Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Immunization, Allergen Challenge, and Sample Collection
2.3. ELISA for OVA-Specific Antibody Assay
2.4. Gene Expression Measurement
2.5. Flow Cytometry
2.6. Histology
2.7. Statistics
3. Results
3.1. Chronically Low-Dose Adjuvant-Free Antigen Administration in Subcutaneous but Not Abdominal Fat Tissue Induced Early B-Cell IgE Class Switching and Reproduced IgE-Mediated Type I Hypersensitivity
3.2. Subcutaneous-Fat-Associated B Cells Are Responsible for Early B-Cell IgE Class Switching
3.3. Subcutaneous Fat in Mouse Withers Region Contains Organized Tertiary Lymphoid Structures
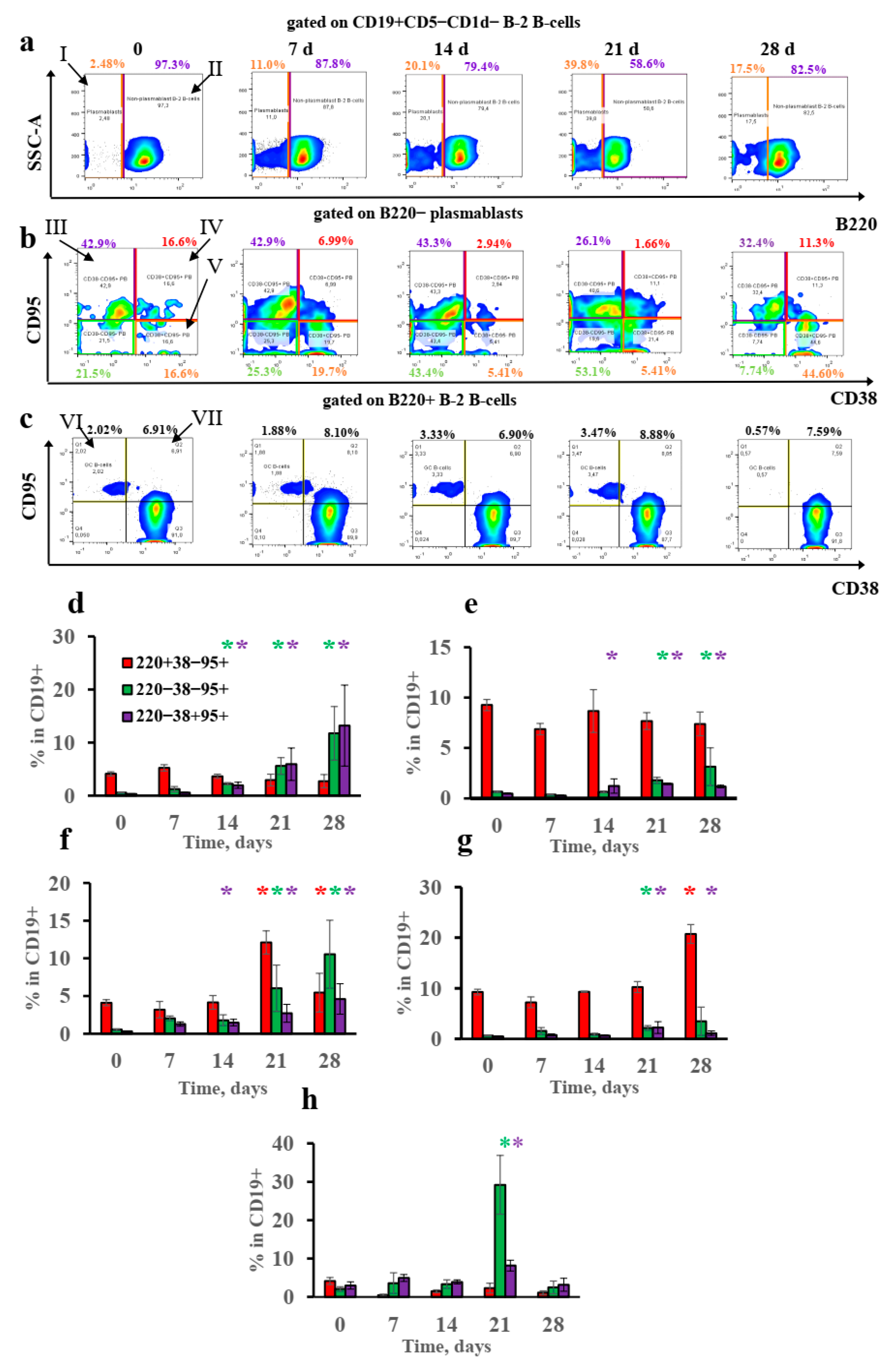
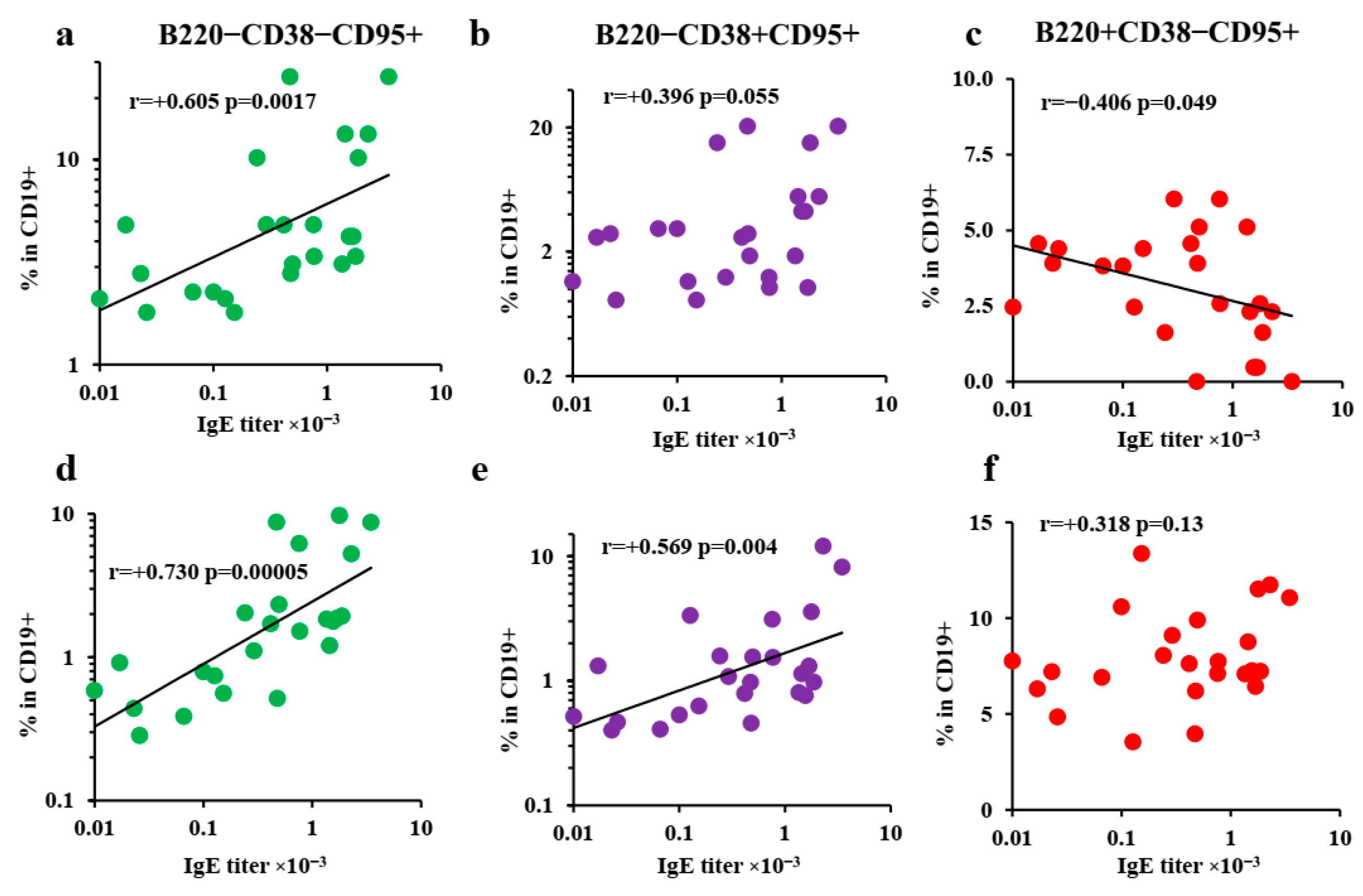
3.4. B-2-Cell-Derived Plasmablasts but Not GCs Are Responsible for IgE Production after Long-Term Antigen Administration
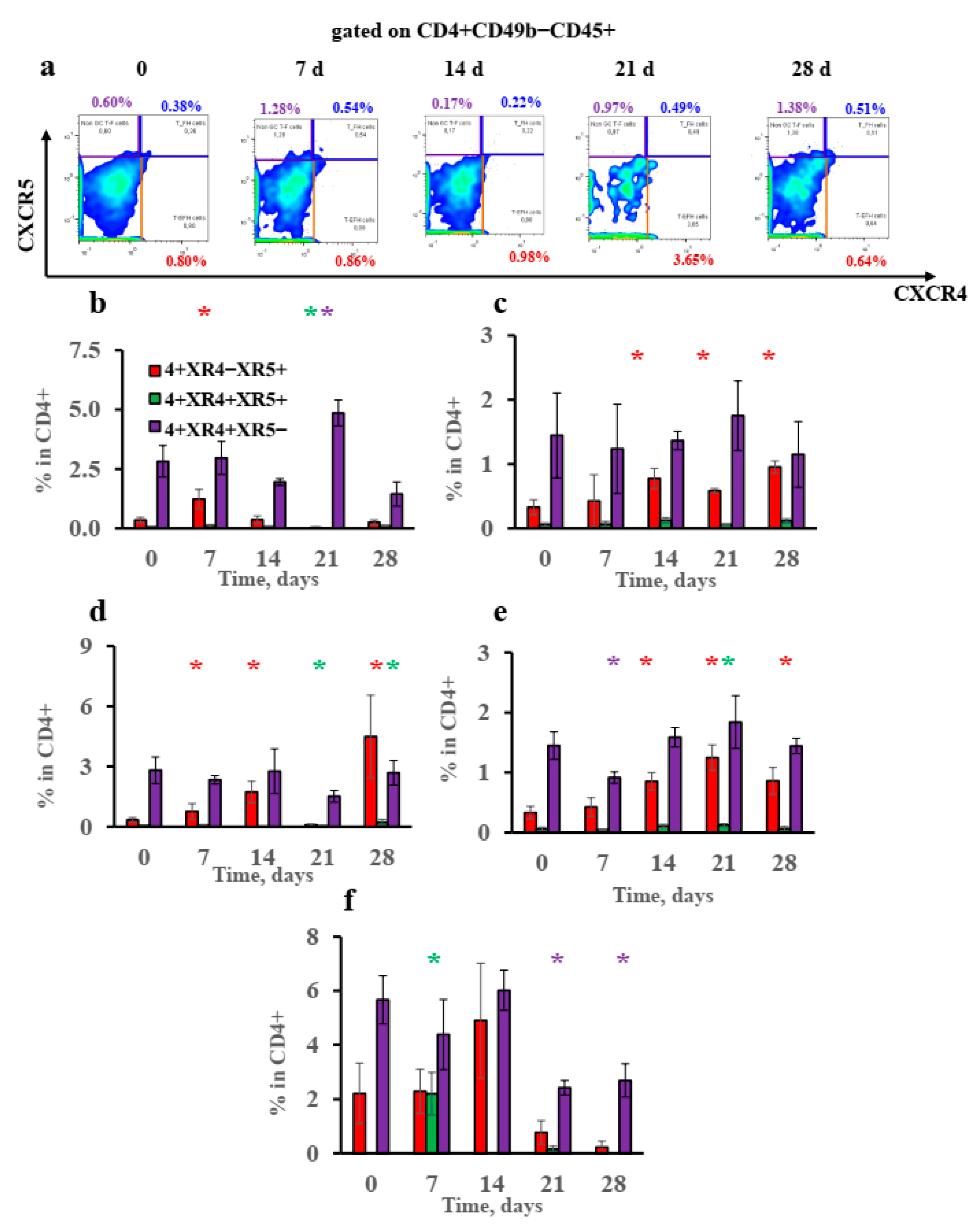
3.5. Extrafollicular T-Helper-Cell Accumulation Results in High IgE Production Accompanied by Minimal Igg1 Production in Response to Low Antigen Doses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ober, C.; Yao, T.-S. The genetics of asthma and allergic disease: A 21st century perspective. Immunol. Rev. 2011, 242, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.D.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Geora, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786. [Google Scholar] [CrossRef] [Green Version]
- Sweerus, K.; Lachowicz-Scroggins, M.; Gordon, E.; LaFemina, M.; Huang, X.; Parikh, M.; Kanegai, C.; Fahy, J.V.; Frank, J.A. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 2017, 139, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrecht, B.N.; Hammad, H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J. Allergy Clin. Immunol. 2014, 134, 499–507. [Google Scholar] [CrossRef]
- Gevaert, P.; Nouri-Aria, K.T.; Wu, H.; Harper, C.E.; Takhar, P.; Fear, D.J.; Acke, F.; De Ruyck, N.; Banfield, G.; Kariyawasam, H.H.; et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy 2013, 68, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Kondo, K.; Toma-Hirano, M.; Kanaya, K.; Suzukawa, K.; Ushio, M.; Suzukawa, M.; Ohta, K.; Yamasoba, T. Local increase in IgE and class switch recombination to IgE in nasal polyps in chronic rhinosinusitis. Clin. Exp. Allergy 2014, 44, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Feldman, S.; Kasjanski, R.; Poposki, J.; Hernandez, D.; Chen, J.N.; Norton, J.E.; Suh, L.; Carter, R.G.; Stevens, W.W.; Peters, A.T.; et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin. Exp. Allergy 2017, 47, 457–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, E.; Ozasa, K.; Temizoz, B.; Ohata, K.; Koo, C.X.; Kanuma, T.; Kusakabe, T.; Kobari, S.; Horie, M.; Morimoto, Y.; et al. Inhaled fine particles induce alveolar macrophage death and interleukin-1a release to promote inducible bronchus-associated lymphoid tissue formation. Immunity 2016, 45, 1299–1310. [Google Scholar] [CrossRef] [Green Version]
- Lindell, D.M.; Berlin, A.A.; Schaller, M.A.; Lukacs, N.W. B cell antigen presentation promotes Th2 responsesand immunopathology during chronic allergic lung disease. PLoS ONE 2008, 3, e3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, L.Y.; Iijima, K.; Hara, K.; Kobayasi, T.; Kephart, G.M.; Kita, H. B Cells Play Key Roles in Th2-type airway immune responses in mice exposed to natural airborne allergens. PLoS ONE 2015, 10, e0121660. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.E.; Ganley-Leal, L.M.; Mehta, A.; Bigornia, S.J.; Mott, M.; Rehman, Q.; Farb, M.G.; Hess, D.T.; Joseph, L.; Gokce, N.; et al. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity 2012, 20, 1372–1378. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Thaller, S.; Blomberg, B.B. Secretion of autoimmune antibodies in the human subcutaneous adipose tissue. PLoS ONE 2018, 13, e0197472. [Google Scholar] [CrossRef] [Green Version]
- Peters, U.; Dixon, A.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niederberger, V.; Niggemann, B.; Kraft, D.; Spitzauer, S.; Valenta, R. Evolution of IgM, IgE and IgG (1-4) antibody responses in early childhood monitored with recombinant allergen components: Implications for class switch mechanisms. Eur. J. Immunol. 2002, 32, 576–584. [Google Scholar] [CrossRef]
- Resch, Y.; Michel, S.; Kabesch, M.; Lupineck, C.; Valenta, R.; Vrtala, S. Different IgE recognition of mite allergen components in asthmatic and non-asthmatic children. J. Allergy Clin. Immunol. 2015, 136, 1083–1091. [Google Scholar] [CrossRef] [Green Version]
- Svirschevskaya, E.; Fattakhova, G.; Khlgatian, S.; Chudakov, D.; Kashirina, E.; Ryasantsev, D.; Kotsareva, O.; Zavriev, S. Direct versus sequential immunoglobulin switch in allergy and antiviral responses. Clin. Immunol. 2016, 170, 31–38. [Google Scholar] [CrossRef]
- Kim, D.I.; Song, M.-K.; Lee, K. Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pulm. Med. 2019, 19, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Saiz, R.; Chu, D.K.; Mandur, T.S.; Walker, T.D.; Gordon, M.E.; Chaudhary, R.; Koening, J.; Saliba, S.; Galipeau, H.J.; Utley, A.; et al. Lifelong memory responses perpetuate humoral Th2 immunity and anaphylaxis in food allergy. J. Allergy Clin. Immunol. 2017, 140, 1604–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-S.; Kim, Y.-K.; Jeon, S.G.; Kim, S.-H.; Kim, S.-S.; Park, H.-W.; Min, K.-U.; Kim, Y.-Y.; Cho, S.-H. Influence of the adjuvants and genetic background on the asthma model using recombinant Der f 2 in mice. Immune Netw. 2013, 13, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arps, V.; Sudowe, S.; Kolsh, E. Antigen dose-dependent differences in IgE antibody production are not due to polarization towards Th1 and Th2 cell subsets. Eur. J. Immunol. 1998, 28, 681–686. [Google Scholar] [CrossRef]
- Kolbe, L.; Heusse, C.H.; Kolsch, E. Isotype-associated recognition of allergen epitopes and its modulation by antigen dose. Immunology 1995, 84, 285–289. [Google Scholar] [PubMed]
- Furuhashi, K.; Chua, Y.L.; Wong, K.H.S.; Zhou, Q.; Lee, D.C.P.; Liong, K.H.; Teo, G.H.; Hutchinson, P.E.; Kemeny, D.M. Priming with high and low respiratory allergen dose induces differential CD4+ T helper type 2 cells and IgE/IgG1 antibody responses in mice. J. Immunol. 2017, 151, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudakov, D.B.; Ryasantsev, D.Y.; Tsaregorotseva, D.S.; Kotsareva, O.D.; Fattakhova, G.V.; Svirschevskaya, E.V. Tertiary lymphoid structure related B-cell IgE isotype switching and secondary lymphoid organ linked IgE production in mouse allergy model. BMC Immunol. 2020, 21, 45. [Google Scholar] [CrossRef]
- Nials, A.T.; Uddin, S. Mouse models of allergic asthma: Acute and chronic allergen challenge. Dis. Model. Mech. 2008, 1, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Talay, O.; Yan, D.; Brightbill, H.D.; Straney, E.M.; Zhou, M.; Ladi, E.; Lee, W.P.; Egen, J.; Austin, C.D.; Xu, M.; et al. IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nat. Immunol. 2012, 13, 396–404. [Google Scholar] [CrossRef]
- Yang, Z.; Sullivan, B.M.; Allen, C.D.C. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity 2012, 36, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, C.-A.M.; Targ, S.; Allen, C.D.C. IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J. Exp. Med. 2020, 217, e20190472. [Google Scholar] [CrossRef] [Green Version]
- Kitayama, D.; Sakamoto, A.; Arima, M.; Hatano, M.; Miyazaki, M.; Tokuhisa, T. A role for Bcl6 in sequential class switch recombination to IgE in B cells stimulated with IL-4 and IL-21. Mol. Immunol. 2008, 45, 1337–1345. [Google Scholar] [CrossRef]
- Stebegg, M.; Kumar, S.D.; Silva-Cayetano, A.; Fonseca, F.R.; Linterman, M.A.; Graca, L. Regulation of the Germinal Center Response. Front. Immunol. 2018, 9, 2469. [Google Scholar] [CrossRef] [Green Version]
- Wade-Vallance, A.K.; Allen, C.D.C. Intrinsic and extrinsic regulation of IgE B cell responses. Curr. Opin. Immunol. 2021, 72, 221–229. [Google Scholar] [CrossRef]
- Corrado, A.; Ramonell, R.P.; Woodruff, M.C.; Tipton, C.; Wise, S.; Levy, J.; DelGaudio, J.; Kuruvilla, M.E.; Magliocca, K.R.; Tomar, D.; et al. Extrafollicular IgD+ B cells generate IgE antibody secreting cells in the nasal mucosa. Mucosal Immunol. 2021, 14, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef] [PubMed]
- Ramadani, F.; Upton, N.; Hobson, P.; Chan, Y.-C.; Mzinza, D.; Bowen, H.; Kerridge, C.; Sutton, B.J.; Fear, D.J.; Gould, H.J. Intrinsic properties of germinal center-derived B cells promote their enhanced class switching to IgE. Allergy 2015, 70, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, Y.; Sielski, R.; Kawakami, T. Mouse body temperature measurement using infrared thermometer during passive systemic anaphylaxis and food allergy evaluation. J. Vis. Exp. 2018, 14, 58391. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Hu, Z.; Shi, X.; Li, X.; Zhan, X.; Li, X.-D.; Wang, J.; Choi, J.H.; Wang, K.-W.; Purrington, T.; et al. MAVS, cGAS, and endogenous retroviruses in T-independent B cell responses. Science 2014, 346, 1486–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinall, S.M.; Gonzalez-Fernandez, M.; Noelle, R.J.; Waldschmidth, T.J. Identification of Murine GC B cell subsets defined by the expression of surface isotypes and differentiation antigens. J. Immunol. 2000, 164, 5729–5738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finke, D.; Baribaud, F.; Diggelmann, H.; Acha-Orbea, H. Extrafollicular plasmablast b cells play a key role in carrying retroviral infection to peripheral organs. Immunology 2001, 166, 6266–6275. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.A.; Ernst, D.N.; Baumgarth, N. Single and coexpression of CXCR4 and CXCR5 identifies CD4 T helper cells in distinct lymph node niches during influenza virus infection. J. Virol. 2012, 86, 7146–7157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Iijima, K.; Dent, A.L.; Kita, H. Follicular helper T (Tfh) cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol. 2017, 139, 300–313e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faquim-Mauro, E.L.; Coffman, R.L.; Abrahamsohn, I.A.; Macedo, M.S. Cutting edge: Mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J. Immunol. 1999, 163, 3572–3576. [Google Scholar] [PubMed]
- Beutier, H.; Gillis, C.M.; Iannascoli, B.; Godon, O.; England, P.; Sibilano, R.; Reber, L.L.; Galli, S.J.; Cragg, M.S.; Van Rooijen, N.; et al. IgG subclasses determine pathways of anaphylaxis in mice. J. Allergy Clin. Immunol. 2017, 139, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelman, F.D. Anaphylaxis: Lessons from mouse models. J. Allergy Clin. Immunol. 2007, 120, 506–515. [Google Scholar] [CrossRef]
- Khan, S.; Tsai, S.; Winner, D.A. Adipose tissue B cells come of age: The AABs of fat inflammation. Cell Metab. 2019, 30, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Tfh cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roco, J.A.; Mesin, L.; Binder, S.C.; Nefzger, C.; Gonzalez-Figueroa, P.; Canete, P.F.; Ellyard, J.; Shen, Q.; Robert, P.A.; Cappello, J.; et al. Class-switch recombination occurs infrequently in GCs. Immunity 2019, 51, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Schattner, E.; Friedman, S.M. Fas expression and apoptosis in human B cells. Immunol. Res. 1996, 15, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Cattoretti, G.; Buttner, M.; Schaknovich, R.; Kremmer, E.; Alobeid, B.; Niedobitek, G. Nuclear and cytoplasmic AID in extrafollicular and GC B cells. Blood 2006, 107, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.L.; Zhang, Y.; Pallan, L.; Hsu, M.-C.; Khan, M.; Cunningham, A.F.; MacLennan, I.C.M.; Toellner, K.-M. Early B blasts acquire a capacity for Ig class switch recombination that is lost as they become plasmablasts. Eur. J. Immunol. 2011, 41, 3506–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudakov, D.B.; Kotsareva, O.D.; Konovalova, M.V.; Tsaregorodtseva, D.S.; Shevchenko, M.A.; Sergeev, A.A.; Fattakhova, G.V. Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model. Vaccines 2022, 10, 969. https://doi.org/10.3390/vaccines10060969
Chudakov DB, Kotsareva OD, Konovalova MV, Tsaregorodtseva DS, Shevchenko MA, Sergeev AA, Fattakhova GV. Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model. Vaccines. 2022; 10(6):969. https://doi.org/10.3390/vaccines10060969
Chicago/Turabian StyleChudakov, Dmitrii Borisovich, Olga Dmitrievna Kotsareva, Maryia Vladimirovna Konovalova, Daria Sergeevna Tsaregorodtseva, Marina Alexandrovna Shevchenko, Anton Andreevich Sergeev, and Gulnar Vaisovna Fattakhova. 2022. "Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model" Vaccines 10, no. 6: 969. https://doi.org/10.3390/vaccines10060969
APA StyleChudakov, D. B., Kotsareva, O. D., Konovalova, M. V., Tsaregorodtseva, D. S., Shevchenko, M. A., Sergeev, A. A., & Fattakhova, G. V. (2022). Early IgE Production Is Linked with Extrafollicular B- and T-Cell Activation in Low-Dose Allergy Model. Vaccines, 10(6), 969. https://doi.org/10.3390/vaccines10060969





