ChAdOx1 nCoV-19 Immunogenicity and Immunological Response Following COVID-19 Infection in Patients Receiving Maintenance Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Serum Collection for Antibody Testing
2.3. Serological Analysis for Humoral Immunogenecity
2.4. Statistical Evaluation
3. Results
3.1. Participant Characteristics
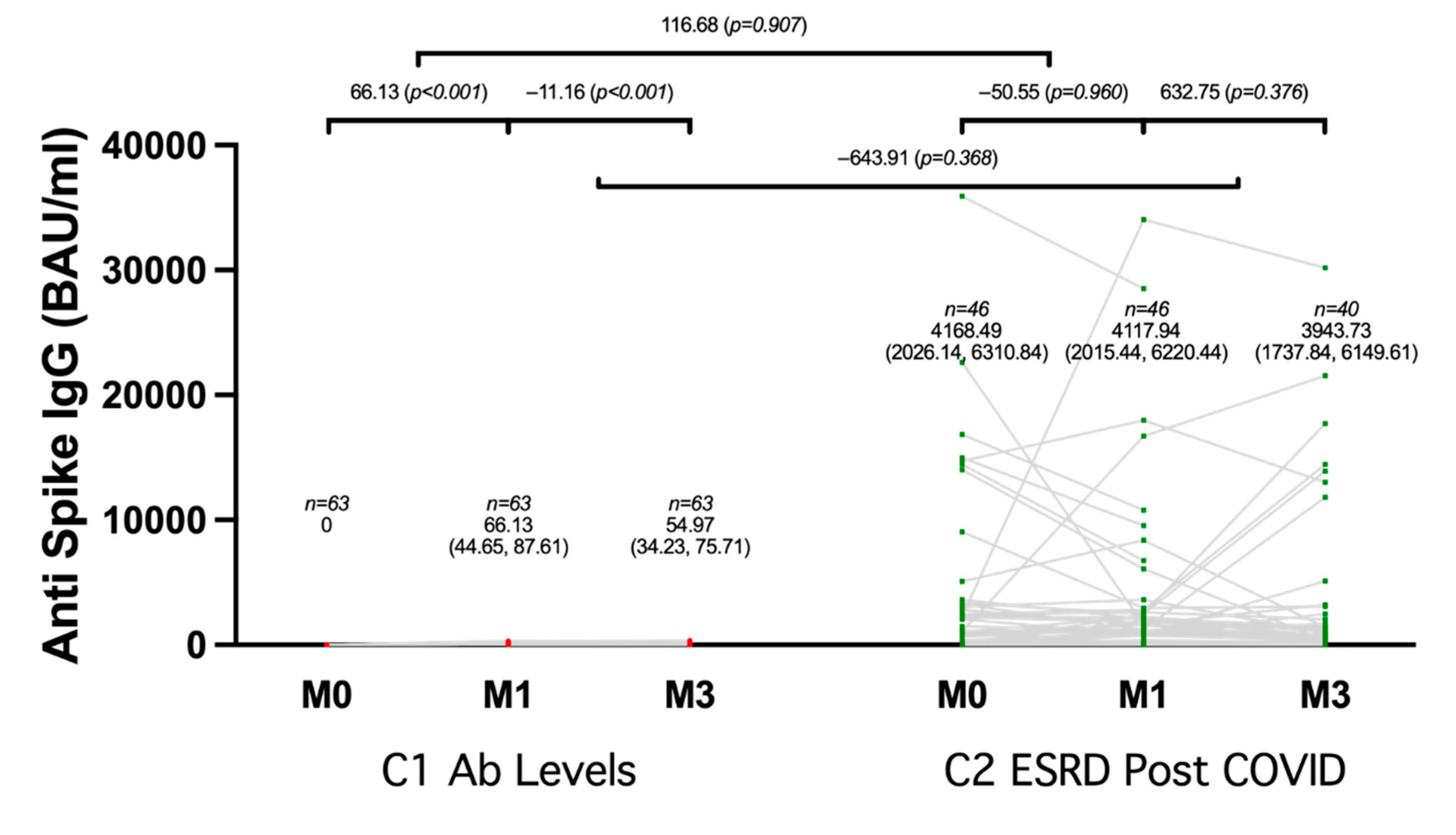
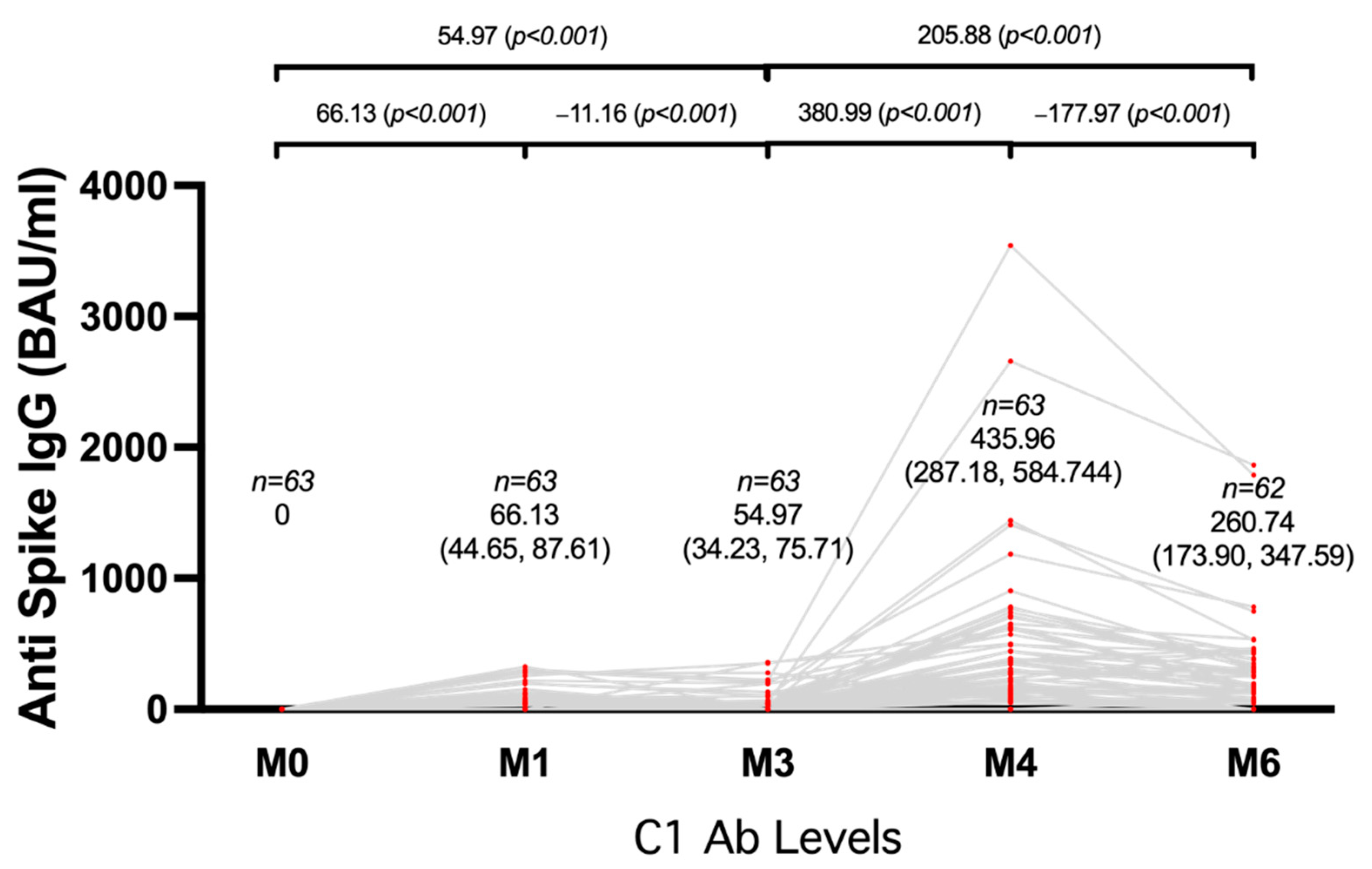
3.2. Humoral Immunogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease 2019 (COVID-19); Situation Report 51; World Health Organization: Geneva, Switzerland, 2019.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Portolés, J.; Marques, M.; López-Sánchez, P.; de Valdenebro, M.; Muñez, E.; Serrano, M.; Malo, R.; García, E.; Cuervas, V. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol. Dial. Transplant. 2020, 35, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef]
- Valeri, A.; Robbins-Juarez, S.; Stevens, J.; Ahn, W.; Rao, M.; Radhakrishnan, J.; Gharavi, A.; Mohan, S.; Husain, S. Presentation and Outcomes of Patients with ESKD and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1409–1415. [Google Scholar] [CrossRef]
- Ziemba, R.; Campbell, K.; Yang, T.; Schaeffer, S.; Mayo, K.; McGann, P.; Quinn, S.; Roach, J.; Huff, E. Excess Death Estimates in Patients with End-Stage Renal Disease–United States, February–August 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 825–829. [Google Scholar] [CrossRef]
- Nopsopon, T.; Kittrakulrat, J.; Takkavatakarn, K.; Eiamsitrakoon, T.; Kanjanabuch, T.; Pongpirul, K. Covid-19 in end-stage renal disease patients with renal replacement therapies: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009156. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J.; Xue, C.; Yang, B.; Mao, Z.; Ong, A. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: A meta-analysis and systematic review. Ren. Fail. 2020, 43, 1–15. [Google Scholar] [CrossRef]
- Ewer, K.; Barrett, J.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef]
- Folegatti, P.; Ewer, K.; Aley, P.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Barrett, J.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.; Folegatti, P.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Minassian, A.; Ewer, K.; Flaxman, A.; Folegatti, P.; Owens, D.; Voysey, M.; Aley, P.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.; Madhi, S.; Weckx, L.; Folegatti, P.; Aley, P.; Angus, B.; Baillie, V.; Barnabas, S.; Bhorat, Q.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.; Madhi, S.; Weckx, L.; Folegatti, P.; Aley, P.; Angus, B.; Baillie, V.; Barnabas, S.; Bhorat, Q.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Emary, K.; Golubchik, T.; Aley, P.; Ariani, C.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Madhi, S.; Baillie, V.; Cutland, C.; Voysey, M.; Koen, A.; Fairlie, L.; Padayachee, S.; Dheda, K.; Barnabas, S.; Bhorat, Q.; et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Mastalerz-Migas, A.; Steciwko, A.; Brydak, L. Immune response to influenza vaccine in hemodialysis patients with chronic renal failure. Adv. Exp. Med. Biol. 2013, 756, 285–290. [Google Scholar] [CrossRef]
- McGrath, L.; Kshirsagar, A.; Cole, S.; Wang, L.; Weber, D.; Stürmer, T.; Brookhart, M. Influenza vaccine effectiveness in patients on hemodialysis: An analysis of a natural experiment. Arch. Intern. Med. 2012, 172, 548–554. [Google Scholar] [CrossRef][Green Version]
- Buti, M.; Viladomiu, L.; Jardi, R.; Olmos, A.; Rodriguez, J.; Bartolome, J.; Esteban, R.; Guardia, J. Long-term immunogenicity and efficacy of hepatitis B vaccine in hemodialysis patients. Am. J. Nephrol. 1992, 12, 144–147. [Google Scholar] [CrossRef]
- Patel, N.; Assimon, M.; Bruni, E.; McNutt, L.; Mason, D. Incidence and Clinical Predictors of Nonresponse to Hepatitis B Vaccination among Patients Receiving Hemodialysis: Importance of Obesity. South Med. J. 2015, 108, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Stein, G.; Bhupalam, S.; Havlichek, D. Immunogenicity of 13-Valent Conjugate Pneumococcal Vaccine in Patients 50 Years and Older with End-Stage Renal Disease and on Dialysis. Clin. Vaccine Immunol. 2016, 23, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Goupil, R.; Benlarbi, M.; Beaubien-Souligny, W.; Nadeau-Fredette, A.; Chatterjee, D.; Goyette, G.; Gunaratnam, L.; Lamarche, C.; Tom, A.; Finzi, A.; et al. Short-term antibody response after 1 dose of BNT162b2 vaccine in patients receiving hemodialysis. CMAJ 2021, 193, E793–E800. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2021, 9, 360. [Google Scholar] [CrossRef]
- Frater, J.; Ewer, K.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.; Ali, M.; Ansari, M.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef]
- Raja, N.; Rajagopalan, A.; Arunachalam, J.; Prasath, A.; Durai, R.; Rajendran, M. Humoral response to viral vector COVID-19 vaccine in hemodialysis patients. Kidney Res. Clin. Pract. 2022, 4, 342–350. [Google Scholar] [CrossRef]
- Billany, R.; Selvaskandan, H.; Adenwalla, S.; Hull, K.; March, D.; Burton, J.; Bishop, N.; Carr, E.; Beale, R.; Tang, J.; et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: A call to arms. Kidney Int. 2021, 99, 1492–1494. [Google Scholar] [CrossRef]
- Yadav, A.; Gondil, V.; Singla, M.; Goyal, A.; Kaushal, R.; Chauhan, M.; Jha, V. Humoral Response to One and Two Doses of ChAdOx1-S Vaccine in Patients on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1875–1876. [Google Scholar] [CrossRef]
- Haase, M.; Lesny, P.; Anderson, M.; Cloherty, G.; Stec, M.; Haase-Fielitz, A.; Haarhaus, M.; Santos-Araújo, C.; Veiga, P.; Macario, F. Humoral immunogenicity and tolerability of heterologous ChAd/BNT compared with homologous BNT/BNT and ChAd/ChAd SARS-CoV-2 vaccination in hemodialysis patients: A multicenter prospective observational study. J. Nephrol. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Watcharananan, S.; Jaru-Ampornpan, P.; Sahawongcharoen, S.; Naitook, N.; Himananto, O.; Jongkaewwattana, A.; Setthaudom, C.; Rattanasiri, S.; Phuphuakrat, A.; Thakkinstian, A.; et al. Comparison of the immunogenicity of ChAdOx1 nCoV-19 vaccine against the wild-type and delta variants in kidney transplant recipients and healthy volunteers. Am. J. Transplant. 2022, 22, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.; Starr, N.; Chan, G.; Dempsey, E.; Heffernan, E.; Newman, E.; O’Neill, J.; Hannan, M.; Lynch, B.; Joyce, E. Humoral response to SARS-CoV-2 adenovirus vector vaccination (ChAdOx1 nCoV-19 [AZD1222]) in heart transplant recipients aged 18 to 70 years of age. J. Heart Lung Transplant. 2022, 41, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.; Koen, A.; Izu, A.; Fairlie, L.; Cutland, C.; Baillie, V.; Padayachee, S.; Dheda, K.; Barnabas, S.; Bhorat, Q.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: An interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021, 8, e568–e580. [Google Scholar] [CrossRef]
- Chang, J.; Chiou, J.; Hung, C.; Liu, M.; Chang, H.; Hong, S.; Wang, C.; Lin, Y.; Hsieh, Y.; Chung, C.; et al. Humoral Immunogenicity and Reactogenicity of the Standard ChAdOx1 nCoV-19 Vaccination in Taiwan. Vaccines 2022, 10, 312. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.; Stoesser, N.; Matthews, P.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.; Newton, J.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Zitt, E.; Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Mutschlechner, B.; Ulmer, H.; Benda, M.; Sprenger-Mähr, H.; Winder, T.; Lhotta, K. The Safety and Immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 Vaccine in Hemodialysis Patients. Front. Immunol. 2021, 12, 704773. [Google Scholar] [CrossRef]
- Broseta, J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.; Marcos, M.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef]
- Longlune, N.; Nogier, M.; Miedougé, M.; Gabilan, C.; Cartou, C.; Seigneuric, B.; Del Bello, A.; Marion, O.; Faguer, S.; Izopet, J.; et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol. Dial. Transplant. 2021, 36, 1704–1709. [Google Scholar] [CrossRef]
- Duarte, R.; Roldão, M.; Figueiredo, C.; Luz, I.; Ferrer, F.; Gonçalves, H.; Sofia, F.; Lopes, K. Humoral response to BNT162b2 mRNA COVID-19 vaccine in peritoneal and hemodialysis patients: A comparative study. Ther. Apher. Dial. 2021. [Google Scholar] [CrossRef] [PubMed]
- Strengert, M.; Becker, M.; Ramos, G.; Dulovic, A.; Gruber, J.; Juengling, J.; Lürken, K.; Beigel, A.; Wrenger, E.; Lonnemann, G.; et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on haemodialysis. EBioMedicine 2021, 70, 103524. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Charmetant, X.; Barba, T.; Koppe, L.; Pelletier, C.; Kalbacher, E.; Chalencon, E.; Mathias, V.; Ovize, A.; Cart-Tanneur, E.; et al. The ROMANOV study found impaired humoral and cellular immune responses to SARS-CoV-2 mRNA vaccine in virus-unexposed patients receiving maintenance hemodialysis. Kidney Int. 2021, 100, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Speer, C.; Göth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kälble, F.; Kreysing, M.; Reichel, P.; et al. Early Humoral Responses of Hemodialysis Patients after COVID-19 Vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef]
- Melin, J.; Svensson, M.; Albinsson, B.; Winqvist, O.; Pauksens, K. Humoral and cellular response to SARS-CoV-2 BNT162b2 mRNA vaccine in hemodialysis patients. BMC Immunol. 2021, 22, 70. [Google Scholar] [CrossRef]
- Simon, B.; Rubey, H.; Treipl, A.; Gromann, M.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Safranow, K.; Wojciechowska-Koszko, I.; Roszkowska, P.; Dziedziejko, V.; Myślak, M.; Różański, J.; Ciechanowski, K.; Stompór, T.; Przybyciński, J.; et al. SARS-CoV-2 mRNA Vaccine-Induced Cellular and Humoral Immunity in Hemodialysis Patients. Biomedicines 2022, 10, 636. [Google Scholar] [CrossRef]
- Dheir, H.; Tocoglu, A.; Toptan, H.; Pinar, M.; Demirci, T.; Koroglu, M.; Yaylaci, S.; Genc, A.; Genc, A.; Firat, N.; et al. Short and mid-term SARS-CoV-2 antibody response after inactivated COVID-19 vaccine in hemodialysis and kidney transplant patients. J. Med. Virol. 2022, 94, 3176–3183. [Google Scholar] [CrossRef]
- Lesny, P.; Anderson, M.; Cloherty, G.; Stec, M.; Haase-Fielitz, A.; Haarhaus, M.; Santos, C.; Lucas, C.; Macario, F.; Haase, M. Immunogenicity of a first dose of mRNA- or vector-based SARS-CoV-2 vaccination in dialysis patients: A multicenter prospective observational pilot study. J. Nephrol. 2021, 34, 975–983. [Google Scholar] [CrossRef]
- Clarke, C.; Prendecki, M.; Dhutia, A.; Gan, J.; Edwards, C.; Prout, V.; Lightstone, L.; Parker, E.; Marchesin, F.; Griffith, M.; et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021, 99, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Labriola, L.; Scohy, A.; Seghers, F.; Perlot, Q.; De Greef, J.; Desmet, C.; Romain, C.; Morelle, J.; Yombi, J.; Kabamba, B.; et al. A Longitudinal, 3-Month Serologic Assessment of SARS-CoV-2 Infections in a Belgian Hemodialysis Facility. Clin. J. Am. Soc. Nephrol. 2021, 16, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Gurevich, M.; Falb, R.; Dreyer-Alster, S.; Sonis, P.; Mandel, M. SARS-CoV-2 antibody dynamics and B-cell memory response over time in COVID-19 convalescent subjects. Clin. Microbiol. Infect. 2021, 27, 1349.e1341–1349.e1346. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.; Juno, J.; Wang, J.; Selva, K.; Reynaldi, A.; Tan, H.; Lee, W.; Wragg, K.; Kelly, H.; Esterbauer, R.; et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.; Zhu, F.; Ong, S.; Young, B.; Fong, S.; Le Bert, N.; Tan, C.; Tiu, C.; Zhang, J.; Tan, S.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Rosati, M.; Terpos, E.; Ntanasis-Stathopoulos, I.; Agarwal, M.; Bear, J.; Burns, R.; Hu, X.; Korompoki, E.; Donohue, D.; Venzon, D.; et al. Sequential Analysis of Binding and Neutralizing Antibody in COVID-19 Convalescent Patients at 14 Months After SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 793953. [Google Scholar] [CrossRef]
- Huynh, A.; Arnold, D.; Smith, J.; Moore, J.; Zhang, A.; Chagla, Z.; Harvey, B.; Stacey, H.; Ang, J.; Clare, R.; et al. Characteristics of Anti-SARS-CoV-2 Antibodies in Recovered COVID-19 Subjects. Viruses 2021, 13, 697. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, H.; Ye, B.; Zhao, M.; Zhan, J.; Dong, S.; Guo, Y.; Zhao, Y.; Li, M.; Liu, S.; et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clin. Infect. Dis. 2021, ciab884. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Drouot, L.; Lamulle, J.; Hanoy, M.; Edet, S.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; et al. SARS-CoV-2-specific Humoral and Cellular Immunities in Kidney Transplant Recipients and Dialyzed Patients Recovered From Severe and Nonsevere COVID-19. Transplant. Direct. 2021, 7, e792. [Google Scholar] [CrossRef]
- Snyman, J.; Hwa, S.; Krause, R.; Muema, D.; Reddy, T.; Ganga, Y.; Karim, F.; Leslie, A.; Sigal, A.; Ndung’u, T. Similar antibody responses against SARS-CoV-2 in HIV uninfected and infected individuals on antiretroviral therapy during the first South African infection wave. Clin. Infect. Dis. 2021, ciab758. [Google Scholar] [CrossRef]
- Mahallawi, W.; Alzahrani, M.; Alahmadey, Z. Durability of the humoral immune response in recovered COVID-19 patients. Saudi J. Biol. Sci. 2021, 28, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Pałgan, K. Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination. Cells 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Department of Disease Control. COVID-19 Vaccination in Thailand; Department of Disease Control: Atlanta, GA, USA, 2022. [Google Scholar]
- World Health Organization. The Oxford/AstraZeneca (ChAd)x1-S [Recombinant] Vaccine) COVID-19 Vaccine: What You Need to Know; World Health Organization: Geneva, Switzerland, 2022.
| Characteristics | Total | Vaccinated | Recovered | p-Value |
|---|---|---|---|---|
| Participants | 109 | 63 | 46 | |
| Age, years (mean ± SD) | 54.93 ± 15.28 | 57.63 ± 14.83 | 51.22 ± 15.26 | 0.03 |
| BMI, kg/m2 (mean ± SD) | 23.86 ± 4.80 | 24.61 ± 5.19 | 22.83 ± 4.03 | 0.06 |
| Comorbidities | 94 (86.24%) | 57 (90.48%) | 37 (80.43%) | 0.16 |
| Diabetes mellitus | 49 (44.95%) | 33 (52.38%) | 16 (34.78%) | 0.08 |
| Hypertension | 89 (81.65%) | 56 (88.89%) | 33 (71.74%) | 0.03 |
| Coronary artery disease | 4 (3.67%) | 3 (4.76%) | 1 (2.17%) | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasithsirikul, W.; Nopsopon, T.; Phutrakool, P.; Suwanwattana, P.; Kantagowit, P.; Pongpirul, W.; Jongkaewwattana, A.; Pongpirul, K. ChAdOx1 nCoV-19 Immunogenicity and Immunological Response Following COVID-19 Infection in Patients Receiving Maintenance Hemodialysis. Vaccines 2022, 10, 959. https://doi.org/10.3390/vaccines10060959
Prasithsirikul W, Nopsopon T, Phutrakool P, Suwanwattana P, Kantagowit P, Pongpirul W, Jongkaewwattana A, Pongpirul K. ChAdOx1 nCoV-19 Immunogenicity and Immunological Response Following COVID-19 Infection in Patients Receiving Maintenance Hemodialysis. Vaccines. 2022; 10(6):959. https://doi.org/10.3390/vaccines10060959
Chicago/Turabian StylePrasithsirikul, Wisit, Tanawin Nopsopon, Phanupong Phutrakool, Pawita Suwanwattana, Piyawat Kantagowit, Wannarat Pongpirul, Anan Jongkaewwattana, and Krit Pongpirul. 2022. "ChAdOx1 nCoV-19 Immunogenicity and Immunological Response Following COVID-19 Infection in Patients Receiving Maintenance Hemodialysis" Vaccines 10, no. 6: 959. https://doi.org/10.3390/vaccines10060959
APA StylePrasithsirikul, W., Nopsopon, T., Phutrakool, P., Suwanwattana, P., Kantagowit, P., Pongpirul, W., Jongkaewwattana, A., & Pongpirul, K. (2022). ChAdOx1 nCoV-19 Immunogenicity and Immunological Response Following COVID-19 Infection in Patients Receiving Maintenance Hemodialysis. Vaccines, 10(6), 959. https://doi.org/10.3390/vaccines10060959







