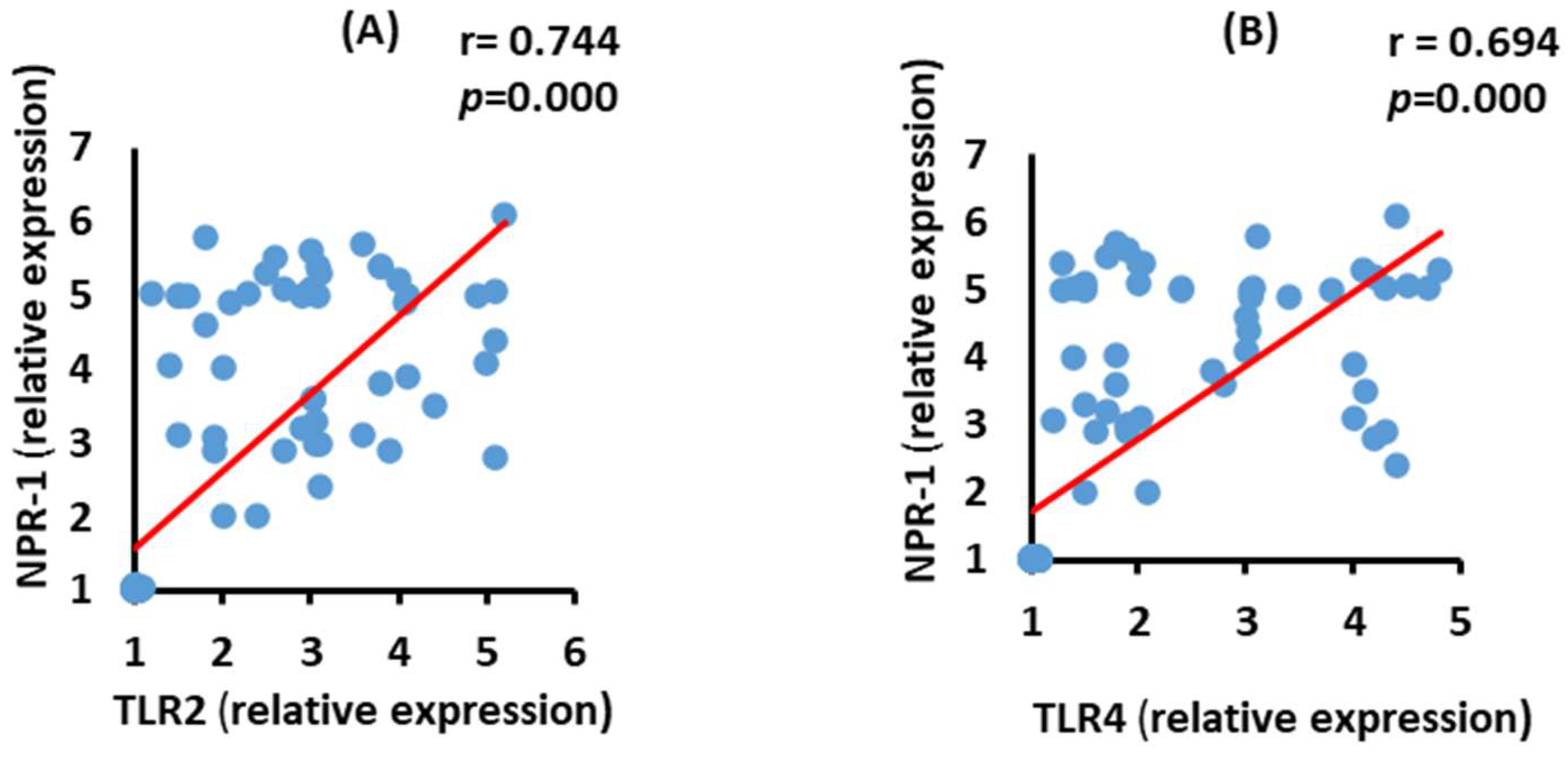
3.8. Correlation between NRP-1 and TLR2 and TLR4 mRNA Expression in the Moderate COVID-19 Patients
3.9. Correlation between NRP-2 and TLR2 and TLR4 mRNA Expression in the Severe COVID-19 Patients
3.10. Correlation between Renal and Cardiac Biomarkers with ACE2 and NRP-1 mRNA Expressions in Moderate COVID-19 Patients
3.11. Correlation between Renal and Cardiac Biomarkers with ACE2 and NRP-1 in Severe COVID-19 Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, S.R.; Navas-Martin, S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2021.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, A.C.; Bishop, A.; Yao, Y.; Kemp, F.; Ren, J.; Chen, H.; Xu, X.; Berkhout, B.; Van Der Hoek, L.; Jones, I.M. Interaction of severe acute respiratory syndrome-coronavirus and NL63 coronavirus spike proteins with angiotensin converting enzyme-2. J. Gen. Virol. 2008, 89, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A Human Homolog of Angiotensin-converting Enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, C.A.; Rolain, J.-M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Peacock, T.P.; Goldhill, D.H.; Zhou, J.; Baillon, L.; Frise, R.; Swann, O.C.; Kugathasan, R.; Penn, R.; Brown, J.C.; Sanchez-David, R.Y.; et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 2021, 6, 899–909. [Google Scholar] [CrossRef]
- Zhuang, M.; Cheng, Y.; Zhang, J.; Jiang, X.; Wang, L.; Deng, J.; Wang, P. Increasing host cellular receptor—angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J. Med. Virol. 2020, 92, 2693–2701. [Google Scholar] [CrossRef]
- Mayi, B.S.; Leibowitz, J.A.; Woods, A.T.; Ammon, K.A.; Liu, A.E.; Raja, A. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 2021, 17, e1009153. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2021, 94, 869–877. [Google Scholar] [CrossRef]
- Boozari, M.; Butler, A.E.; Sahebkar, A. Impact of curcumin on toll-like receptors. J. Cell. Physiol. 2019, 234, 12471–12482. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nature reviews. Microbiology 2021, 19, 155–170. [Google Scholar] [PubMed]
- Hossain, G.; Akter, S.; Uddin, J. Emerging Role of Neuropilin-1 and Angiotensin-Converting Enzyme-2 in Renal Carcinoma-Associated COVID-19 Pathogenesis. Infect. Dis. Rep. 2021, 13, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Patibandla, S.; Gupta, K.; Alsayouri, K. Cardiac Enzymes. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545216/ (accessed on 11 August 2021).
- Schmiechen, N.J.; Han, C.; Milzman, D.P. ED Use of Rapid Lactate to Evaluate Patients with Acute Chest Pain. Ann. Emerg. Med. 1997, 30, 571–577. [Google Scholar] [CrossRef]
- Yun, D.D.; Alpert, J.S. Acute Coronary Syndromes. Cardiology 1997, 88, 223–237. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed on 20 July 2021).
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66–75. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Henry, T.J. Clinical Chemistry Principles and Techniques, 2nd ed.; Harper and Row Publishers: New York, NY, USA, 1974. [Google Scholar]
- Kaplan, A. Urea. Clinical Chemistry PBL; The C.V. Mosby Co.: St Louis, MO, USA; Toronto, ON, Canada; Princeton, NJ, USA, 1984; pp. 418, 437, 1257–1260. [Google Scholar]
- Buhl, S.N.; Jackson, K.Y. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lac-tate-to-pyruvate and Pyruvate-to-lactate in human serum at 25, 30- and 37-degree C. Clin. Chem. 1978, 24, 828. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- Ahnach, M.; Zbiri, S.; Nejjari, S.; Ousti, F.; Elkettani, C. C-reactive protein as an early predictor of COVID-19 severity. J. Med. Biochem. 2020, 39, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.W.; Zeng, B.Y.; Dai, Y.T.; Liang, X.J.; Zhang, L.J.; Ahmad, R.; Su, X.T. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2022, 614, 547–555. [Google Scholar] [CrossRef]
- Fazal, M. C-Reactive Protein a Promising Biomarker of COVID-19 Severity. Korean J. Clin. Lab. Sci. 2021, 53, 201–207. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. kidney disease is asso-ciated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Ahmadian, E.; Hosseiniyan Khatibi, S.M.; Razi Soofiyani, S.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Zununi Vahed, S. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef]
- Buonaguro, F.M.; Ascierto, P.A.; Morse, G.D.; Buonaguro, L.; Puzanov, I.; Tornesello, M.L.; Bréchot, C.; Gallo, R.C. COVID-19: Time for a paradigm change. Rev. Med. Virol. 2020, 30, e2134. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Relationships among Lymphocyte Subsets, Cytokines, and the Pulmonary Inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020, 189, 428–437. [Google Scholar] [CrossRef]
- Fan, C.; Lu, W.; Li, K.; Ding, Y.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis In-fection in COVID-19Patients. Front. Med. 2021, 7, 563893. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Wang, B.; Lv, L.-L.; Liu, B.-C. Pathogenesis of Acute Kidney Injury in Coronavirus Disease 2019. Front. Physiol. 2021, 12, 586589. [Google Scholar] [CrossRef] [PubMed]
- Imig, J.D.; Ryan, M.J. Immune and Inflammatory Role in Renal Disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinellu, A.; Sotgia, S.; Fois, A.G.; Mangoni, A.A. Serum CK-MB, COVID-19 severity and mortality: An updated systematic review and meta-analysis with meta-regression. Adv. Med. Sci. 2021, 66, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, W.; Zhang, T.; Wang, Z.; Li, J.; Zhu, M.; Liang, Y.; You, W.; Li, K.; Ding, R.; et al. Implications of cardiac markers in risk-stratification and management for COVID-19 patients. Crit. Care 2021, 25, 624. [Google Scholar] [CrossRef]
- An, W.; Kang, J.S.; Wang, Q.; Kim, T.E. Cardiac biomarkers and COVID-19: A systematic review and me-ta-analysis. J. Infect. Public Health 2021, 14, 1191–1197. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Q.; Xu, J. Changes of Laboratory Cardiac Markers and Mechanisms of Cardiac Injury in Coronavirus Disease 2019. BioMed Res. Int. 2020, 2020, 7413673. [Google Scholar] [CrossRef]
- Sultan, R.H.; Abdallah, M.; Ali, T.M.; Ahmed, A.E.; Assal, H.H.; Elesawy, B.H.; Ahmed, O.M. The Associations between Cytokine Levels, Kidney and Heart Function Biomarkers, and Expression Levels of Angiotensin-Converting Enzyme-2 and Neuropilin-1 in COVID-19 Patients. Vaccines 2022, 10, 1045. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Freeman, T.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Rivero, A.; Mora, C.; Muros, M.; García, J.; Herrera, H.; Navarro-González, J. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin. Sci. 2009, 116, 479–492. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Phar-Macological Res. 2020, 157, 104833. [Google Scholar] [CrossRef] [PubMed]
- Issa, H.; Eid, A.H.; Berry, B.; Takhviji, V.; Khosravi, A.; Mantash, S.; Nehme, R.; Hallal, R.; Karaki, H.; Dhayni, K.; et al. Combination of angiotensin (1-7) agonists and convalescent plasma as a new strategy to overcome angiotensin converting enzyme 2 (ACE2) inhibition for the treatment of COVID-19. Front. Med. 2021, 8, 620990. [Google Scholar] [CrossRef]
- Ziegler, C.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response during SARS-CoV-2 Infection: Lessons from the Past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef]
- Browne, E.P. The Role of Toll-Like Receptors in Retroviral Infection. Microorganisms 2020, 8, 1787. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Feng, Z. The Role of Toll-Like Receptor Signaling in the Progression of Heart Failure. Mediat. Inflamm. 2018, 2018, 9874109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Karmakar, S.; Babu, S.P. TLR2 and TLR4 mediated host immune responses in major infectious dis-eases: A review. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2016, 20, 193–204. [Google Scholar]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Infect. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, J.; Zhang, D.; Xu, Z.; Ji, J.; Wen, C. Cytokine Storm in COVID-19: The Current Evidence and Treat-ment Strategies. Front. Immunol. 2020, 11, 1708. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Basu-Ray, I.; Almaddah, N.K.; Adeboye, A.; Soos, M.P. Cardiac Manifestations of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bugert, C.L.; Kwiat, V.; Valera, I.C.; Bugert, J.J.; Parvatiyar, M.S. Cardiovascular Injury Due to SARS-CoV-2. Curr. Clin. Microbiol. Rep. 2021, 8, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19induces cy-tokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Testani, J.M. The kidney in heart failure: An update. Eur. Heart J. 2015, 36, 1437–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef]
- Faour, W.H.; Choaib, A.; Issa, E.; Choueiry, F.E.; Shbaklo, K.; Alhajj, M.; Sawaya, R.T.; Harhous, Z.; Alefishat, E.; Nader, M. Mechanisms of COVID-19-induced kidney injury and current pharmacotherapies. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2022, 71, 39–56. [Google Scholar] [CrossRef] [PubMed]


| Healthy Controls | Moderate Patients | Severe Patients | |
|---|---|---|---|
| (n = 50) | (n = 50) | (n = 50) | |
| Age (Year) | 52.12 ± 1.22 | 58.24 ± 1.24 | 57.36 ± 1.43 |
| Gender, no. (%) | |||
| Male | 25 (50%) | 25 (50%) | 29 (58%) |
| Female | 25 (50%) | 25 (50%) | 21 (42%) |
| BMI (kg/m2) | 24.25 ± 0.35 | 24.73 ± 0.48 | 25.03 ± 0.52 |
| CRP (mg/dL) | 1.97 ± 0.19 | 61.90 ± 6.05 + *** | 73.92 ± 3.42 *** |
| Creatinine (mg/dL) | 0.89 ± 0.03 | 0.85 ± 0.06 +++ | 1.75 ± 0.30 ** |
| Urea (mg/dL) | 25.1 ± 0.93 | 48.68 ± 5.49 +++ ** | 79.52 ± 9.14 *** |
| CK-MB (U/L) | 1.29 ± 0.05 | 4.07 ± 0.29 *** | 3.55 ± 0.20 *** |
| LDH (U/L) | 261.6 ± 3.00 | 339.24 ± 25.96 ++ ** | 418.7 ± 26.12 *** |
| Troponin I (ng/mL) | 0.02 ± 0.002 | 0.03 ± 0.003 | 0.04 ± 0.01 *** |
| Renal Biomarkers | Cardiac Biomarkers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine | Urea | CK-MB | LDH | Troponin I | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| ACE2 | −0.113 | 0.262 | 0.193 | 0.054 | 0.543 *** | 0.000 | 0.023 | 0.823 | 0.260 ** | 0.009 |
| NRP-1 | −0.018 | 0.86 | 0.334 ** | 0.001 | 0.653 *** | 0.000 | 0.198 * | 0.048 | 0.261 ** | 0.009 |
| Renal Biomarkers | Cardiac Biomarkers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine | Urea | CK-MB | LDH | Troponin I | ||||||
| r | p | r | p | r | p | r | p | r | p | |
| ACE2 | 0.204 * | 0.041 | 0.438 *** | 0.000 | 0.586 *** | 0.000 | 0.557 *** | 0.000 | 0.147 | 0.144 |
| NRP-1 | 0.300 ** | 0.002 | 0.443 *** | 0.000 | 0.624 *** | 0.000 | 0.328 ** | 0.001 | 0.301 ** | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, R.H.; Elesawy, B.H.; Ali, T.M.; Abdallah, M.; Assal, H.H.; Ahmed, A.E.; Ahmed, O.M. Correlations between Kidney and Heart Function Bioindicators and the Expressions of Toll-Like, ACE2, and NRP-1 Receptors in COVID-19. Vaccines 2022, 10, 1106. https://doi.org/10.3390/vaccines10071106
Sultan RH, Elesawy BH, Ali TM, Abdallah M, Assal HH, Ahmed AE, Ahmed OM. Correlations between Kidney and Heart Function Bioindicators and the Expressions of Toll-Like, ACE2, and NRP-1 Receptors in COVID-19. Vaccines. 2022; 10(7):1106. https://doi.org/10.3390/vaccines10071106
Chicago/Turabian StyleSultan, Rabab Hussain, Basem H. Elesawy, Tarek M. Ali, Maged Abdallah, Hebatallah Hany Assal, Amr E. Ahmed, and Osama M. Ahmed. 2022. "Correlations between Kidney and Heart Function Bioindicators and the Expressions of Toll-Like, ACE2, and NRP-1 Receptors in COVID-19" Vaccines 10, no. 7: 1106. https://doi.org/10.3390/vaccines10071106
APA StyleSultan, R. H., Elesawy, B. H., Ali, T. M., Abdallah, M., Assal, H. H., Ahmed, A. E., & Ahmed, O. M. (2022). Correlations between Kidney and Heart Function Bioindicators and the Expressions of Toll-Like, ACE2, and NRP-1 Receptors in COVID-19. Vaccines, 10(7), 1106. https://doi.org/10.3390/vaccines10071106







