Abstract
Chile is among the most successful nations worldwide in terms of its COVID-19 vaccine rollout. By 31 December 2021, 84.1% of the population was fully vaccinated, and 56.1% received booster doses using different COVID-19 vaccines. In this context, we aimed to estimate the prevalence of anti-SARS-CoV-2 antibodies following the infection and vaccination campaign. Using a three-stage stratified sampling, we performed a population-based cross-sectional serosurvey based on a representative sample of three Chilean cities. Selected participants were blood-sampled on-site and answered a short COVID-19 and vaccination history questionnaire using Wantai SARS-CoV-2 Ab ELISA to determine seroprevalence. We recruited 2198 individuals aged 7–93 between 5 October and 25 November 2021; 2132 individuals received COVID-19 vaccinations (97%), 67 (3.1%) received one dose, 2065 (93.9%) received two doses, and 936 received the booster jab (42.6%). Antibody seroprevalence reached 97.3%, ranging from 40.9% among those not vaccinated to 99.8% in those with booster doses (OR = 674.6, 154.8–2938.5). SARS-CoV-2 antibodies were associated with vaccination, previous COVID-19 diagnosis, age group, and city of residence. In contrast, we found no significant differences in the type of vaccine used, education, nationality, or type of health insurance. We found a seroprevalence close to 100%, primarily due to the successful vaccination program, which strongly emphasizes universal access.
1. Introduction
Latin American countries have been severely impacted by the COVID-19 pandemic, partly due to health and socioeconomic inequities and political instability [1], and Chile was not an exception. By the end of June 2020, the country had one of the worst outbreaks in the world. However, the prompt implementation of an aggressive vaccination strategy [2,3] and population compliance [4] transformed the country into a successful example of the COVID-19 vaccine rollout.
The vaccination process started in late December 2020 with health workers and teachers. In February, the massive vaccine rollout began prioritizing the elderly population [5]. By the end of 2021, 84.1% of the Chilean population had been fully vaccinated, and 56.1% had received the booster dose. Despite the rapid advance in vaccination during the second great wave of COVID-19, the outbreak of SARS-CoV-2 associated with the Lambda and mainly Gamma variants could not be controlled [6]. However, the later circulation of the Delta variant, predominantly during the last southern spring (October–December), did not reach the magnitude of previous waves.
One of the striking aspects of the vaccination campaign in Chile is the concomitant use of different vaccine technologies. Of 44.4 million doses administered in 2021 (up to 4 January 2022), 55.8% were the inactivated Sinovac’s CoronaVac. Nucleic Acid mRNA vaccines represented 35.8% (Pfizer-BioNTech’s BNT162b2), and 8.4% was viral vector vaccines (7.1% Oxford-AstraZeneca’s AZD1222, and 1.3% CanSino Biologics’ Ad5-nCoV).
The most frequent vaccine schedule used in Chile at the moment of the study was heterologous and differed by age group. In people aged 55 and older, combined two doses of inactivated vaccine with a booster viral vector vaccine; in youngers, the booster was with the mRNA vaccine. The second most frequent scheme was homologous, using two doses and booster of mRNA vaccines (BNT162b2). Since September 2021, the health authority extended the vaccination campaign to children between 6 and 17 and from December to children from 3 years of age with CoronaVac for both groups. Two weeks later, the health authority approved BNT162b2 for children aged five and older.
This study aims to estimate the seroprevalence of antibodies in individuals with SARS-CoV-2 due to natural or vaccine-induced infection. Our main contribution to the literature is our use of a population-based serosurvey in an emerging country with high vaccine coverage and various vaccine schemes.
2. Materials and Methods
We performed a population-based cross-sectional serosurvey based on a previous study of a representative sample of the cities of Santiago, Coquimbo/La Serena, and Talca [7]. This study used a stratified sampling of three stages, census district, block, and dwelling for Coquimbo–La Serena and Talca, and municipality, block, and dwelling for the city of Santiago. All former participants were contacted, and those who refused to participate or were not located were replaced in a standardized manner. Each participant answered a questionnaire, and a blood sample was taken through venipuncture to determine antibodies through the Wantai SARS-CoV-2 Ab ELISA test following the manufacturer’s protocol. For those who presented difficulties with venipuncture, we offered a lateral flow immunoassay, which required capillary blood sampling (Cellex qSARS-CoV-2 IgG/IgM Cassette Rapid Test kit; compared to Rt-PCR-positive, percent agreement is 93.8%, and negative percent agreement is 96%).
Participants completed an on-site questionnaire regarding basic demographic and socioeconomic variables, self-reported COVID-19 infection by asking for PCR confirmation, history of COVID-19 exposure, symptoms, and questions about vaccination (the number of doses, type of vaccine, and administration time). Seroprevalence was expressed as a proportion of the sampled population. The association between seroprevalence and risk factors or vaccine use was expressed as odds ratios (ORs) with a 95% confidence interval. Data were analyzed using STATA statistical software (StataCorp. 2017. Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX, USA). This research followed the generic protocol of World Health Organization Unity studies [8,9]. Protocols were approved by the Ethics Committee of Universidad del Desarrollo, Universidad Catolica de Norte, and Universidad de Talca.
3. Results
Between October 5th and November 25th, 2021, 2198 individuals in 1131 dwellings were recruited in Santiago (1204–54.8%), Coquimbo–La Serena (536–24.4%), and Talca (458–20.8%). A quarter of the households in the first study were not recruited due to refusal or a change of residence. These cases were replaced by recruiting new dwellings, following the same sampling method. The average age of the participants was 43.7 years (SD 19.1), and 833 were males (37.9%). We collected 2176 serum samples and used rapid tests on 22 participants (Table 1).

Table 1.
Total sample SARS-CoV-2 seroprevalence by subgroup.
The overall sample seroprevalence was 97.3%, which was significantly higher in Santiago and Talca than in Coquimbo–La Serena (98.4%, 98.5%, and 93.9%, respectively). The odds ratio (OR) provides information to compare the seroprevalence of each category of risk factors, including vaccines, using one of them as a reference. As seen in Table 1, there were no significant differences between sexes, and concerning age, individuals aged less than 10 years had a significantly lower seroprevalence than other age groups. From the age of 20, the seroprevalence exceeded 97%, reaching almost 100% in the 30–39 group. Previous COVID-19 history (self-reported) was present in 12.1% of the sampled individuals. Among them, the presence of antibodies was approximately 100% and was significantly higher than in people who did not have this antecedent. Previous COVID-19 history was more frequent in people between 30 and 60 years old than in those younger and older.
Concerning vaccine coverage, 97% of the enrolled subjects had received at least one dose of the COVID-19 vaccine, and this figure was higher in Santiago and Talca than in Coquimbo–La Serena and in people older than 20 years old. There was a significant association between antibodies and vaccination history (Table 2). Seroprevalence doubled in people with one dose compared to those not vaccinated and increased with more doses, reaching almost 100% in subjects who received the booster jab (Mantel–Haenszel chi-square = 395.5030; p-value < 0.0001). Having two doses (or a complete basal schedule using Ad5-nCoV) increases the probability of having antibodies by 12 times compared with one dose while having a booster dose increases it by 52 times.

Table 2.
SARS-CoV-2 seroprevalence by number of doses and type of vaccine used.
The seroprevalence differences in the time elapsed between the last dose of vaccine and the blood sample obtained for this study were not significant. However, the highest seroprevalence was between 15 and 180 days (Table 3).

Table 3.
Seroprevalence of SARS-CoV-2 by time elapsed since the last dose of vaccine.
4. Discussion
The results show a high seroprevalence of antibodies in the subjects studied, strongly associated with vaccination, consistent with the immunization coverage registered by the Chilean Ministry of Health for the three cities. At the end of October, during the fieldwork, reported vaccine coverage was 83.8% for the first dose, 76.9% for two doses, and 29.9% for the booster jab [10]. The cumulative incidence reported by the Chilean MOH at that time was 7059 per 100,000 inhabitants in Coquimbo–La Serena; 11,724 in Santiago; and 10,076 in Talca. The SARS-CoV-2 Delta variant represented 97% of the cases. Figure 1 shows the COVID-19 epi-curve in the studied cities, indicating the fieldwork period; Figure 2 shows the results of genomic surveillance of SARS-CoV-2 variants of concern performed by the Institute of Public Health in Chile [6].
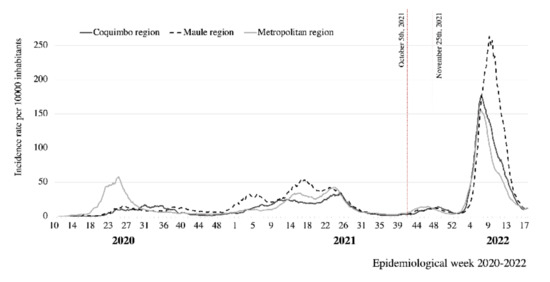
Figure 1.
COVID-19 epidemic curve in three regions of Chile, 2020–2022. Source: [10].
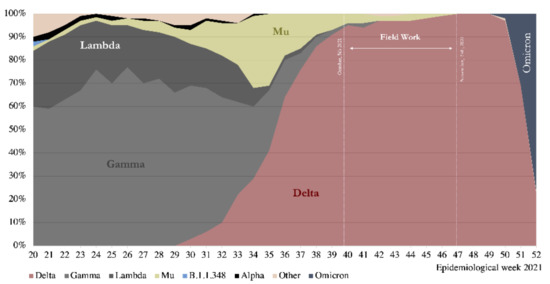
Figure 2.
Genomic Surveillance for SARS-CoV-2 Variants of Concern circulating in Chile, 2021. Source: [6].
Our results show a higher seroprevalence than that reported in other population-based seroprevalence studies. In India, between June and July 2021, seropositivity in adults was 66.7% [11]; in Switzerland, in the same period, it reached 64.4%, with 30% due to natural infection [12], while in Greece in June 2021, it was 55.7% [13]. This difference may be due to the high vaccination coverage achieved in Chile. On the other hand, the study carried out in India, similar to our results, shows an antibody gradient according to the number of vaccine doses (62% among those not vaccinated; 81% with one vaccine dose, and 89.8% with a full scheme) [11].
Coquimbo–La Serena showed a significantly lower seroprevalence than the other two cities, which could be explained by the higher proportion of unvaccinated individuals in that city obtained in our study (5%, versus 2.5% in Santiago and 2% in Talca). Another factor could be that the accumulated incidence in Coquimbo was lower than that in the other cities at the time of the study.
The lower seroprevalence observed in children under 10 years of age is probably due to their late incorporation into the vaccination campaign, which as of 27 September 2021, covered children between 6 and 11 years of age. We also found an association between seroprevalence and history of COVID-19, but with lower significance than vaccination.
The lack of association of seroprevalence with education, health insurance (public or private), and nationality is consistent with the strategy of the health authority. The early purchase of jabs and the implementation of a universal vaccination campaign allowed access to all people, taking care of vulnerable and hard-to-reach populations, including migrants. In contrast, the Geneva study showed significant differences in vaccination coverage related to education, leading to higher seroprevalence among more educated people [12].
The seroprevalence among vaccinated subjects (any dose) reached 99.1%, while that among unvaccinated subjects was 40.9%. We observed a gradient effect between the prevalence of antibodies and the number of vaccine doses received. Those with the booster doses reach almost 100% seroprevalence, independent of the type of vaccine used.
Regarding the type of vaccines applied, the most significant antibody variation occurred in those who received only one dose. In contrast, those who completed the two-dose schedules had a similar seroprevalence, regardless of the type of vaccine administered or the age-related vaccine schedule defined by the health authority. The mRNA-exclusive scheme was available to health care workers in intensive care units at the beginning of the vaccine rollout. Later there was open to the general public when most elders were already vaccinated. Then, the people who received mRNA are on average younger than those who received the heterologous regimen. Finally, seroprevalence was even more homogeneously high in those with booster doses.
The prevalence of antibodies in unvaccinated people demonstrates the burden of infection achieved with successive pandemic waves. In our previous survey, at the end of 2020, the seroprevalence was 10.4%, with differences between the three cities. There are still differences among the cities, although they all have very high seroprevalence.
Our study had some limitations. The rejection rate of former participants could be associated with the vaccination status; therefore, our results might have shown higher vaccine uptake than officially reported. However, this does not preclude comparative analysis between vaccinated and unvaccinated participants and the different vaccine schemes. Further analysis will include weighted seroprevalence and neutralizing antibody responses.
In sum, our results show a prevalence of antibodies close to 100% in the population aged seven years and older, primarily due to the successful vaccination program, which strongly emphasizes universal access. We also observed that seroprevalence remained very high 180 days after the last vaccine dose. However, this might not be enough to prevent the recent summer resurgence of cases resulting from the circulation of the Omicron variant, even though it is expected to have less impact on hospitalizations and deaths. We still need to learn about SARS-CoV-2 transmission, the protection conferred by vaccines, and their relationship with the evolution of the virus.
Author Contributions
Conceptualization, X.A., C.G., G.I., M.R.-S., L.N.-F. and P.V.; Data curation, C.G., M.A., P.R., G.I. and C.C.-L.; Formal analysis, M.A., P.R. and G.I.; Funding acquisition, X.A. and M.R.-S.; Investigation, C.G., C.P., L.J.C., R.Q.-G., J.C., M.S., J.H. and C.V.; Methodology, X.A., C.G., M.A., P.R., G.I., C.C.-L., L.J.C. and P.V.; Project administration, X.A., C.G. and P.V.; Resources, C.G., M.R.-S., C.P., L.J.C., R.Q.-G., L.N.-F., M.S., J.H. and C.V.; Software, C.G., M.A., P.R., G.I., J.C. and C.C.-L.; Supervision, X.A., C.G., M.R.-S., L.N.-F., C.V. and P.V.; Validation, M.A., P.R., G.I., L.J.C., J.H. and C.V.; Visualization, X.A., M.A., P.R., G.I., L.J.C. and J.H.; Writing—original draft, X.A. and M.A.; Writing—review and editing, X.A., C.G., M.A., P.R., G.I., M.R.-S., L.N.-F., L.J.C., C.C.-L. and P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been supported by WHO Unity Studies, a global seroepidemiological standardization initiative, with funding to the WHO by the COVID-19 Solidarity Response Fund and the German Federal Ministry of Health (BMG) COVID-19 Research and Development Fund.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committees of the three Universities involved: Universidad del Desarrollo, Santiago, Chile (protocol code 2020-54; 31 August 2021); Universidad de Talca, Talca Chile (protocol code 34-2021, 3 November 2021); and Facultad de Medicina of the Universidad Católica del Norte, Coquimbo, Chile (protocol code UCN REV. N°38/2021, 7 September 2021). All methods were carried out in accordance with relevant guidelines and regulations in ethical approval and consent to participate.
Informed Consent Statement
Informed consent was obtained from all subjects—if subjects were under 18, from a parent and/or legal guardian.
Data Availability Statement
Study datasets are available in a data repository (https://doi.org/10.5281/zenodo.5773152) (accessed on 13 March 2022) and/or available from the authors upon reasonable request.
Acknowledgments
The authors acknowledge the WHO for the donation of tests to measure SARS-CoV-2 antibodies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lancet, T. COVID-19 in Latin America—emergency and opportunity. Lancet 2021, 398, 93. [Google Scholar] [CrossRef]
- Aguilera, X.; Mundt, A.P.; Araos, R.; Weitzel, T. The story behind Chile’s rapid rollout of COVID-19 vaccination. Travel Med. Infect. Dis. 2021, 42, 102092. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Dintrans, P.V.; Maddaleno, M. The successful COVID-19 vaccine rollout in Chile: Factors and challenges. Vaccine X 2021, 9, 100114. [Google Scholar] [CrossRef] [PubMed]
- García, L.Y.; Cerda, A.A. Contingent assessment of the COVID-19 vaccine. Vaccine 2020, 38, 5424–5429. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Salud. Gob.cl—Yo Me Vacuno. Gob Chile. 2021. Available online: https://www.gob.cl/yomevacuno/ (accessed on 4 January 2022).
- Ministerio de Salud. Variantes SARS-CoV-2. 2021. Available online: https://vigilancia.ispch.gob.cl/app/varcovid (accessed on 4 January 2022).
- Vial, P.; González, C.; Icaza, G.; Ramirez-Santana, M.; Quezada-Gaete, R.; Núñez-Franz, L.; Apablaza, M.; Vial, C.; Rubilar, P.; Correa, J.; et al. Seroprevalence, spatial distribution, and social determinants of SARS-CoV-2 in three urban centers of Chile. BMC Infect. Dis. 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Lewis, H.C.; Subissi, L.; Nardone, A.; Valenciano, M.; Cheng, B.; Glonti, K.; Williams, B.; Abejirinde, I.O.; Simniceanu, A.; et al. Early epidemiological investigations: World Health Organization UNITY protocols provide a standardized and timely international investigation framework during the COVID-19 pandemic. Influ. Other Respir. Viruses 2022, 16, 7–13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for Coronavirus 2019 (COVID-19) Infection; 26 May 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ministerio de Ciencia y Tecnología. Datos-COVID19. GitHub n.d. Available online: https://github.com/MinCiencia/Datos-COVID19 (accessed on 14 January 2022).
- Murhekar, M.V.; Bhatnagar, T.; Thangaraj, J.W.V.; Saravanakumar, V.; Kumar, M.S.; Selvaraju, S.; Rade, K.; Kumar, C.P.G.; Sabarinathan, R.; Asthana, S.; et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June–July 2021: A population-based cross-sectional study. PLOS Med. 2021, 18, e1003877. [Google Scholar] [CrossRef] [PubMed]
- Stringhini, S.; Zaballa, M.-E.; Pullen, N.; Perez-Saez, J.; de Mestral, C.; Loizeau, A.J.; Lamour, J.; Pennacchio, F.; Wisniak, A.; Dumont, R.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021. Eurosurveillance 2021, 26, 2100830. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Krumbholz, B.; Mavrouli, M.; Tseroni, M.; Gamaletsou, M.N.; Botsa, E.; Anastassopoulou, C.; Gikas, A.; Fournarakou, E.; Kavieri, M.; et al. A study of the evolution of the third COVID-19 pandemic wave in the Athens metropolitan area, Greece, through two cross-sectional seroepidemiological surveys: March, June 2021. J. Med Virol. 2021, 94, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).