Knowledge of University Students in Health Care Settings on Vaccines and Vaccinations Strategies: Impact Evaluation of a Specific Educational Training Course during the COVID-19 Pandemic Period in Italy
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
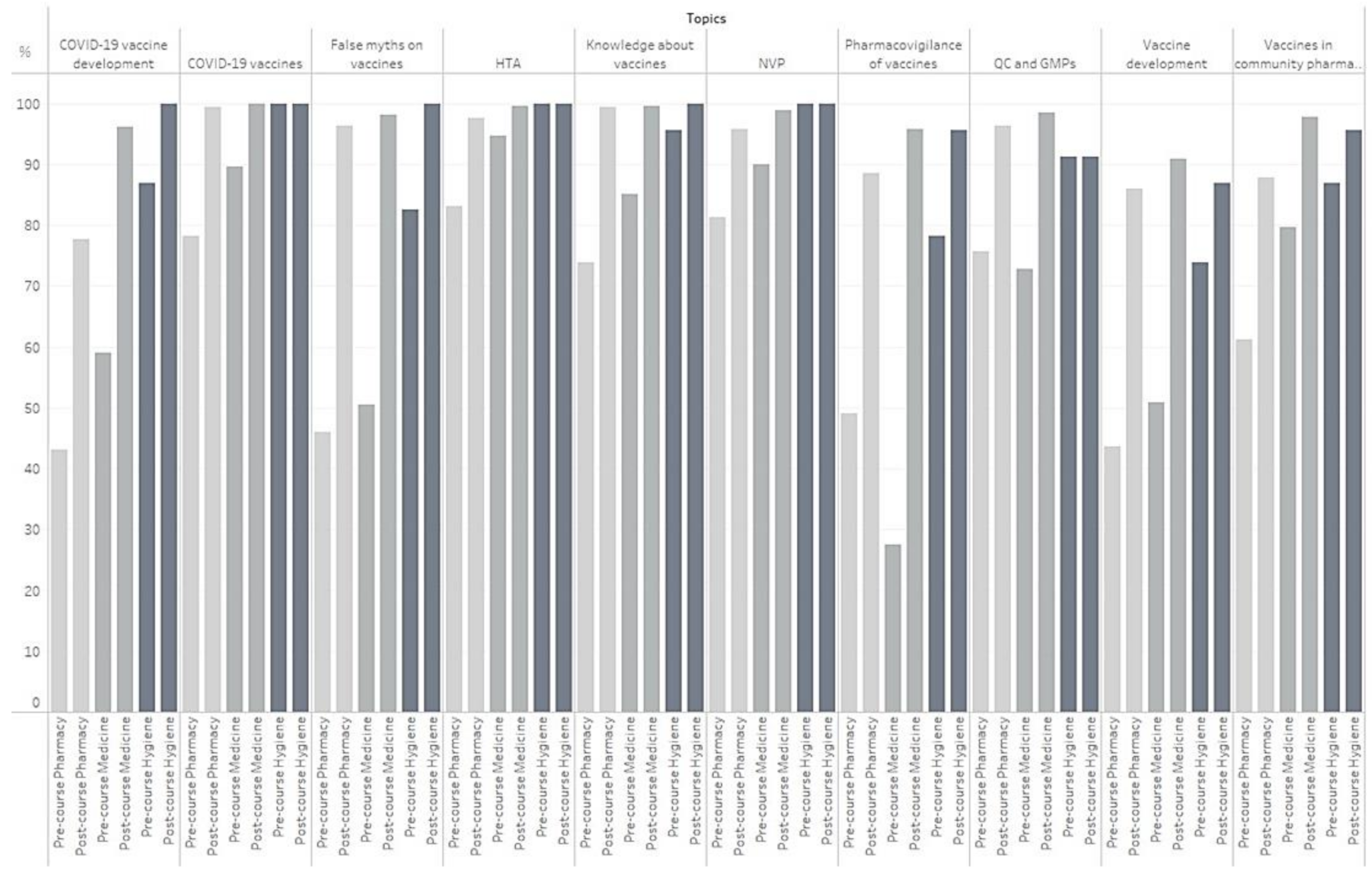
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- From the Centers for Disease Control and Prevention. Achievements in Public Health, 1900–1999: Changes in the Public Health System. JAMA 2000, 283, 735–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11 (Suppl. 4), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Gagnon, D.; Ouakki, M.; Bettinger, J.A.; Guay, M.; Halperin, S.; Wilson, K.; Graham, J.; Witteman, H.O.; MacDonald, S.; et al. Canadian Immunization Research Network. Understanding Vaccine Hesitancy in Canada: Results of a Consultation Study by the Canadian Immunization Research Network. PLoS ONE 2016, 11, e0156118. [Google Scholar] [CrossRef] [PubMed]
- Eskola, J.; Duclos, P.; Schuster, M.; MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. How to deal with vaccine hesitancy? Vaccine 2015, 33, 4215–4217. [Google Scholar] [CrossRef] [PubMed]
- Giambi, C.; Fabiani, M.; D’Ancona, F.; Ferrara, L.; Fiacchini, D.; Gallo, T.; Martinelli, D.; Pascucci, M.G.; Prato, R.; Filia, A.; et al. Parental vaccine hesitancy in Italy—Results from a national survey. Vaccine 2018, 36, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Omer, S.B.; Bednarczyk, R.A.; Halsey, N.A.; Moulton, L.H.; Salmon, D.A. Parents’ source of vaccine information and impact on vaccine attitudes, beliefs, and nonmedical exemptions. Adv. Prev. Med. 2012, 2012, 932741. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.; Muhlhans, S.; Gaedicke, G. Teaching vaccine safety communication to medical students and health professionals. Curr. Drug. Saf. 2015, 10, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Afonso, N.; Kavanagh, M.; Swanberg, S. Improvement in attitudes toward influenza vaccination in medical students following an integrated curricular intervention. Vaccine 2014, 32, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Newall, A.; Heywood, A.E. Knowledge, attitudes and practices of Australian medical students towards influenza vaccination. Vaccine 2016, 34, 6193–6199. [Google Scholar] [CrossRef] [PubMed]
- Bechini, A.; Moscadelli, A.; Sartor, G.; Shtylla, J.; Guelfi, M.R.; Bonanni, P.; Boccalini, S. Impact assessment of an educational course on vaccinations in a population of medical students. J. Prev. Med. Hyg. 2019, 60, E171–E177. [Google Scholar] [CrossRef] [PubMed]
- Marotta, C.; Raia, D.D.; Ventura, G.; Casuccio, N.; Dieli, F.; D’Angelo, C.; Restivo, V.; Costantino, C.; Vitale, F.; Casuccio, A. Improvement in vaccination knowledge among health students following an integrated extra curricular intervention, an explorative study in the University of Palermo. J. Prev. Med. Hyg. 2017, 58, E93–E98. [Google Scholar] [PubMed]
- Kernéis, S.; Jacquet, C.; Bannay, A.; May, T.; Launay, O.; Verger, P.; Pulcini, C.; EDUVAC Study Group. Vaccine Education of Medical Students: A Nationwide Cross-sectional Survey. Am. J. Prev. Med. 2017, 53, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Vorsters, A.; Tack, S.; Hendrickx, G.; Vladimirova, N.; Bonanni, P.; Pistol, A.; Metlicar, T.; Pasquin, M.J.; Mayer, M.A.; Aronsson, B.; et al. A summer school on vaccinology: Responding to identified gaps in pre-service immunisation training of future health care workers. Vaccine 2010, 28, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.D.; Bechini, A.; Buscemi, P.; Bonanni, P.; On Behalf of the Working Group Dhs; Boccalini, S. Reasons for the Intention to Refuse COVID-19 Vaccination and Their Association with Preferred Sources of Information in a Nationwide, Population-Based Sample in Italy, before COVID-19 Vaccines Roll Out. Vaccines 2022, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Wang, W.; Zhang, R.; Du, M.; Shan, L.; Li, Y.; Wang, X.; Liu, Y.; Zhang, W.; et al. Effect of an educational intervention on human papillomavirus (HPV) knowledge and attitudes towards HPV vaccines among healthcare workers (HCWs) in Western China. Hum. Vaccin. Immunother. 2021, 17, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Sabella, E.A.; Roma, P.; De Giglio, O.; Caggiano, G.; Tafuri, S.; Da Molin, G.; Ferracuti, S.; Montagna, M.T.; Liguori, G.; et al. Knowledge and Acceptance of COVID-19 Vaccination among Undergraduate Students from Central and Southern Italy. Vaccines 2021, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Paterson, P.; Meurice, F.; Stanberry, L.R.; Glismann, S.; Rosenthal, S.L.; Larson, H.J. Vaccine hesitancy and healthcare providers. Vaccine 2016, 34, 6700–6706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; While, A.E.; Norman, I.J. Nurses’ vaccination against pandemic H1N1 influenza and their knowledge and other factors. Vaccine 2012, 30, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
| Year of Study | |||||||
|---|---|---|---|---|---|---|---|
| Faculty | Students N = 449 (%) | Males N = 148 (%) | Females N = 301 (%) | III | IV | V or More | Postgraduation |
| Pharmacy | 165 (36.8) | 38 (25.7) | 127 (42.2) | 15 | 44 | 106 | - |
| Medicine | 261 (58.1) | 101 (68.2) | 160 (53.1) | 7 | 11 | 243 | - |
| Hygiene | 23 (5.1) | 9 (6.1) | 14 (4.7) | - | - | - | 23 |
| Faculty | N Students (%) | Score Pre | Score Post | p-Value | Δ% |
|---|---|---|---|---|---|
| Pharmacy, mean ± SD | 165 (36.8) | 19.3 ± 4.3 | 26.9 ± 3.0 | <0.001 * | +27.3% |
| Medicine, mean ± SD | 261 (58.1) | 22.0 ± 4.4 | 30.7 ± 2.9 | <0.001 * | +27.6% |
| Hygiene, mean ± SD | 23 (5.1) | 26.3 ± 2.7 | 29.6 ± 3.4 | <0.001 * | +10.8% |
| Overall, mean ± SD | 449 | 21.2 ± 4.6 | 29.2 ± 3.4 | <0.001 * | +27.3% |
| Faculty | N Students N = 449 (%) | Score Pre | p-Value | Score Post | p-Value | p-Value Pre-/Post-ETA |
|---|---|---|---|---|---|---|
| Pharmacy | ||||||
| Males, mean ± SD | 38 (25.7) | 18.1 ± 4.3 | 0.052 | 25.9 ± 3.5 | 0.035 | <0.001 |
| Females, mean ± SD | 127 (42.2) | 19.6 ± 4.2 | 27.1 ± 2.8 | <0.001 | ||
| Medicine | ||||||
| Males, mean ± SD | 101 (68.2) | 21.6 ± 4.7 | 0.191 | 30.7 ± 2.5 | 0.991 | <0.001 |
| Females, mean ± SD | 160 (53.2) | 22.3 ± 4.2 | 30.7 ± 3.1 | <0.001 | ||
| Hygiene | ||||||
| Males, mean ± SD | 9 (6.1) | 26.6 ± 2.6 | 0.730 | 29.8 ± 2.7 | 0.854 | 0.012 |
| Females, mean ± SD | 14 (4.7) | 26.1 ± 2.9 | 29.6 ± 2.1 | 0.005 |
| Incorrect Answers | Overall N = 449 (%) | Pharmacy N = 165 (%) | Medicine N = 261 (%) | Hygiene N = 23 (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Question 1—Vaccines: | 152 (33.9) | 23 (5.1) | 52 (31.5) | 16 (9.7) | 94 (36.0) | 7 (2.7) | 6 (26.1) | - |
| Are comparable in all respects to drugs | 93 (20.7) | 15 (3.3) | 29 (17.6) | 10 (6.1) | 58 (22.2) | 5 (1.9) | 6 (26.1) | - |
| Allow to treat people | 8 (1.8) | - | 1 (0.6) | - | 7 (2.7) | - | - | - |
| All act only at the level of the individual vaccinated subject | 51 (11.4) | 8 (1.8) | 22 (13.3) | 6 (3.6) | 29 (11.1) | 2 (0.8) | - | - |
| Question 2—Combined vaccines are: | 48 (10.7) | 5 (1.1) | 25 (15.2) | 3 (1.8) | 22 (8.4) | 2 (0.8) | 1 (4.4) | - |
| Produced using the recombinant DNA technique | 25 (5.6) | 3 (0.7) | 13 (7.9) | 3 (1.8) | 11 (4.2) | - | 1 (4.4) | - |
| Different vaccines administered in the same vaccination session but in different anatomical sites | 10 (2.2) | 1 (0.2) | 3 (1.8) | - | 7 (2.7) | 1 (0.4) | - | - |
| Very effective but unfortunately they always result in an exponential increase in adverse events | 13 (2.9) | 1 (0.2) | 9 (5.5) | - | 4 (1.5) | 1 (0.4) | - | - |
| Question 3—Flu vaccines actually available in Italy are: | 160 (35.6) | 23 (5.1) | 81 (49.1) | 18 (10.9) | 77 (29.5) | 5 (1.9) | 2 (8.7) | - |
| Whole inactivated virus | 127 (28.3) | 18 (4.0) | 67 (40.6) | 14 (8.5) | 58 (22.2) | 4 (1.5) | 2 (8.7) | - |
| Based on anatoxins | 9 (2.0) | 5 (1.1) | 3 (1.8) | 4 (2.4) | 6 (2.3) | 1 (0.4) | - | - |
| Based on polysaccharides | 24 (5.4) | - | 11 (6.7) | - | 13 (5.0) | - | - | - |
| Question 4—What was the first SARS-CoV-2 vaccine to be approved by EMA? | 8 (1.8) | 2 (0.4) | 6 (3.6) | - | 2 (0.8) | 2 (0.8) | - | - |
| Moderna | 2 (0.5) | - | 2 (1.2) | - | - | - | - | - |
| Oxford—AstraZeneca | 4 (0.9) | 1 (0.2) | 4 (2.4) | - | - | 1 (0.4) | - | - |
| Sanofi—GSK | 1 (0.2) | - | - | - | 1 (0.4) | - | - | - |
| J and J (Johnson and Johnson) | 1 (0.2) | 1 (0.2) | - | - | 1 (0.4) | 1 (0.4) | - | - |
| Question 5—The Pfizer-Biontech vaccine differs from the Moderna vaccine in one of the following options: | 127 (28.3) | 21 (4.7) | 66 (40.0) | 14 (8.5) | 60 (23.0) | 7 (2.7) | 1 (4.4) | - |
| It does not need to be reconstituted with the physiological solution. unlike the Moderna vaccine which must be reconstituted | 17 (3.8) | 10 (2.2) | 10 (6.1) | 8 (4.9) | 7 (2.9) | 2 (0.8) | - | - |
| It exploits the mechanism of messenger RNA (mRNA). unlike the Moderna which is made up of purified antigens | 60 (13.4) | 2 (0.5) | 35 (21.2) | 2 (1.2) | 24 (9.2) | - | 1 (4.4) | - |
| It cannot be administered to subjects over 55 years of age | 6 (1.3) | 2 (0.5) | 5 (3.0) | 1 (0.6) | 1 (0.4) | 1 (0.4) | - | - |
| None of the above | 44 (9.8) | 7 (1.6) | 16 (9.7) | 3 (1.8) | 28 (10.7) | 4 (1.5) | - | - |
| Question 6—How many doses of Pfizer-Biontech vaccine can be obtained from each vial. according to the latest AIFA legislation? | 117 (26.1) | 3 (0.7) | 57 (34.6) | 2 (1.2) | 59 (22.6) | 1 (0.4) | 0 (4.4) | - |
| 1 dose | 30 (6.7) | 2 (0.5) | 16 (9.7) | 2 (1.2) | 14 (5.4) | - | - | - |
| 3 doses | 24 (5.4) | - | 14 (8.5) | - | 10 (3.8) | - | - | - |
| 4 doses | 15 (3.3) | - | 11 (6.7) | - | 4 (1.5) | - | - | - |
| 5 doses | 48 (10.7) | 1 (0.2) | 16 (9.7) | - | 31 (11.9) | 1 (0.4) | 1 (4.4) | - |
| Question 7—Development phases of drugs and vaccines in which safety is also assessed are: | 176 (39.2) | 26 (5.8) | 92 (55.8) | 13 (7.9) | 80 (30.6) | 9 (3.4) | 4 (17.4) | 4 (17.4) |
| Phases 1 and 2 | 62 (13.8) | 10 (2.2) | 20 (12.1) | 3 (1.8) | 39 (14.9) | 4 (1.5) | 3 (13.1) | 3 (13.1) |
| Phases 2 and 3 | 59 (13.1) | 10 (2.2) | 29 (17.6) | 6 (3.6) | 29 (11.1) | 3 (1.1) | 1 (4.4) | 1 (4.4) |
| Phase 4 | 55 (12.3) | 6 (1.3) | 43 (26.1) | 4 (2.4) | 12 (4.6) | 2 (0.8) | - | - |
| Question 8—The selection of adjuvants is performed: | 212 (47.2) | 67 (14.9) | 77 (46.7) | 30 (18.2) | 126 (48.3) | 28 (10.7) | 9 (39.2) | 9 (39.2) |
| In phase 3 | 41 (9.1) | 7 (1.6) | 12 (7.3) | 2 (1.2) | 28 (10.7) | 4 (1.5) | 1 (4.4) | 1 (4.4) |
| After vaccine authorisation | 13 (2.9) | 2 (0.5) | 8 (4.9) | 2 (1.2) | 5 (1.9) | - | - | - |
| In phase 1-2 | 158 (35.2) | 58 (12.9) | 57 (34.5) | 26 (15.8) | 93 (35.6) | 24 (9.2) | 8 (34.8) | 8 (34.8) |
| Question 9—The first legislative rules on the development of vaccines date back to: | 318 (70.8) | 123 (27.4) | 133 (80.6) | 66 (40.0) | 171 (65.5) | 42 (16.1) | 14 (60.9) | 15 (9.0) |
| 1950 | 184 (41.0) | 61 (13.6) | 76 (46.1) | 31 (18.8) | 103 (39.5) | 30 (11.5) | 5 (21.7) | - |
| 1970 | 97 (21.6) | 16 (3.6) | 46 (27.9) | 9 (5.5) | 47 (18.0) | 6 (2.3) | 4 (17.4) | 1 (4.4) |
| 1802 | 37 (8.2) | 46 (10.2) | 11 (6.7) | 26 (15.8) | 21 (8.1) | 6 (2.3) | 5 (21.7) | 14 (60.9) |
| Question 10—Which of these is one of the main ethical challenges in vaccination against SARS-CoV-2? | 246 (54.8) | 55 (12.2) | 135 (76.8) | 43 (26.1) | 107 (41.0) | 12 (4.6) | 4 (17.4) | - |
| Splitting of the doses | 54 (12.0) | 20 (4.5) | 26 (15.8) | 14 (8.5) | 28 (10.7) | 6 (2.3) | - | - |
| Adverse effects | 62 (13.8) | 16 (3.6) | 31 (18.8) | 15 (9.1) | 31 (11.9) | 1 (0.4) | - | - |
| mRNA technology | 130 (29.0) | 19 (4.2) | 78 (42.3) | 14 (8.5) | 48 (18.4) | 5 (1.9) | 4 (17.4) | - |
| Question 11—Which groups of subjects were excluded from pre-marketing testing of SARS-CoV-2 vaccines? | 67 (14.9) | 8 (1.8) | 26 (15.8) | 5 (3.0) | 41 (15.7) | 3 (1.1) | - | - |
| Developing countries | 43 (9.6) | 6 (1.4) | 13 (7.9) | 4 (2.4) | 30 (11.5) | 2 (0.8) | - | - |
| Elderly people | 10 (2.2) | - | 5 (3.0) | - | 5 (1.9) | - | - | - |
| Obese patients | 14 (3.1) | 2 (0.5) | 8 (4.9) | 1 (0.6) | 6 (2.3) | 1 (0.4) | - | - |
| Question 12—Authorisation of the Comirnaty vaccine has been granted: | 342 (76.2) | 174 (38.8) | 129 (78.2) | 131 (79.4) | 197 (75.5) | 37 (14.1) | 16 (69.6) | 6 (26.1) |
| Simultaneously on a global level | 76 (16.9) | 16 (3.6) | 38 (23.0) | 12 (7.3) | 37 (14.2) | 4 (1.5) | 1 (4.4) | - |
| Firstly by the FDA in the USA | 229 (51.0) | 157 (35.0) | 70 (42.4) | 118 (71.5) | 144 (55.2) | 33 (12.5) | 15 (65.2) | 6 (26.1) |
| First. the Chinese government | 37 (8.2) | 1 (0.2) | 21 (12.7) | 1 (0.6) | 16 (6.1) | - | - | - |
| Question 13—How is the Quality Unit structured? | 155 (34.5) | 12 (2.7) | 44 (26.6) | 4 (2.4) | 105 (40.2) | 6 (2.3) | 6 (26.1) | 2 (8.7) |
| Quality Control + Pharmacovigilance | 139 (31.0) | 12 (2.7) | 36 (21.8) | 4 (2.4) | 97 (37.2) | 6 (2.3) | 6 (26.1) | 2 (8.7) |
| Quality Assurance + Device monitoring | 7 (1.6) | - | 4 (2.4) | - | 3 (1.1) | - | - | - |
| None of the above | 9 (2.0) | - | 4 (2.4) | - | 5 (1.9) | - | - | - |
| Question 14—GMP stands for: | 268 (59.7) | 161 (35.9) | 116 (70.3) | 108 (65.4) | 141 (54.0) | 44 (16.9) | 11 (47.8) | 9 (39.1) |
| Standards of good manufacturing | 12 (2.7) | 4 (0.9) | 9 (5.5) | 4 (2.4) | 3 (1.1) | - | - | - |
| Good Manufacturing Practices | 251 (55.9) | 156 (34.7) | 103 (62.4) | 104 (63.0) | 137 (52.5) | 43 (16.5) | 11 (47.8) | 9 (39.1) |
| Standards of good production practice | 5 (1.1) | 1 (0.2) | 4 (2.4) | - | 1 (0.4) | 1 (0.4) | - | - |
| Question 15—The materials for the production of a vaccine: | 20 (4.5) | 8 (1.8) | 11 (6.7) | 3 (1.8) | 9 (3.4) | 4 (1.5) | - | 1 (4.4) |
| Include only the raw materials purchased | 4 (0.9) | - | 1 (0.6) | - | 3 (1.1) | - | - | - |
| Include only packaging materials | 4 (0.9) | - | 3 (1.8) | - | 1 (0.4) | - | - | - |
| Are not analysed upon arrival and are stored at controlled temperature and humidity | 12 (2.7) | 8 (1.8) | 7 (4.2) | 3 (1.8) | 5 (1.9) | 4 (1.5) | - | 1 (4.4) |
| Question 16—In the pharmacy vaccines can be found: | 266 (59.2) | 123 (27.4) | 111 (67.3) | 92 (55.7) | 145 (55.6) | 26 (10.0) | 10 (43.5) | 5 (21.8) |
| Only in the refrigerator | 204 (45.4) | 111 (24.7) | 91 (55.2) | 88 (53.3) | 105 (40.2) | 19 (7.3) | 8 (34.8) | 4 (17.4) |
| Only outside the refrigerator | 1 (0.2) | - | 1 (0.6) | - | - | - | - | - |
| All the above | 61 (13.6) | 12 (2.7) | 19 (11.5) | 4 (2.4) | 40 (15.3) | 7 (2.7) | 2 (8.7) | 1 (4.4) |
| Question 17—The most frequent temperature range for thermolabile vaccines is: | 108 (24.1) | 9 (2.0) | 48 (29.1) | 2 (1.2) | 59 (22.6) | 7 (2.7) | 1 (4.4) | - |
| Below −15 °C | 86 (19.2) | 7 (1.6) | 39 (23.6) | 2 (1.2) | 46 (17.6) | 5 (1.9) | 1 (4.4) | - |
| Between 15 °C and 25 °C | 10 (2.2) | - | 5 (3.0) | - | 5 (1.9) | - | - | - |
| Between 8 °C and 15 °C | 12 (2.7) | 2 (0.5) | 4 (2.4) | - | 8 (3.1) | 2 (0.8) | - | - |
| Question 18—The cold chain includes: | 92 (6.9) | 35 (7.6) | 55 (33.3) | 29 (17.6) | 34 (13.0) | 5 (1.9) | 3 (13.1) | - |
| A final report of the load temperatures along the entire route and during storage in the pharmacy | 20 (4.5) | 6 (1.3) | 11 (6.7) | 3 (1.8) | 7 (2.7) | 3 (1.1) | 2 (8.7) | - |
| Constant temperature monitoring by drivers and control centres | 11 (2.5) | 4 (0.9) | 7 (4.2) | 3 (1.8) | 4 (1.5) | 1 (0.4) | - | - |
| The use of temperature-controlled equipment | 61 (13.6) | 24 (5.4) | 37 (22.4) | 23 (13.9) | 23 (8.8) | 1 (0.4) | 1 (4.4) | - |
| Question 19—AEFI stands for: | 116 (25.8) | 8 (1.8) | 47 (28.5) | 2 (1.2) | 64 (24.5) | 6 (2.3) | 5 (21.8) | - |
| Association of Italian Exhibitions and Fairs | 32 (7.1) | 5 (1.1) | 21 (12.7) | 2 (1.2) | 10 (3.8) | 3 (1.1) | 1 (4.4) | - |
| Adverse Events Following Injection | 77 (17.2) | 3 (0.7) | 21 (12.7) | - | 52 (19.9) | 3 (1.1) | 4 (17.4) | - |
| None of the above | 7 (1.6) | - | 5 (3.0) | - | 2 (0.8) | - | - | - |
| Question 20—Which of these features is NOT used to classify an AEFI: | 227 (50.6) | 93 (20.7) | 102 (61.8) | 73 (44.2) | 118 (45.2) | 18 (6.9) | 7 (30.4) | 2 (8.7) |
| Errors in vaccination | 182 (40.5) | 48 (10.7) | 64 (38.8) | 36 (21.8) | 111 (42.5) | 11 (4.2) | 7 (30.4) | 1 (4.4) |
| Defects in the quality of the vaccine | 23 (5.1) | 10 (2.2) | 18 (10.9) | 5 (3.0) | 5 (1.9) | 5 (1.9) | - | - |
| Characteristics of the vaccine | 22 (4.9) | 35 (7.8) | 20 (12.1) | 32 (19.4) | 2 (0.8) | 2 (0.8) | - | 1 (4.4) |
| Question 21—To perform the causality assessment of an AEFI. the following is used: | 91 (20.3) | 43 (9.6) | 82 (49.7) | 25 (15.2) | 108 (41.4) | 16 (6.1) | 8 (34.8) | 2 (8.7) |
| CIOMS/RUCAM algorithm | 16 (3.6) | 9 (2.0) | 13 (7.9) | 3 (1.8) | 47 (18.0) | 4 (1.5) | 2 (8.7) | 2 (8.7) |
| Schumock and Thornton algorithm | 25 (5.6) | 15 (3.3) | 24 (14.6) | 12 (7.3) | 31 (11.9) | 3 (1.1) | 1 (4.4) | - |
| Naranjo scale | 50 (11.1) | 19 (4.2) | 45 (27.3) | 10 (6.1) | 30 (11.5) | 9 (3.4) | 5 (21.7) | - |
| Question 22—Which of the following statements is correct? | 89 (19.8) | 23 (5.1) | 85 (51.5) | 13 (7.9) | 89 (34.1) | 8 (3.1) | 3 (13.1) | 2 (8.7) |
| Formaldehyde is used in vaccines as an adjuvant | 55 (12.3) | 15 (3.3) | 53 (32.1) | 10 (6.1) | 47 (18.0) | 5 (1.9) | 1 (4.4) | - |
| The same amount of formaldehyde produced by an infant is present in vaccines | 7 (1.6) | 3 (0.7) | 6 (3.6) | 1 (0.6) | 14 (5.4) | 1 (0.4) | 1 (4.4) | 1 (4.4) |
| No vaccine contains formaldehyde | 27 (6.0) | 5 (1.1) | 26 (15.8) | 2 (1.2) | 28 (10.7) | 2 (0.8) | 1 (4.4) | 1 (4.4) |
| Question 23—Which of the following statements is correct? | 202 (45.0) | 22 (4.9) | 106 (64.2) | 13 (10.6) | 91 (15.7) | 9 (3.4) | 5 (21.8) | - |
| At two months the child’s immune system is not already able to respond to vaccination | 68 (15.1) | 13 (2.9) | 29 (17.6) | 6 (3.6) | 38 (14.6) | 7 (2.7) | 1 (4.4) | - |
| Vaccines weaken the immune system if administered too early | 13 (2.9) | - | 8 (4.9) | - | 3 (1.1) | - | 2 (8.7) | - |
| The newborn’s immune system is fragile and cannot be subjected to more than ten vaccinations in the first year of life | 121 (27.0) | 9 (2.0) | 69 (41.8) | 7 (4.2) | 50 (19.2) | 2 (0.8) | 2 (8.7) | - |
| Question 24—Which of the following statements is correct? | 114 (25.4) | 10 (2.2) | 58 (35.1) | 6 (3.6) | 53 (20.3) | 4 (1.5) | 3 (13.1) | - |
| Aluminium salts are used in vaccines as a preservative | 85 (18.9) | 9 (2.0) | 39 (23.6) | 6 (3.6) | 43 (16.5) | 3 (1.1) | 3 (13.1) | - |
| The aluminum injected into the muscle with vaccines enters the blood immediately | 7 (1.6) | - | 4 (2.4) | - | 3 (1.1) | - | - | - |
| Vaccines must not contain aluminium salts | 22 (4.9) | 1 (0.2) | 15 (9.1) | - | 7 (2.7) | 1 (0.4) | - | - |
| Question 25—According to the Italian National Immunization Plan 2017–2019. which of these vaccinations are recommended in pregnancy? | 129 (28.7) | 22 (4.9) | 57 (34.6) | 17 (10.3) | 72 (27.6) | 5 (1.9) | - | - |
| Hepatitis B | 29 (6.5) | - | 11 (6.7) | - | 18 (6.9) | - | - | - |
| Varicella (Chickenpox) | 12 (2.7) | 1 (0.2) | 7 (4.2) | 1 (0.6) | 5 (1.9) | - | - | - |
| Measles-Mumps-Rubella | 88 (19.6) | 21 (4.7) | 39 (23.6) | 16 (9.7) | 49 (18.8) | 5 (1.9) | - | - |
| Question 26—Which of the following vaccines are mandatory for school attendance under Law 119/2017 in Italy? | 49 (10.9) | 8 (1.8) | 28 (17.0) | 6 (3.6) | 21 (8.0) | 2 (0.8) | - | - |
| Anti-meningococcal | 39 (8.7) | 3 (0.7) | 20 (12.1) | 2 (1.2) | 19 (7.3) | 1 (0.4) | - | - |
| Anti-influenza | 6 (1.3) | 4 (0.9) | 6 (3.6) | 4 (2.4) | - | - | - | - |
| Anti-pneumococcal | 4 (0.9) | 1 (0.2) | 2 (1.2) | - | 2 (0.8) | 1 (0.4) | - | - |
| Question 27—The impact of vaccination programmes is assessed through: | 64 (14.3) | 31 (6.9) | 28 (17.0) | 26 (15.8) | 34 (13.0) | 5 (1.9) | 2 (8.7) | - |
| Monitoring the hospitalizations trend | 30 (6.7) | 17 (3.8) | 14 (8.5) | 17 (10.3) | 16 (6.1) | - | - | - |
| Monitoring of vaccination coverage | 23 (5.1) | 10 (2.2) | 9 (5.5) | 6 (3.6) | 13 (5.0) | 4 (1.5) | 1 (4.4) | - |
| Monitoring the trend of mandatory disease notifications | 11 (2.5) | 4 (0.9) | 5 (3.0) | 3 (1.8) | 5 (1.9) | 1 (0.4) | 1 (4.4) | - |
| Question 28—A vaccine. to be included in the National Plan for Vaccine Prevention and. therefore. be offered actively and free of charge: | 48 (10.7) | 5 (1.1) | 34 (20.6) | 4 (2.4) | 14 (5.4) | 1 (0.4) | - | - |
| It is sufficient that it is not too expensive | - | 2 (0.5) | - | 2 (1.2) | - | - | - | - |
| It is sufficient that has proven effective | 18 (4.0) | 1 (0.2) | 17 (10.3) | 1 (0.6) | 1 (0.4) | - | - | - |
| It is sufficient that it has been shown to be safe | 30 (6.7) | 2 (0.5) | 17 (10.3) | 1 (0.6) | 13 (5.0) | 1 (0.4) | - | - |
| Question 29—The HTA applied to vaccinations includes assessing: | 72 (16.0) | 3 (0.7) | 41 (24.9) | 2 (1.2) | 31 (11.9) | 1 (0.4) | - | - |
| Organizational aspects | 34 (7.6) | 1 (0.2) | 17 (10.3) | 1 (0.6) | 17 (6.5) | - | - | - |
| The ethical aspects | 20 (4.5) | 1 (0.2) | 12 (7.3) | 1 (0.6) | 8 (3.1) | - | - | - |
| Possible alternative interventions | 18 (4.0) | 1 (0.2) | 12 (7.3) | - | 6 (2.3) | 1 (0.4) | - | - |
| Question 30—The economic evaluations of vaccinations show that: | 59 (13.1) | 19 (4.2) | 42 (25.5) | 15 (9.1) | 17 (6.5) | 3 (1.1) | - | 1 (4.4) |
| Vaccinations are only a cost to the NHS | 15 (3.3) | 5 (1.1) | 10 (6.1) | 3 (1.8) | 5 (1.9) | 1 (0.4) | - | 1 (4.4) |
| There is no need to carry out economic assessments for vaccination | 22 (4.9) | 1 (0.2) | 18 (10.9) | 1 (0.6) | 4 (1.5) | - | - | - |
| Only in some rare cases vaccination is cost-effective | 22 (4.9) | 13 (2.9) | 14 (8.5) | 11 (6.7) | 8 (3.1) | 2 (0.8) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccalini, S.; Vannacci, A.; Crescioli, G.; Lombardi, N.; Del Riccio, M.; Albora, G.; Shtylla, J.; Masoni, M.; Guelfi, M.R.; Bonanni, P.; et al. Knowledge of University Students in Health Care Settings on Vaccines and Vaccinations Strategies: Impact Evaluation of a Specific Educational Training Course during the COVID-19 Pandemic Period in Italy. Vaccines 2022, 10, 1085. https://doi.org/10.3390/vaccines10071085
Boccalini S, Vannacci A, Crescioli G, Lombardi N, Del Riccio M, Albora G, Shtylla J, Masoni M, Guelfi MR, Bonanni P, et al. Knowledge of University Students in Health Care Settings on Vaccines and Vaccinations Strategies: Impact Evaluation of a Specific Educational Training Course during the COVID-19 Pandemic Period in Italy. Vaccines. 2022; 10(7):1085. https://doi.org/10.3390/vaccines10071085
Chicago/Turabian StyleBoccalini, Sara, Alfredo Vannacci, Giada Crescioli, Niccolò Lombardi, Marco Del Riccio, Giuseppe Albora, Jonida Shtylla, Marco Masoni, Maria Renza Guelfi, Paolo Bonanni, and et al. 2022. "Knowledge of University Students in Health Care Settings on Vaccines and Vaccinations Strategies: Impact Evaluation of a Specific Educational Training Course during the COVID-19 Pandemic Period in Italy" Vaccines 10, no. 7: 1085. https://doi.org/10.3390/vaccines10071085
APA StyleBoccalini, S., Vannacci, A., Crescioli, G., Lombardi, N., Del Riccio, M., Albora, G., Shtylla, J., Masoni, M., Guelfi, M. R., Bonanni, P., & Bechini, A. (2022). Knowledge of University Students in Health Care Settings on Vaccines and Vaccinations Strategies: Impact Evaluation of a Specific Educational Training Course during the COVID-19 Pandemic Period in Italy. Vaccines, 10(7), 1085. https://doi.org/10.3390/vaccines10071085












