Assessment of Anti m-RNA SARS-CoV-2 (BNT162b2) Antibody Titres in Mother and Child Pairs of Breastfeeding Women Vaccinated Post-Delivery
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
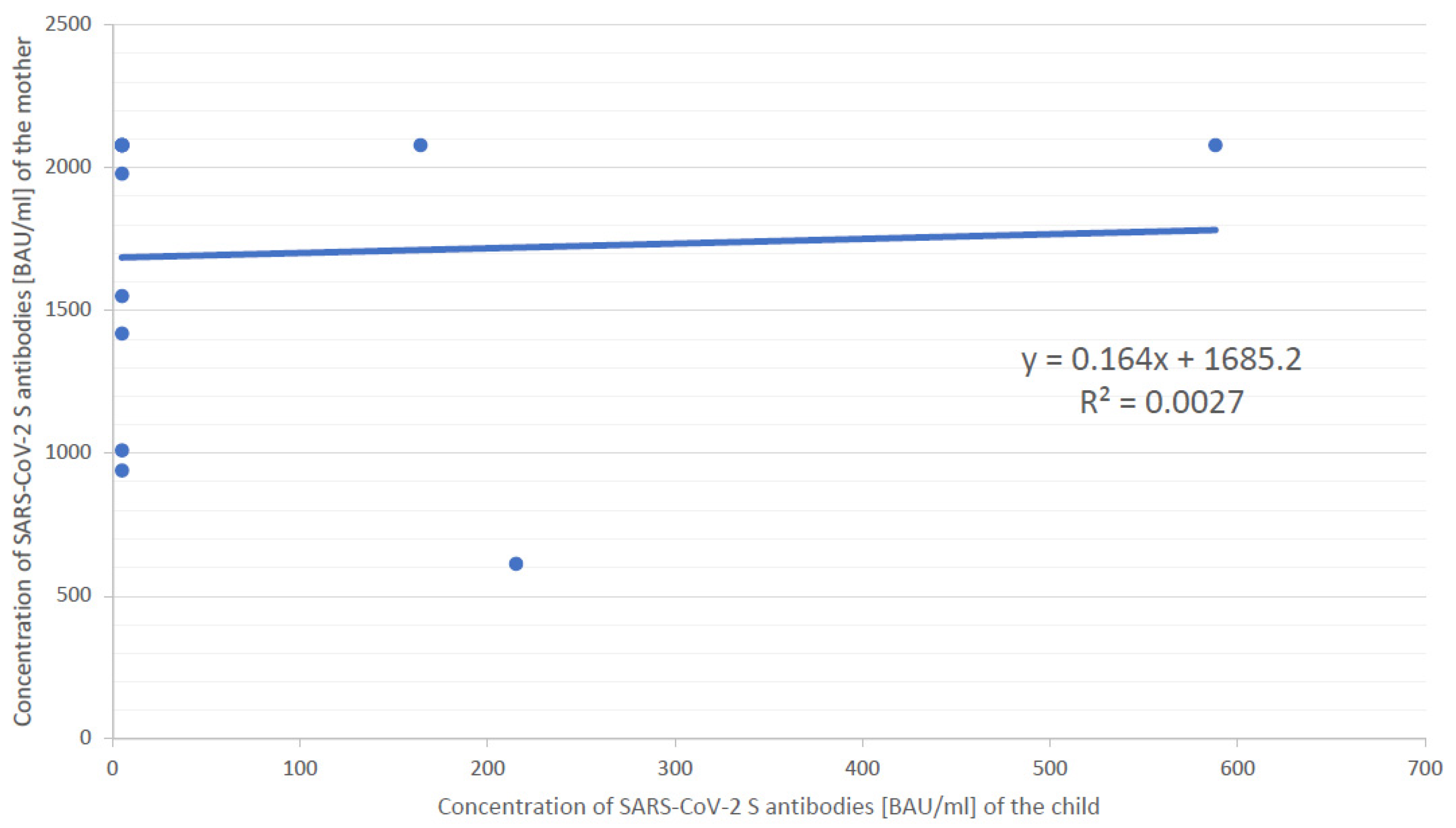
3. Results
4. Discussion
5. Conclusions
- The breastfeeding women vaccinated with BNT162b2 demonstrate anti-SARS-CoV-2 antibodies between 6 and 10 weeks after vaccination.
- It cannot be concluded from this study that breastfeeding by women vaccinated against COVID-19 during lactation does not lead to a passive immune response in their children. Confirmation of the conclusion requires studies on a larger population with a uniform feeding schedule, including in particular exclusively breastfed children.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 7 May 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Adhikari, E.H.; Spong, C.Y. COVID-19 Vaccination in Pregnant and Lactating Women. JAMA 2021, 325, 1039. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.S.A.G.; Clemens, D.R.A.; Pressman, P.; Zaigham, B.M.; Kadkhoda, K.; Davies, K.J.A.; Naidu, A.S. COVID-19 during Pregnancy and Postpartum. J. Diet. Suppl. 2022, 19, 115–142. [Google Scholar] [CrossRef]
- Halasa, N.B.; Olson, S.M.; Staat, M.A.; Newhams, M.M.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Cameron, M.A.; Pannaraj, P.S.; Bline, K.E.; et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19–Associated Hospitalization in Infants Aged <6 Months—17 States, July 2021–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Edlow, A.G. COVID-19 vaccine response in pregnant and lactating women: A cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Falsaperla, R.; Leone, G.; Familiari, M.; Ruggieri, M. COVID-19 vaccination in pregnant and lactating women: A systematic review. Expert Rev. Vaccines 2021, 20, 1619–1628. [Google Scholar] [CrossRef]
- Maertens, K.; Orije, M.R.P.; Van Damme, P.; Leuridan, E. Vaccination during pregnancy: Current and possible future recommendations. Eur. J. Pediatr. 2020, 179, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bränn, E.; Edvinsson, Å.; Punga, A.R.; Sundström-Poromaa, I.; Skalkidou, A. Inflammatory and anti-inflammatory markers in plasma: From late pregnancy to early postpartum. Sci. Rep. 2019, 9, 1863. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trend, S.; de Jong, E.; Lloyd, M.L.; Kok, C.H.; Richmond, P.; Doherty, D.A.; Simmer, K.; Kakulas, F.; Strunk, T.; Currie, A. Leukocyte Populations in Human Preterm and Term Breast Milk Identified by Multicolour Flow Cytometry. PLoS ONE 2015, 10, e0135580. [Google Scholar] [CrossRef] [Green Version]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Geddes, D.T. Immune Cell–Mediated Protection of the Mammary Gland and the Infant during Breastfeeding. Adv. Nutr. Int. Rev. J. 2015, 6, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Christensen, N.; Bruun, S.; Søndergaard, J.; Christesen, H.T.; Fisker, N.; Zachariassen, G.; Sangild, P.T.; Husby, S. Breastfeeding and Infections in Early Childhood: A Cohort Study. Pediatrics 2020, 146, e20191892. [Google Scholar] [CrossRef] [PubMed]
- Verd, S.; Ramakers, J.; Vinuela, I.; Martin-Delgado, M.-I.; Prohens, A.; Díez, R. Does breastfeeding protect children from COVID-19? An observational study from pediatric services in Majorca, Spain. Int. Breastfeed. J. 2021, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Luxi, N.; Giovanazzi, A.; Capuano, A.; Crisafulli, S.; Cutroneo, P.M.; Fantini, M.P.; Ferrajolo, C.; Moretti, U.; Poluzzi, E.; Raschi, E.; et al. COVID-19 Vaccination in Pregnancy, Paediatrics, Immunocompromised Patients, and Persons with History of Allergy or Prior SARS-CoV-2 Infection: Overview of Current Recommendations and Pre- and Post-Marketing Evidence for Vaccine Efficacy and Safety. Drug Saf. 2021, 44, 1247–1269. [Google Scholar] [CrossRef]
- Nassar, A.H.; Visser, G.H.A.; Nicholson, W.K.; Ramasauskaite, D.; Kim, Y.H.; Barnea, E.R.; Escobar, M.F.; Pacagnella, R.; Theron, G.; Wright, A. FIGO Statement: Vaccination in pregnancy. Int. J. Gynecol. Obstet. 2021, 152, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2–Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2022, 226, 236.e1–236.e14. [Google Scholar] [CrossRef] [PubMed]
- Ciapponi, A.; Bardach, A.; Mazzoni, A.; Alconada, T.; Anderson, A.S.; Argento, F.J.; Ballivian, J.; Bok, K.; Comandé, D.; Erbelding, E.; et al. Safety of components and platforms of COVID-19 vaccines considered for use in pregnancy: A rapid review. Vaccine 2021, 39, 5891–5908. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age from 18 to 45 | 18 < age < 45 |
| Taking two doses of BNT162b2 (Comirnaty, Pfizer–BioNTech) vaccine after childbirth | Vaccination before childbirth |
| Negative history of SARS-CoV-2 infection prior to mother or child vaccination | Failure to complete the full vaccination schedule with BNT162b2 (Comirnaty, Pfizer–BioNTech) |
| Time from taking the second dose of the vaccine is 6 to 12 weeks | Time from the second dose of vaccine less than 6 or more than 12 weeks |
| No immunodeficiency | SARS-CoV-2 infection confirmed by PCR or antigen test before immunization of the mother or child |
| Single pregnancy | Congenital or acquired immunodeficiencies |
| Childbirth after 37 weeks of pregnancy | Use of immunosuppressive drugs |
| Physiological delivery and caesarean section | Multiple pregnancy |
| Active lactation and breastfeeding | Birth defects |
| Informed consent to participate in the study | Premature delivery <38 weeks of pregnancy |
| Lack of informed consent to participate in the study |
| Frequency (n)/% | Including the Mother of the Child with Positive IgG Antibodies (n)/% | |
|---|---|---|
| How often have you been breastfeeding? | ||
| a. Main feeding | 1 (8%) | 0 (0%) |
| b. Several times a day: during the day and at night | 9 (69%) | 2 (15%) |
| c. Twice/ three times per day | 3 (23%) | 0 (0%) |
| d. Sporadically | 0 (0%) | 0 (0%) |
| Are you employed? | ||
| a. Yes | 1 (8%) | 0 (0%) |
| b. Yes, I work online | 7 (54%) | 2 (15%) |
| c. No | 5 (38%) | 1 (8%) |
| Was any close family contact with suspected or diagnosed with COVID-19 person after childbirth? | ||
| a. No | 4 (31%) | 2 (15%) |
| b. Yes | 9 (69%) | 1 (8%) |
| Did the household members was diagnosed for COVID-19? | ||
| a. Yes | 3 (23%) | 3 (23%) |
| b. No | 10 (77%) | 0 (0%) |
| Concentration of SARS-CoV-2 S Antibodies [BAU/mL] of the Mother (Mean 1252 (95% CI = 736–1769) BAU/mL) | Mother’s Age [Years] (34.84 ± 2.70 Years) | Time from the 2nd Dose of Vaccination to Blood Collection [Weeks] | Concentration of SARS-CoV-2 S Antibodies [BAU/mL] of the Child (Mean 322, (95% CI = −253–897) BAU/mL) | Child’s Age [Months] (Mean 15.76 ± 7.49 Months) | Child’s Gender (31% Male 69% Female) |
|---|---|---|---|---|---|
| 1420 | 32 | 6 | <4.81 | 14 | female |
| 614 | 33 | 7 | 215.00 | 28 | female |
| >2080 | 35 | 7 | <4.81 | 13 | male |
| 1010 | 40 | 7 | <4.81 | 23 | female |
| >2080 | 32 | 7 | <4.81 | 11 | female |
| >2080 | 35 | 12 | <4.81 | 10 | male |
| >2080 | 35 | 8 | <4.81 | 29 | male |
| 940 | 36 | 9 | <4.81 | 24 | female |
| 1550 | 33 | 7 | <4.81 | 10 | male |
| 1980 | 32 | 10 | <4.81 | 15 | female |
| >2080 | 36 | 8 | <4.81 | 8 | female |
| >2080 | 34 | 10 | 164.00 | 10 | female |
| >2080 | 40 | 10 | 588.00 | 10 | female |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kociszewska-Najman, B.; Jaskólska, M.; Taradaj, K.; Sibanda, E.; Ginda, T. Assessment of Anti m-RNA SARS-CoV-2 (BNT162b2) Antibody Titres in Mother and Child Pairs of Breastfeeding Women Vaccinated Post-Delivery. Vaccines 2022, 10, 1089. https://doi.org/10.3390/vaccines10071089
Kociszewska-Najman B, Jaskólska M, Taradaj K, Sibanda E, Ginda T. Assessment of Anti m-RNA SARS-CoV-2 (BNT162b2) Antibody Titres in Mother and Child Pairs of Breastfeeding Women Vaccinated Post-Delivery. Vaccines. 2022; 10(7):1089. https://doi.org/10.3390/vaccines10071089
Chicago/Turabian StyleKociszewska-Najman, Bożena, Magdalena Jaskólska, Karol Taradaj, Elopy Sibanda, and Tomasz Ginda. 2022. "Assessment of Anti m-RNA SARS-CoV-2 (BNT162b2) Antibody Titres in Mother and Child Pairs of Breastfeeding Women Vaccinated Post-Delivery" Vaccines 10, no. 7: 1089. https://doi.org/10.3390/vaccines10071089
APA StyleKociszewska-Najman, B., Jaskólska, M., Taradaj, K., Sibanda, E., & Ginda, T. (2022). Assessment of Anti m-RNA SARS-CoV-2 (BNT162b2) Antibody Titres in Mother and Child Pairs of Breastfeeding Women Vaccinated Post-Delivery. Vaccines, 10(7), 1089. https://doi.org/10.3390/vaccines10071089





