First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Strategy
2.2. Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Identification and Selection of Studies
3.2. Characteristics of the Studies
3.3. Characteristics of the Studies
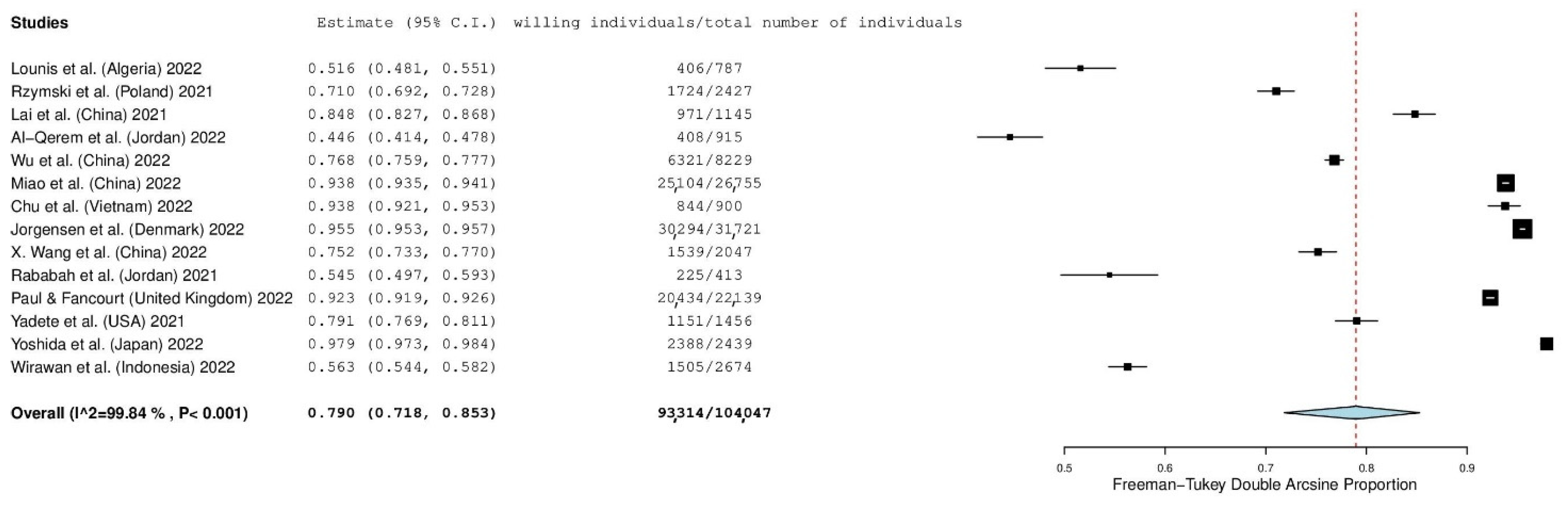
3.4. Individuals’ Willingness and Refusal to Accept a First COVID-19 Booster Dose
3.5. Impact of the “Unsure” Response Option
3.6. Impact of the Sampling Method
3.7. Impact of the Countries
3.8. Meta-Regression Analysis
3.9. Predictors of Individuals’ Willingness to Accept a First COVID-19 Booster Dose
4. Discussion
4.1. Willingness and Refusal of Individuals to Accept a First COVID-19 Booster Dose
4.2. Subgroup Analyses
4.3. Predictors of Individuals’ Willingness to Accept a First COVID-19 Booster Dose
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, H.-Y.; Jian, M.-J.; Chang, C.-K.; Lin, J.-C.; Yeh, K.-M.; Chen, C.-W.; Hsieh, S.-S.; Hung, K.-S.; Tang, S.-H.; Perng, C.-L.; et al. Emergency SARS-CoV-2 Variants of Concern: Novel Multiplex Real-Time RT-PCR Assay for Rapid Detection and Surveillance. Microbiol. Spectr. 2022, 10, e0251321. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Pant, A.B. Mitigating COVID-19 in the Face of Emerging Virus Variants, Breakthrough Infections and Vaccine Hesitancy. J. Autoimmun. 2022, 127, 102792. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, S.; Ishak, A.; Dhawan, N.; Poudel, S.; Shrestha, P.S.; Singh, P.; Xie, E.; Tahir, P.; Marzaban, S.; Michel, J.; et al. Characteristics of COVID-19 Breakthrough Infections among Vaccinated Individuals and Associated Risk Factors: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kaelber, D.C.; Xu, R.; Berger, N.A. COVID-19 Breakthrough Infections, Hospitalizations and Mortality in Fully Vaccinated Patients with Hematologic Malignancies: A Clarion Call for Maintaining Mitigation and Ramping-up Research. Blood Rev. 2022, 54, 100931. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.; Kronig, I.; Neher, R.A.; Eckerle, I.; Vetter, P.; Kaiser, L. Novel SARS-CoV-2 Variants: The Pandemics within the Pandemic. Clin. Microbiol. Infect. 2021, 27, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased Mortality in Community-Tested Cases of SARS-CoV-2 Lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Chan, K.-H.; Hung, I.F.-N. Safety and Efficacy of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Different Vaccines at Phase 3. Vaccines 2021, 9, 989. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Callaway, E. COVID Vaccine Boosters: The Most Important Questions. Nature 2021, 596, 178–180. [Google Scholar] [CrossRef]
- Tanne, J.H. COVID-19: Moderna Plans Booster Doses to Counter Variants. BMJ 2021, 372, n232. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a Third Dose of the BNT162b2 MRNA COVID-19 Vaccine for Preventing Severe Outcomes in Israel: An Observational Study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an MRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Frenck, R.W.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef]
- Jantarabenjakul, W.; Sodsai, P.; Chantasrisawad, N.; Jitsatja, A.; Ninwattana, S.; Thippamom, N.; Ruenjaiman, V.; Tan, C.W.; Pradit, R.; Sophonphan, J.; et al. Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers. Vaccines 2022, 10, 639. [Google Scholar] [CrossRef]
- Clements, J.M. Knowledge and Behaviors Toward COVID-19 Among US Residents During the Early Days of the Pandemic: Cross-Sectional Online Questionnaire. JMIR Public Health Surveill. 2020, 6, e19161. [Google Scholar] [CrossRef]
- Gallè, F.; Sabella, E.A.; Roma, P.; Ferracuti, S.; Da Molin, G.; Diella, G.; Montagna, M.T.; Orsi, G.B.; Liguori, G.; Napoli, C. Knowledge and Lifestyle Behaviors Related to COVID-19 Pandemic in People over 65 Years Old from Southern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10872. [Google Scholar] [CrossRef]
- Abebe, H.; Shitu, S.; Mose, A. Understanding of COVID-19 Vaccine Knowledge, Attitude, Acceptance, and Determinates of COVID-19 Vaccine Acceptance Among Adult Population in Ethiopia. Infect. Drug Resist. 2021, 14, 2015–2025. [Google Scholar] [CrossRef]
- Gallè, F.; Sabella, E.A.; Roma, P.; De Giglio, O.; Caggiano, G.; Tafuri, S.; Da Molin, G.; Ferracuti, S.; Montagna, M.T.; Liguori, G.; et al. Knowledge and Acceptance of COVID-19 Vaccination among Undergraduate Students from Central and Southern Italy. Vaccines 2021, 9, 638. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Santos, W.M.d.; Secoli, S.R.; Püschel, V.A.d.A. The Joanna Briggs Institute Approach for Systematic Reviews. Rev. Lat.-Am. Enferm. 2018, 26. [Google Scholar] [CrossRef] [Green Version]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-Analysis of Prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Wallace, B.C.; Schmid, C.H.; Lau, J.; Trikalinos, T.A. Meta-Analyst: Software for Meta-Analysis of Binary, Continuous and Diagnostic Data. BMC Med. Res. Methodol. 2009, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Al-Qerem, W.; Al Bawab, A.Q.; Hammad, A.; Ling, J.; Alasmari, F. Willingness of the Jordanian Population to Receive a COVID-19 Booster Dose: A Cross-Sectional Study. Vaccines 2022, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Vu Thi, H.; Nguyen Thi, Y.V.; Nguyen, M.-A.; Bui, N.-L.; Hoang, V.T.; Hoang Nam, D.; Do, D.-L.; Than, V.T.; Al-Tawfiq, J.A. Willingness to Receive COVID-19 Vaccine Booster Doses for Adults and Their Children in Vietnam. J. Hum. Behav. Soc. Environ. 2022, 1–13. [Google Scholar] [CrossRef]
- Jørgensen, F.J.; Nielsen, L.H.; Petersen, M.B. Willingness to Take the Booster Vaccine in a Nationally Representative Sample of Danes. Vaccines 2022, 10, 425. [Google Scholar] [CrossRef]
- Lai, X.; Zhu, H.; Wang, J.; Huang, Y.; Jing, R.; Lyu, Y.; Zhang, H.; Feng, H.; Guo, J.; Fang, H. Public Perceptions and Acceptance of COVID-19 Booster Vaccination in China: A Cross-Sectional Study. Vaccines 2021, 9, 1461. [Google Scholar] [CrossRef]
- Lounis, M.; Bencherit, D.; Rais, M.A.; Riad, A. COVID-19 Vaccine Booster Hesitancy (VBH) and Its Drivers in Algeria: National Cross-Sectional Survey-Based Study. Vaccines 2022, 10, 621. [Google Scholar] [CrossRef]
- Miao, Y.; Li, Y.; Zhang, W.; Wu, J.; Gu, J.; Wang, M.; Wei, W.; Ye, B.; Miao, C.; Tarimo, C.S.; et al. The Psychological Experience of COVID-19 Vaccination and Its Impact on the Willingness to Receive Booster Vaccines among the Chinese Population: Evidence from a National Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 5464. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Fancourt, D. Predictors of Uncertainty and Unwillingness to Receive the COVID-19 Booster Vaccine: An Observational Study of 22,139 Fully Vaccinated Adults in the UK. Lancet Reg. Health-Eur. 2022, 14, 100317. [Google Scholar] [CrossRef] [PubMed]
- Rababa’h, A.M.; Abedalqader, N.N.; Ababneh, M. Jordanians’ Willingness to Receive Heterologous Prime-Boost COVID-19 Vaccination and Vaccine Boosters. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7516–7525. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Poniedziałek, B.; Fal, A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines 2021, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Pei, M.; Li, X.; Li, N. Willingness of the General Public to Receive A COVID-19 Vaccine Booster—China, April–May 2021. China CDC Wkly. 2022, 4, 66–70. [Google Scholar] [CrossRef]
- Wirawan, G.B.S.; Harjana, N.P.A.; Nugrahani, N.W.; Januraga, P.P. Health Beliefs and Socioeconomic Determinants of COVID-19 Booster Vaccine Acceptance: An Indonesian Cross-Sectional Study. Vaccines 2022, 10, 724. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; Deng, Z.; Yin, D.; Shen, Q.; Zeng, J.; Xie, Y.; Xu, M.; Yang, M.; Jiang, S.; et al. Acceptance of COVID-19 Booster Vaccination Based on the Protection Motivation Theory: A Cross-sectional Study in China. J. Med. Virol. 2022, in press. [Google Scholar] [CrossRef]
- Yadete, T.; Batra, K.; Netski, D.M.; Antonio, S.; Patros, M.J.; Bester, J.C. Assessing Acceptability of COVID-19 Vaccine Booster Dose among Adult Americans: A Cross-Sectional Study. Vaccines 2021, 9, 1424. [Google Scholar] [CrossRef]
- Yoshida, M.; Kobashi, Y.; Kawamura, T.; Shimazu, Y.; Nishikawa, Y.; Omata, F.; Zhao, T.; Yamamoto, C.; Kaneko, Y.; Nakayama, A.; et al. Factors Associated with COVID-19 Vaccine Booster Hesitancy: A Retrospective Cohort Study, Fukushima Vaccination Community Survey. Vaccines 2022, 10, 515. [Google Scholar] [CrossRef]
- Nehal, K.R.; Steendam, L.M.; Campos Ponce, M.; van der Hoeven, M.; Smit, G.S.A. Worldwide Vaccination Willingness for COVID-19: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 1071. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Intention of Healthcare Workers to Accept COVID-19 Vaccination and Related Factors: A Systematic Review and Meta-Analysis. Asian Pac. J. Trop. Med. 2021, 14, 543. [Google Scholar] [CrossRef]
- Galanis, P.; Vraka, I.; Siskou, O.; Konstantakopoulou, O.; Katsiroumpa, A.; Kaitelidou, D. Willingness, Refusal and Influential Factors of Parents to Vaccinate Their Children against the COVID-19: A Systematic Review and Meta-Analysis. Prev. Med. 2022, 157, 106994. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Jones, A.; Lesser, I.; Daly, M. International Estimates of Intended Uptake and Refusal of COVID-19 Vaccines: A Rapid Systematic Review and Meta-Analysis of Large Nationally Representative Samples. Vaccine 2021, 39, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Zintel, S.; Flock, C.; Arbogast, A.L.; Forster, A.; von Wagner, C.; Sieverding, M. Gender Differences in the Intention to Get Vaccinated against COVID-19: A Systematic Review and Meta-Analysis. J. Public Health 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Siskou, O.; Konstantakopoulou, O.; Katsiroumpa, A.; Kaitelidou, D. Predictors of COVID-19 Vaccination Uptake and Reasons for Decline of Vaccination: A Systematic Review. MedRxiv 2021. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Joshi, A.D.; Drew, D.A.; Merino, J.; Ma, W.; Lo, C.-H.; Kwon, S.; Wang, K.; Graham, M.S.; Polidori, L.; et al. Racial and Ethnic Differences in COVID-19 Vaccine Hesitancy and Uptake. MedRxiv 2021. [Google Scholar] [CrossRef]
- Schrading, W.A.; Trent, S.A.; Paxton, J.H.; Rodriguez, R.M.; Swanson, M.B.; Mohr, N.M.; Talan, D.A.; Project COVERED Emergency Department Network; Bahamon, M.; Carlson, J.N.; et al. Vaccination Rates and Acceptance of SARS-CoV-2 Vaccination among U.S. Emergency Department Health Care Personnel. Acad. Emerg. Med. 2021, 28, 455–458. [Google Scholar] [CrossRef]
- Sakou, I.-I.; Tsitsika, A.K.; Papaevangelou, V.; Tzavela, E.C.; Greydanus, D.E.; Tsolia, M.N. Vaccination Coverage among Adolescents and Risk Factors Associated with Incomplete Immunization. Eur. J. Pediatr. 2011, 170, 1419–1426. [Google Scholar] [CrossRef]
- Bish, A.; Yardley, L.; Nicoll, A.; Michie, S. Factors Associated with Uptake of Vaccination against Pandemic Influenza: A Systematic Review. Vaccine 2011, 29, 6472–6484. [Google Scholar] [CrossRef]
- Jiménez-García, R.; Hernández-Barrera, V.; de Andres, A.L.; Jimenez-Trujillo, I.; Esteban-Hernández, J.; Carrasco-Garrido, P. Gender Influence in Influenza Vaccine Uptake in Spain: Time Trends Analysis (1995–2006). Vaccine 2010, 28, 6169–6175. [Google Scholar] [CrossRef]
- Pulcini, C.; Massin, S.; Launay, O.; Verger, P. Factors Associated with Vaccination for Hepatitis B, Pertussis, Seasonal and Pandemic Influenza among French General Practitioners: A 2010 Survey. Vaccine 2013, 31, 3943–3949. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Anderson, E.R. Brave Men and Timid Women? A Review of the Gender Differences in Fear and Anxiety. Clin. Psychol. Rev. 2009, 29, 496–505. [Google Scholar] [CrossRef]
- Biswas, M.R.; Alzubaidi, M.S.; Shah, U.; Abd-Alrazaq, A.A.; Shah, Z. A Scoping Review to Find Out Worldwide COVID-19 Vaccine Hesitancy and Its Underlying Determinants. Vaccines 2021, 9, 1243. [Google Scholar] [CrossRef]
- Wake, A.D. The Willingness to Receive COVID-19 Vaccine and Its Associated Factors: “Vaccination Refusal Could Prolong the War of This Pandemic”—A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 2609–2623. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Xu, R.H.; Wong, E.L.; Hung, C.; Feng, D.; Feng, Z.; Yeoh, E.; Wong, S.Y. Public Preference for COVID-19 Vaccines in China: A Discrete Choice Experiment. Health Expect. 2020, 23, 1543–1578. [Google Scholar] [CrossRef] [PubMed]
- Pogue, K.; Jensen, J.L.; Stancil, C.K.; Ferguson, D.G.; Hughes, S.J.; Mello, E.J.; Burgess, R.; Berges, B.K.; Quaye, A.; Poole, B.D. Influences on Attitudes Regarding Potential COVID-19 Vaccination in the United States. Vaccines 2020, 8, 582. [Google Scholar] [CrossRef]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated with US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef]
- Seale, H.; Heywood, A.E.; Leask, J.; Sheel, M.; Durrheim, D.N.; Bolsewicz, K.; Kaur, R. Examining Australian Public Perceptions and Behaviors towards a Future COVID-19 Vaccine. BMC Infect. Dis. 2021, 21, 120. [Google Scholar] [CrossRef]
- Yang, F.; Li, X.; Su, X.; Xiao, T.; Wang, Y.; Hu, P.; Li, H.; Guan, J.; Tian, H.; Wang, P.; et al. A Study on Willingness and Influencing Factors to Receive COVID-19 Vaccination among Qingdao Residents. Hum. Vaccines Immunother. 2021, 17, 408–413. [Google Scholar] [CrossRef]
- Palamenghi, L.; Barello, S.; Boccia, S.; Graffigna, G. Mistrust in Biomedical Research and Vaccine Hesitancy: The Forefront Challenge in the Battle against COVID-19 in Italy. Eur. J. Epidemiol. 2020, 35, 785–788. [Google Scholar] [CrossRef]
- Sallam, M.; Dababseh, D.; Eid, H.; Al-Mahzoum, K.; Al-Haidar, A.; Taim, D.; Yaseen, A.; Ababneh, N.A.; Bakri, F.G.; Mahafzah, A. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. Vaccines 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, S.; Osborne, V.; Lynn, E.; Shakir, S. Postmarketing Studies: Can They Provide a Safety Net for COVID-19 Vaccines in the UK? BMJ Evid.-Based Med. 2022, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mehraeen, E.; Karimi, A.; Barzegary, A.; Vahedi, F.; Afsahi, A.M.; Dadras, O.; Moradmand-Badie, B.; Seyed Alinaghi, S.A.; Jahanfar, S. Predictors of Mortality in Patients with COVID-19–a Systematic Review. Eur. J. Integr. Med. 2020, 40, 101226. [Google Scholar] [CrossRef]
- Sepandi, M.; Taghdir, M.; Alimohamadi, Y.; Afrashteh, S.; Hosamirudsari, H. Factors Associated with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2020, 49, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Roberts, M.K.; Herring, T.E.; Alschuler, K.N. Willingness to Obtain COVID-19 Vaccination in Adults with Multiple Sclerosis in the United States. Mult. Scler. Relat. Disord. 2021, 49, 102788. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Williams, L.; Flowers, P.; McLeod, J.; Young, D.; Rollins, L.; The CATALYST Project Team. Social Patterning and Stability of Intention to Accept a COVID-19 Vaccine in Scotland: Will Those Most at Risk Accept a Vaccine? Vaccines 2021, 9, 17. [Google Scholar] [CrossRef]
- Callaghan, T.; Moghtaderi, A.; Lueck, J.A.; Hotez, P.; Strych, U.; Dor, A.; Fowler, E.F.; Motta, M. Correlates and Disparities of Intention to Vaccinate against COVID-19. Soc. Sci. Med. 2021, 272, 113638. [Google Scholar] [CrossRef]
- Patalon, T.; Gazit, S.; Pitzer, V.E.; Prunas, O.; Warren, J.L.; Weinberger, D.M. Odds of Testing Positive for SARS-CoV-2 Following Receipt of 3 vs 2 Doses of the BNT162b2 MRNA Vaccine. JAMA Intern. Med. 2022, 182, 179. [Google Scholar] [CrossRef]
- Bari, M.S.; Hossain, M.J.; Ahmmed, F.; Sarker, M.M.R.; Khandokar, L.; Chaithy, A.P.; Aziz, F.; Mitra, S.; Emran, T.B.; Islam, M.S.; et al. Knowledge, Perception, and Willingness towards Immunization among Bangladeshi Population during COVID-19 Vaccine Rolling Period. Vaccines 2021, 9, 1449. [Google Scholar] [CrossRef]
- Šljivo, A.; Ćetković, A.; Abdulkhaliq, A.; Kiseljaković, M.; Selimović, A.; Kulo, A. COVID-19 Vaccination Knowledge, Attitudes and Practices among Residents of Bosnia and Herzegovina during the Third Wave of COVID-19 Outbreak. Ann. Ig. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Lu, P.; Kong, D.; Shelley, M. Risk Perception, Preventive Behavior, and Medical Care Avoidance among American Older Adults During the COVID-19 Pandemic. J. Aging Health 2021, 33, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.J.; Amlot, R.; Page, L.; Wessely, S. Public Perceptions, Anxiety, and Behaviour Change in Relation to the Swine Flu Outbreak: Cross Sectional Telephone Survey. BMJ 2009, 339, b2651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-H.; Chen, L.; Pan, Q.-N.; Liu, J.; Zhang, X.; Yi, J.-J.; Chen, C.-M.; Luo, Q.-H.; Tao, P.-Y.; Pan, X.; et al. Vaccination Status, Acceptance, and Knowledge toward a COVID-19 Vaccine among Healthcare Workers: A Cross-Sectional Survey in China. Hum. Vaccines Immunother. 2021, 17, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, F.; Menon, V.; Jensen, J.L.; Shariff, M.A.; Pillai, A.; Venugopal, U.; Kasubhai, M.; Dimitrov, V.; Kanna, B.; Poole, B.D. Knowledge, Attitudes and Perceptions of COVID-19 Vaccination among Healthcare Workers of an Inner-City Hospital in New York. Vaccines 2021, 9, 516. [Google Scholar] [CrossRef]
- Tadele Admasu, F. Knowledge and Proportion of COVID-19 Vaccination and Associated Factors Among Cancer Patients Attending Public Hospitals of Addis Ababa, Ethiopia, 2021: A Multicenter Study. Infect. Drug Resist. 2021, 14, 4865–4876. [Google Scholar] [CrossRef]
- Huynh, G.; Nguyen, T.V.; Nguyen, D.D.; Lam, Q.M.; Pham, T.N.; Nguyen, H.T.N. Knowledge About COVID-19, Beliefs and Vaccination Acceptance Against COVID-19 Among High-Risk People in Ho Chi Minh City, Vietnam. Infect. Drug Resist. 2021, 14, 1773–1780. [Google Scholar] [CrossRef]



| Reference | Country | Data Collection Time | Sample Size (N) | Females (%) | Age, Mean (Standard Deviation) | Study Design | Sampling Method | Recruitment Method | Response Rate (%) | Published In |
|---|---|---|---|---|---|---|---|---|---|---|
| Lounis et al. [30] | Algeria | January to March 2022 | 787 | 61.6 | 18–40 years, 58.2%; 41–60 years, 36.8%; >60 years, 5% | Cross-sectional | Snowball | Online survey | NA | Journal |
| Rzymski et al. [34] | Poland | September 2021 | 2427 | 50.7 | <50 years, 62.3%; ≥50 years, 37.7% | Cross-sectional | Snowball | Online survey | NA | Journal |
| Lai et al. [29] | China | June 2021 | 1145 | 50.3 | ≤40 years, 65.7%; 41–59 years, 34.3% | Cross-sectional | Snowball | Online survey | NA | Journal |
| Al-Qerem et al. [26] | Jordan | October to December 2021 | 915 | NR | 18–29 years, 45.7%; ≥30 years, 54.3% | Cross-sectional | Convenience | Online survey | NA | Journal |
| Wu et al. [37] | China | October 2021 | 8229 | 69.0 | 26–45 years, 78.5%; ≥46 years, 21.5% | Cross-sectional | Snowball | Online survey | NA | Journal |
| Miao et al. [31] | China | August 2021 | 26,755 | 52.6 | <40 years, 83.8%; ≥40 years, 16.2% | Cross-sectional | Snowball | Online survey | NA | Journal |
| Chu et al. [27] | Vietnam | November 2021 | 900 | 25.7 | 18–44 years, 91.8%; ≥45 years, 8.2% | Cross-sectional | Convenience | Online survey | NA | Journal |
| Jørgensen et al. [28] | Denmark | December 2021 | 31,721 | NR | NR | Cross-sectional | Stratified random | Online survey | 25 | Journal |
| Wang et al. [35] | China | April to May 2021 | 2047 | 59.3 | 18–44 years, 64.7%; ≥45 years, 35.3% | Cross-sectional | Snowball | Online survey | NA | Journal |
| Rababa’h et al. [33] | Jordan | August 2021 | 413 | 76 | 18–39 years, 74.6%; ≥40 years, 25.4% | Cross-sectional | Convenience | Online survey | NA | Journal |
| Paul et al. [32] | United Kingdom | November to December 2021 | 22,139 | 51 | 18–44 years, 44%; ≥45 years, 56% | Cross-sectional | Convenience | Online survey | NA | Journal |
| Yadete et al. [38] | USA | July 2021 | 1456 | 49.7 | NR | Cross-sectional | Convenience | Online survey | NA | Journal |
| Yoshida et al. [39] | Japan | September to October 2021 | 2439 | 58.3 | 52.6 (19.3) | Cohort | Convenience | Online survey | NR | Journal |
| Wirawan et al. [36] | Indonesia | December 2021 to January 2022 | 2674 | 58 | 29 (24–35) a | Cross-sectional | Convenience | Online survey | NA | Journal |
| Reference | Question/Statement to Measure Patients’ Willingness | Response Scale a | Willingness Results (%) |
|---|---|---|---|
| Lounis et al. [30] | Are you willing to receive the potential additional dose of the COVID-19 vaccine if it would be made available? | Yes (Y), unsure (U), no (N) | Yes: 51.6 Unsure: 23.4 No: 25 |
| Rzymski et al. [34] | Are you willing to receive the potential additional dose of the COVID-19 vaccine if it would be made available? | Yes (Y), unsure (U), no (N) | Yes: 71.0 Unsure: 4.3 No: 24.7 |
| Lai et al. [29] | If a COVID-19 booster is recommended as a supplement to the current vaccination schedule, would you accept it? | Yes (Y), no (N) | Yes: 84.8 No: 15.2 |
| Al-Qerem et al. [26] | Are you willing to take the booster dose? | Yes (Y), unsure (U), no (N) | Yes: 44.6 Unsure: 24.7 No: 30.7 |
| Wu et al. [37] | To what extent do you want to take the booster COVD-19 vaccine? | Yes, definitely (Y), unsure but tend to be willing (U), unsure but tend to be unwilling (U), definitely no (N) | Yes: 76.8 Unsure: 22.2 No: 1.0 |
| Miao et al. [31] | Are you willing to take the booster dose? | Very willing (Y), willing (Y), fair (U), unwilling (U), very unwilling (U), don’t know (U) | Yes: 93.8 Unsure: 6.2 |
| Chu et al. [27] | If an extra dose of COVID-19 vaccine is available, would you get it? | Definitely yes (Y), probably yes (Y), probably no (N), definitely no (N) | Yes: 93.7 No: 6.3 |
| Jørgensen et al. [28] | Will you accept the booster vaccine? | I have received the booster dose (Y), I have not yet received the invitation to the booster dose, but I wish to be vaccinated with the booster dose (Y), I have received the invitation to the booster dose, and I wish to be vaccinated, but have not yet been vaccinated with the booster dose (Y), I have received an invitation to the booster dose, but a do not wish the booster dose (N), I have not yet received the invitation to the booster dose, and I do not wish to be vaccinated with the booster dose (N), Do not want to answer (N) | Yes: 95.5 No: 4.5 |
| Wang et al. [35] | Are you willing to receive the potential additional dose of the COVID-19 vaccine if it would be made available? | Yes (Y), no (N) | Yes: 75.2 No: 24.8 |
| Rababa’h et al. [33] | Do you accept receiving the COVID-19 booster vaccine? | Yes (Y), unsure (U), no (N) | Yes: 54.5 Unsure: NR No: NR |
| Paul et al. [32] | How likely do you think you are to get a COVID-19 booster vaccine if/when you are offered one? | A scale from 1 (very unlikely) to 6 (very likely); 5–6 (Y), 3–4 (U), 1–2 (N) | Yes: 92.3 Unsure: 4.1 No: 3.6 |
| Yadete et al. [38] | Do you accept receiving the COVID-19 booster vaccine? | Yes (Y), no (N) | Yes: 79.1 No: 20.9 |
| Yoshida et al. [39] | Do you accept receiving the COVID-19 booster vaccine? | Yes (Y), no (N) | Yes: 97.9 No: 2.1 |
| Wirawan et al. [36] | Will you accept the booster vaccine? | I have received the booster dose (Y), A scale from 1 (would not accept) to 5 (certainly would accept); 5 (Y), 1–4 (N) | Yes: 56.3 No: 43.7 |
| Reference | Older Age | Males | Married | Higher Educational Level | Ethnicity | Higher Income | Healthcare Workers | Residence | Chronic Comorbidities | History of COVID-19 Infection | Hospitalization | Higher Risk Perception of Infection | Higher Severity Perception of COVID-19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lounis et al. [30] | ↑ | ↑ | NS | ↓ | - | - | ↓ | NS | ↑ | ↑ | NS | - | - |
| Rzymski et al. [34] | ↑ | ↓ | - | NS | - | - | - | NS | ↑ | ↑ | - | - | - |
| Lai et al. [29] | ↓ | NS | NS | ↓ | - | - | - | NS | NS | ↑ | - | NS | NS |
| Al-Qerem et al. [26] | NS | - | - | NS | - | - | - | NS | - | - | - | NS | NS |
| Wu et al. [37] | ↑ | - | - | - | - | - | ↑ | - | - | - | - | ↑ | ↓ |
| Miao et al. [31] | ↑ | ↓ | ↑ | ↑ | - | - | - | NS | ↓ | - | - | - | - |
| Chu et al. [27] | NS | NS | NS | ↑ | NS | NS | NS | NS | NS | NS | - | - | - |
| Jørgensen et al. [28] | ↑ | NS | - | NS | - | - | - | - | - | - | - | - | ↑ |
| Wang et al. [35] | ↓ | ↑ | - | ↓ | - | NS | ↓ | NS | - | - | - | - | - |
| Paul et al. [32] | ↑ | NS | NS | ↑ | NS | ↑ | NS | NS | ↑ | NS | - | ↑ | ↑ |
| Yoshida et al. [39] | ↑ | NS | - | - | - | - | - | - | - | - | - | - | - |
| Wirawan et al. [36] | ↑ | NS | - | ↑ | - | ↑ | - | - | - | NS | - | ↑ | ↑ |
| Positive association a | 8/12 | 2/10 | 1/5 | 4/10 | 0/2 | 2/4 | 1/5 | 0/8 | 3/6 | 3/6 | 0/1 | 3/5 | 3/6 |
| Negative association b | 2/12 | 2/10 | 0/5 | 3/10 | 0/2 | 0/4 | 2/5 | 0/8 | 1/6 | 0/6 | 0/1 | 0/5 | 1/6 |
| No association c | 2/12 | 6/10 | 4/5 | 3/10 | 2/2 | 2/4 | 2/5 | 8/8 | 2/6 | 3/6 | 1/1 | 2/5 | 2/6 |
| Reference | Infection in Family | Mortality in Family | Influenza Vaccine | Confidence in Healthcare System/Government/Physicians | Confidence in COVID-19 Vaccines | Confidence in COVID-19 Booster Doses | Compliance with Prevention Measures | Adverse Reactions and Discomfort Experienced after Previous Doses | Concerns for Serious Adverse Reactions to COVID-19 Booster Doses | ||||
| Lounis et al. [30] | NS | NS | NS | ↑ | ↑ | ↑ | - | - | - | ||||
| Rzymski et al. [34] | - | - | ↑ | - | - | ↑ | - | ↓ | ↓ | ||||
| Lai et al. [29] | - | - | - | - | - | ↑ | - | - | ↓ | ||||
| Miao et al. [31] | - | - | - | ↑ | - | - | - | ↓ | - | ||||
| Wang et al. [35] | - | - | ↑ | - | ↑ | - | - | - | - | ||||
| Paul et al. [32] | - | - | - | NS | - | - | ↑ | - | - | ||||
| Al-Qerem et al. [26] | - | - | - | - | - | - | - | ↓ | - | ||||
| Wu et al. [37] | - | - | - | - | - | - | - | ↓ | - | ||||
| Wirawan et al. [36] | NS | - | - | - | - | ↑ | - | - | - | ||||
| Positive association a | 0/2 | 0/1 | 2/3 | 2/3 | 2/2 | 4/4 | 1/1 | 0/4 | 0/2 | ||||
| Negative association b | 0/2 | 0/1 | 0/3 | 0/3 | 0/2 | 0/4 | 0/1 | 4/4 | 2/2 | ||||
| No association c | 2/2 | 1/1 | 1/3 | 1/3 | 0/2 | 0/4 | 0/1 | 0/4 | 0/2 | ||||
| Reference | Desire to Travel Abroad | Harm in Immune System | Further Vaccination Is Unnecessary | Low Safety of COVID-19 Booster Doses | Knowledge Level | Initial Uncertainty and Unwillingness to Accept the First COVID-19 Vaccine | |||||||
| Lounis et al. [30] | ↑ | ↓ | - | - | - | - | |||||||
| Rzymski et al. [34] | - | - | ↓ | - | - | - | |||||||
| Lai et al. [29] | - | - | - | ↓ | - | - | |||||||
| Al-Qerem et al. [26] | - | - | - | - | NS | - | |||||||
| Paul et al. [32] | - | - | - | - | NS | ↓ | |||||||
| Positive association a | 1/1 | 0/1 | 0/1 | 0/1 | 0/2 | 0/1 | |||||||
| Negative association b | 0/1 | 1/1 | 1/1 | 1/1 | 0/2 | 1/1 | |||||||
| No association c | 0/1 | 0/1 | 0/1 | 0/1 | 2/2 | 0/1 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galanis, P.; Vraka, I.; Katsiroumpa, A.; Siskou, O.; Konstantakopoulou, O.; Katsoulas, T.; Mariolis-Sapsakos, T.; Kaitelidou, D. First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors. Vaccines 2022, 10, 1097. https://doi.org/10.3390/vaccines10071097
Galanis P, Vraka I, Katsiroumpa A, Siskou O, Konstantakopoulou O, Katsoulas T, Mariolis-Sapsakos T, Kaitelidou D. First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors. Vaccines. 2022; 10(7):1097. https://doi.org/10.3390/vaccines10071097
Chicago/Turabian StyleGalanis, Petros, Irene Vraka, Aglaia Katsiroumpa, Olga Siskou, Olympia Konstantakopoulou, Theodoros Katsoulas, Theodoros Mariolis-Sapsakos, and Daphne Kaitelidou. 2022. "First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors" Vaccines 10, no. 7: 1097. https://doi.org/10.3390/vaccines10071097
APA StyleGalanis, P., Vraka, I., Katsiroumpa, A., Siskou, O., Konstantakopoulou, O., Katsoulas, T., Mariolis-Sapsakos, T., & Kaitelidou, D. (2022). First COVID-19 Booster Dose in the General Population: A Systematic Review and Meta-Analysis of Willingness and Its Predictors. Vaccines, 10(7), 1097. https://doi.org/10.3390/vaccines10071097









