Cost-Effectiveness of Extending the National Influenza Vaccination Program in South Korea: Does Vaccination of Older Adults Provide Health Benefits to the Entire Population?
Abstract
:1. Introduction
2. Methods
- (1)
- Target population of current NIP
- ·
- Children aged 6 months to 12 years
- ·
- Pregnant women
- ·
- Adults aged 65 years or more
- (2)
- Target population of proposed policy
- ·
- Current target population
- ·
- Individuals aged 50–64 years
2.1. Model Framework
2.2. Parameters and Data
2.2.1. Vaccine Parameters
2.2.2. Disease Burden and Health Outcomes
2.2.3. Utilities
2.3. Costs
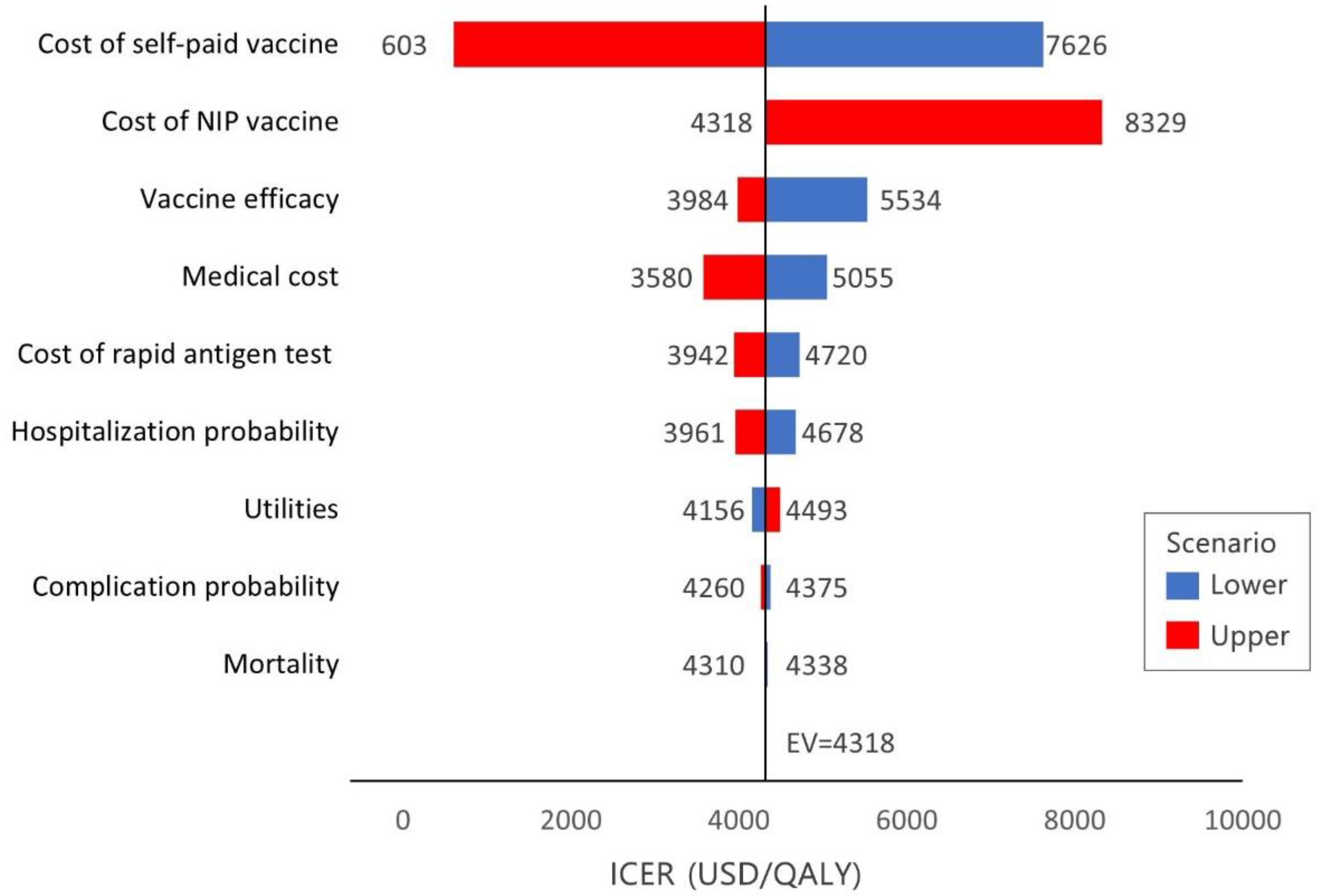
2.4. Sensitivity and Scenario Analyses
3. Results
3.1. Base Case
3.2. Scenario Analyses
3.3. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 3 December 2021).
- Park, M.; Wu, P.; Goldstein, E.; Kim, W.J.; Cowling, B.J. Influenza-associated excess mortality in South Korea. Am. J. Prev. Med. 2016, 50, e111–e119. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Kang, D.R.; Lee, D.H.; Choi, Y.J.; Tchoe, B.; Nam, C.M.; Kim, H.J.; Lee, J.K.; Jun, B.Y.; Youm, Y.; et al. Socioeconomic burden of influenza in the Republic of Korea, 2007–2010. PLoS ONE 2013, 8, e84121. [Google Scholar]
- Wang, Q.; Yue, N.; Zheng, M.; Wang, D.; Duan, C.; Yu, X.; Zhang, X.; Bao, C.; Jin, H. Influenza vaccination coverage of population and the factors influencing influenza vaccination in mainland China: A meta-analysis. Vaccine 2018, 36, 7262–7269. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.B.; Thompson, W.W.; Meltzer, M.I.; Reeve, G.R.; Talamonti, W.J.; Cox, N.J.; Lilac, H.A.; Hall, H.; Klimov, A.; Fukuda, K. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000, 284, 1655–1663. [Google Scholar] [CrossRef]
- Campbell, D.S.; Rumley, M.H. Cost-effectiveness of the influenza vaccine in a healthy, working-age population. J. Occup. Environ. Med. 1997, 39, 408–414. [Google Scholar] [CrossRef]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef]
- Maciosek, M.V.; Solberg, L.I.; Coffield, A.B.; Edwards, N.M.; Goodman, M.J. Influenza vaccination: Health impact and cost effectiveness among adults aged 50 to 64 and 65 and older. Am. J. Prev. Med. 2006, 31, 72–79. [Google Scholar] [CrossRef]
- Trucchi, C.; Paganino, C.; Orsi, A.; Amicizia, D.; Tisa, V.; Piazza, M.F.; Gallo, D.; Simonetti, S.; Buonopane, B.; Icardi, G.; et al. Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults≥ 50 years-old. BMC Health Serv. Res. 2019, 19, 585. [Google Scholar] [CrossRef]
- Bridges, C.B.; Cox, N.; Fukuda, K.; Singleton, J.A.; Strikas, R.A.; Winquist, A. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Available online: https://stacks.cdc.gov/view/cdc/5679 (accessed on 4 May 2022).
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 influenza season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices, United States, 2021–2022 influenza season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare of South Korea. Vaccination Standards and Methods; Enforcement Rule 2010-126; Ministry of Health and Welfare of South Korea: Seoul, Korea, 2010.
- Palache, A. Seasonal influenza vaccine provision in 157 countries (2004–2009) and the potential influence of national public health policies. Vaccine 2011, 29, 9459–9466. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. Korea National Health & Nutrition Examination Survey (KNHANES). 2018–2019. Available online: https://www.koreascience.or.kr/article/JAKO202020363945303.page (accessed on 4 May 2022).
- Gallè, F.; Sabella, E.A.; Roma, P.; Ferracuti, S.; Da Molin, G.; Diella, G.; Montagna, M.T.; Orsi, G.B.; Liguori, G.; Napoli, C. Knowledge and lifestyle behaviors related to COVID-19 pandemic in people over 65 years old from southern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10872. [Google Scholar] [CrossRef] [PubMed]
- Eilers, R.; De Melker, H.; Veldwijk, J.; Krabbe, P. Vaccine preferences and acceptance of older adults. Vaccine 2017, 35, 2823–2830. [Google Scholar] [CrossRef]
- Nichol, K.L. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine 2003, 21, 1769–1775. [Google Scholar] [CrossRef]
- Nichol, K.L. Cost-benefit analysis of a strategy to vaccinate healthy working adults against influenza. Arch. Intern. Med. 2001, 161, 749–759. [Google Scholar] [CrossRef]
- Burckel, E.; Ashraf, T.; Galvão de Sousa Filho, J.P.; Forleo Neto, E.; Guarino, H.; Yauti, C.; de Barros Barreto, F.; Champion, L. Economic impact of providing workplace influenza vaccination. Pharmacoeconomics 1999, 16, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Aballéa, S.; Chancellor, J.; Martin, M.; Wutzler, P.; Carrat, F.; Gasparini, R.; Toniolo-Neto, J.; Drummond, M.; Weinstein, M. The cost-effectiveness of influenza vaccination for people aged 50 to 64 years: An international model. Value Health 2007, 10, 98–116. [Google Scholar] [CrossRef]
- Prosser, L.A.; O’Brien, M.A.; Molinari, N.-A.M.; Hohman, K.H.; Nichol, K.L.; Messonnier, M.L.; Lieu, T.A. Non-traditional settings for influenza vaccination of adults. Pharmacoeconomics 2008, 26, 163–178. [Google Scholar] [CrossRef]
- Aballéa, S.; De Juanes, J.R.; Barbieri, M.; Martin, M.; Chancellor, J.; Oyagüez, I.; Verwee, B.; Largeron, N. The cost effectiveness of influenza vaccination for adults aged 50 to 64 years: A model-based analysis for Spain. Vaccine 2007, 25, 6900–6910. [Google Scholar] [CrossRef]
- Turner, D.; Wailoo, A.; Cooper, N.; Sutton, A.; Abrams, K.; Nicholson, K. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine 2006, 24, 1035–1043. [Google Scholar] [CrossRef]
- Newall, A.T.; Scuffham, P.A.; Kelly, H.; Harsley, S.; MacIntyre, C.R. The cost-effectiveness of a universal influenza vaccination program for adults aged 50–64 years in Australia. Vaccine 2008, 26, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Shin, G.; Kang, D.; Lim, J.-O.; Kim, Y.-K.; Choi, W.S.; Yun, J.-W.; Noh, J.Y.; Song, J.Y.; Kim, W.J. Cost-Effectiveness of Influenza Vaccination Strategies in Adults: Older Adults Aged≥ 65 Years, Adults Aged 50–64 Years, and At-Risk Adults Aged 19–64 Years. Vaccines 2022, 10, 445. [Google Scholar] [CrossRef] [PubMed]
- Van Bellinghen, L.-A.; Meier, G.; Van Vlaenderen, I. The potential cost-effectiveness of quadrivalent versus trivalent influenza vaccine in elderly people and clinical risk groups in the UK: A lifetime multi-cohort model. PLoS ONE 2014, 9, e98437. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Johnstone, J.; Loeb, M. Vaccine herd effect. Scand. J. Infect. Dis. 2011, 43, 683–689. [Google Scholar] [CrossRef]
- Pitman, R.; Nagy, L.; Sculpher, M. Cost-effectiveness of childhood influenza vaccination in England and Wales: Results from a dynamic transmission model. Vaccine 2013, 31, 927–942. [Google Scholar] [CrossRef]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Hansen, E.; Day, T. Optimal control of epidemics with limited resources. J. Math. Biol. 2011, 62, 423–451. [Google Scholar] [CrossRef]
- Osthus, D.; Hickmann, K.S.; Caragea, P.C.; Higdon, D.; Del Valle, S.Y. Forecasting seasonal influenza with a state-space SIR model. Ann. Appl. Stat. 2017, 11, 202. [Google Scholar] [CrossRef]
- Korea, S. Population and Housing Census (Register-Based Census). Available online: http://kostat.go.kr/portal/eng/pressReleases/8/7/index.board (accessed on 2 November 2021).
- Capri, S.; Barbieri, M.; de Waure, C.; Boccalini, S.; Panatto, D. Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population. Hum. Vaccines Immunother. 2018, 14, 1331–1341. [Google Scholar] [CrossRef]
- WHO. FluNet. Available online: https://www.who.int/tools/flunet (accessed on 1 November 2021).
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2018, 2, CD004876. [Google Scholar]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Di Pietrantonj, C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018, 2, CD001269. [Google Scholar] [CrossRef]
- Jefferson, T.; Rivetti, A.; Di Pietrantonj, C.; Demicheli, V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev. 2018, 2, CD004879. [Google Scholar] [CrossRef] [PubMed]
- Cauchemez, S.; Carrat, F.; Viboud, C.; Valleron, A.J.; Boëlle, P. A Bayesian MCMC approach to study transmission of influenza: Application to household longitudinal data. Stat. Med. 2004, 23, 3469–3487. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.M.; Petrou, S.; de Lusignan, S.; Yonova, I.; Keeling, M.J. Seasonal influenza: Modelling approaches to capture immunity propagation. PLoS Comput. Biol. 2019, 15, e1007096. [Google Scholar] [CrossRef] [PubMed]
- Ibuka, Y.; Ohkusa, Y.; Sugawara, T.; Chapman, G.B.; Yamin, D.; Atkins, K.E.; Taniguchi, K.; Okabe, N.; Galvani, A.P. Social contacts, vaccination decisions and influenza in Japan. J. Epidemiol. Community Health 2016, 70, 162–167. [Google Scholar] [CrossRef]
- Elbasha, E.H.; Choi, Y.; Daniels, V.; Goveia, M.G. Cost-effectiveness of routine catch-up hepatitis a vaccination in the United States: Dynamic transmission modeling study. Vaccine 2021, 39, 6315–6321. [Google Scholar] [CrossRef]
- NHISS. National Health Insurance Service–National Sample Cohort (NHIS-NSC); National Health Insurance Service: Wonju, Korea.
- WHO. A Manual for Estimating Disease Burden Associated with Seasonal Influenza. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/178801/9789241549301_eng.pdf (accessed on 4 May 2022).
- Yun, J.-W.; Choi, M.J.; Shin, G.-S.; Lim, J.-O.; Noh, J.Y.; Kim, Y.-K.; Song, J.Y.; Kim, W.J.; Choi, S.-E.; Cheong, H.J. Cost-effectiveness of influenza vaccine strategies for the elderly in South Korea. PLoS ONE 2019, 14, e0209643. [Google Scholar] [CrossRef]
- KCDC. Influenza Weekly/Annual Report. Available online: https://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaMain.do?pblctDtaSeAt=3 (accessed on 29 January 2022).
- Moore, K.; Black, J.; Rowe, S.; Franklin, L. Syndromic surveillance for influenza in two hospital emergency departments. Relationships between ICD-10 codes and notified cases, before and during a pandemic. BMC Public Health 2011, 11, 338. [Google Scholar] [CrossRef]
- Pike, J.; Grosse, S.D. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with human capital estimates: A review. Appl. Health Econ. Health Policy 2018, 16, 765–778. [Google Scholar] [CrossRef]
- Korea, S. Consumer Price Index. Available online: http://kostat.go.kr/incomeNcpi/cpi/index.action (accessed on 20 December 2021).
- Choi, K.-R.; Kim, J.-H.; Yoo, S.-H. Public perspective on constructing sea forests as a public good: A contingent valuation experiment in South Korea. Mar. Policy 2020, 120, 104146. [Google Scholar] [CrossRef]
- KDCA. Announcement of Vaccination Cost according to Regulations on Consignment of Vaccination Services. Available online: https://www.kdca.go.kr/board/board.es?mid=a20505000000&bid=0017 (accessed on 3 April 2021).
- HIRA. Information on Non-Insured Medical Expenses. Available online: https://www.hira.or.kr/re/diag/getNewDiagNondeductibleYadmList.do?pgmid=HIRAA030009000000 (accessed on 20 April 2021).
- Ting, E.E.; Sander, B.; Ungar, W.J. Systematic review of the cost-effectiveness of influenza immunization programs. Vaccine 2017, 35, 1828–1843. [Google Scholar] [CrossRef]
- Data, O. Influenza Vaccination Rates. Available online: https://data.oecd.org/healthcare/influenza-vaccination-rates.htm (accessed on 4 May 2022).
- Kim, Y.-K.; Song, J.Y.; Jang, H.; Kim, T.H.; Koo, H.; Varghese, L.; Han, E. Cost Effectiveness of Quadrivalent Influenza Vaccines Compared with Trivalent Influenza Vaccines in Young Children and Older Adults in Korea. Pharmacoeconomics 2018, 36, 1475–1490. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Kim, B.; Yang, Y.; Suh, D.-C.; Kim, E. Cost effectiveness of influenza vaccination in patients with acute coronary syndrome in Korea. Vaccine 2017, 35, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lim, J. The impact of free vaccination policies under the Korean Influenza National Immunization Program: Trends in influenza vaccination rates in South Korea from 2010 to 2019. PLoS ONE 2022, 17, e0262594. [Google Scholar] [CrossRef]
- Thomas, R.E.; Lorenzetti, D.L. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst. Rev. 2018, 5, CD005188. [Google Scholar] [CrossRef] [PubMed]
- de Boer, P.T.; van Maanen, B.M.; Damm, O.; Ultsch, B.; Dolk, F.C.; Crépey, P.; Pitman, R.; Wilschut, J.C.; Postma, M.J. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev. Pharm. Outcomes Res. 2017, 17, 249–265. [Google Scholar] [CrossRef]
- Newall, A.T.; Chaiyakunapruk, N.; Lambach, P.; Hutubessy, R.C. WHO guide on the economic evaluation of influenza vaccination. Influenza Other Respir. Viruses 2018, 12, 211–219. [Google Scholar] [CrossRef]
- Chun, J.Y.; Park, W.B.; Kim, N.J.; Choi, E.H.; Funk, S.; Oh, M.-d. Estimating contact-adjusted immunity levels against measles in South Korea and prospects for maintaining elimination status. Vaccine 2020, 38, 107–111. [Google Scholar] [CrossRef]
- McBean, A.M.; Babish, J.D.; Warren, J.L. The impact and cost of influenza in the elderly. Arch. Intern. Med. 1993, 153, 2105–2111. [Google Scholar] [CrossRef]
- Nichol, K.; Margolis, K.; Wuorenma, J.; Von Sternberg, T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N. Engl. J. Med. 1994, 331, 778–784. [Google Scholar] [CrossRef]
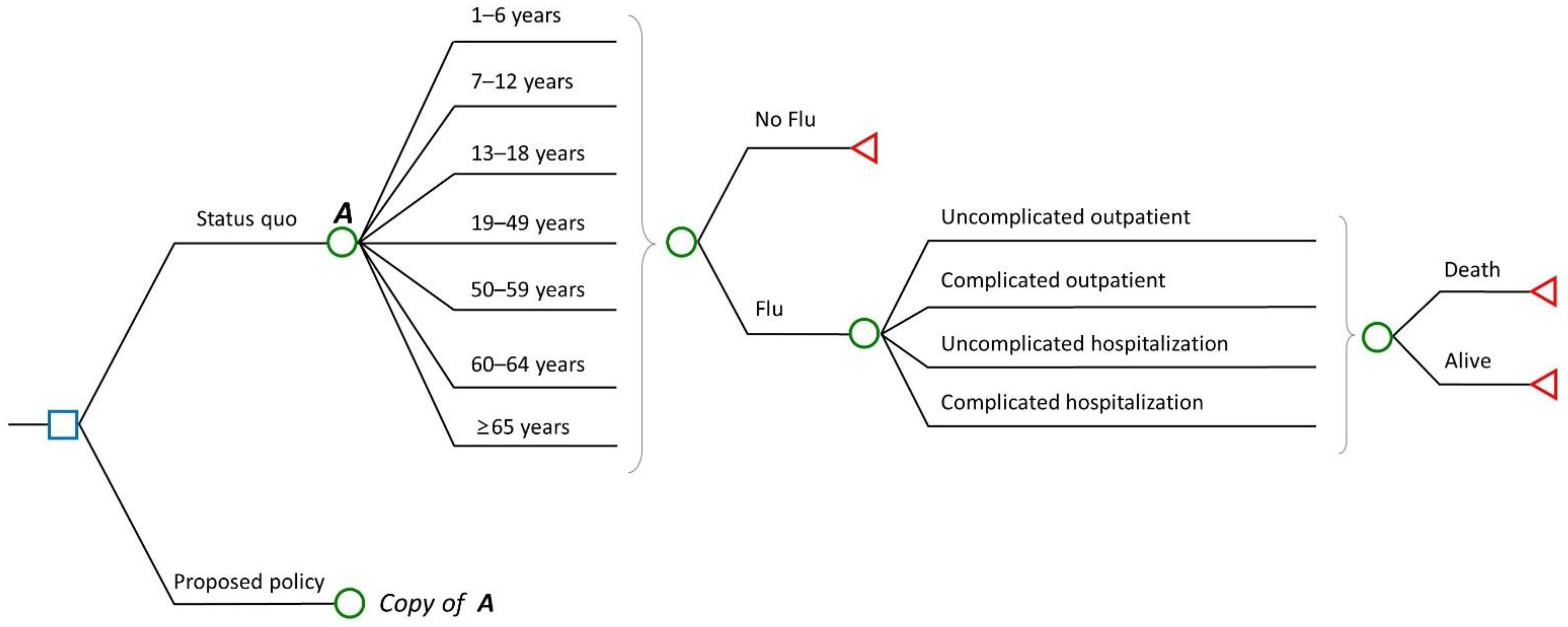

| Variables | Value | Source/Comment |
|---|---|---|
| Population by age group | [36] | |
| 1–6 | 2,297,013 | |
| 7–12 | 2,799,426 | |
| 13–18 | 2,843,464 | |
| 19–49 | 22,532,473 | |
| 50–59 | 8,645,014 | |
| 60–64 | 3,950,469 | |
| ≥65 | 8,496,077 | |
| Immunization rate of influenza vaccine by age group | ||
| Current | [15] | |
| 1–6 | 0.862 | |
| 7–12 | 0.702 | |
| 13–18 | 0.310 | |
| 19–49 | 0.267 | |
| 50–59 | 0.284 | |
| 60–64 | 0.498 | |
| ≥65 | 0.826 | |
| Proposed policy | Assumption | |
| 50–59 | 0.800 | |
| 60–64 | 0.800 | |
| Effectiveness of vaccine by age group | Calculated with reference to Capri et al. [35] using WHO data [37] and Cochrane review [38,39,40] | |
| 1–6 | 0.694 (0.640–0.950) | |
| 7–12 | 0.711 (0.640–0.923) | |
| 13–18 | 0.711 (0.640–0.924) | |
| 19–49 | 0.655 (0.590–0.901) | |
| 50–59 | 0.655 (0.590–0.901) | |
| 60–64 | 0.655 (0.590–0.901) | |
| ≥65 | 0.634 (0.580–0.900) | |
| Transmission coefficient by age group | 0.450 | Fitting |
| Recovery rate | 0.263 | Inverse of the infectious period [41,42] |
| Population mixing matrix between age group | See Ref [43]. | Japanese contact matrix was used |
| Age Group | Range of DSA | Source a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–6 | 7–12 | 13–18 | 19–49 | 50–59 | 60–64 | ≥65 | |||
| Probability | |||||||||
| Influenza case | 0.370 | 0.106 | 0.051 | 0.041 | 0.057 | 0.069 | 0.085 | – | N,I |
| Complication/influenza case | 0.130 | 0.071 | 0.043 | 0.024 | 0.041 | 0.064 | 0.122 | 80–120% | N |
| Hospitalization/influenza case | 0.176 | 0.076 | 0.062 | 0.020 | 0.050 | 0.080 | 0.109 | – | N |
| Death b/influenza case | 0.564 | 0.000 | 0.767 | 1.958 | 5.731 | 28.425 | 389.981 | – | N |
| Utility weight (decrement) | |||||||||
| Baseline utility | 1 c | 1 c | 1 c | 0.973 | 0.957 | 0.943 | 0.887 | 80–120% | [15] |
| Uncomplicated outpatient | −0.35 | −0.35 | −0.35 | −0.35 | −0.35 | −0.35 | −0.35 | 80–120% | [45] |
| Complicated outpatient | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | 80–120% | [45] |
| Uncomplicated hospitalization | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | 80–120% | [45] |
| Complicated hospitalization | −0.5 | −0.5 | −0.5 | −0.5 | −0.5 | −0.5 | −0.5 | 80–120% | [45] |
| Age Group | Base Case | SE | Range of DSA | Source a | |
|---|---|---|---|---|---|
| Cost (USD) | |||||
| Vaccination | |||||
| NIP vaccine dose cost b | 1–12 | 25.69 | – | 25.69–38.56 | [48] |
| 50–64 | 23.45 | – | 23.45–35.18 | Assumption | |
| ≥65 | 23.45 | – | 23.45–35.18 | [48] | |
| Self-paid vaccine c | All | 31.01 | 0.10 | 22.72–39.51 | [49] |
| Direct medical cost | |||||
| Uncomplicated outpatient c | 1–6 | 73.71 | 0.29 | 80%–120% | N |
| 7–12 | 69.36 | 0.27 | 80%–120% | N | |
| 13–18 | 66.44 | 0.23 | 80%–120% | N | |
| 19–49 | 58.28 | 0.08 | 80%–120% | N | |
| 50–59 | 59.03 | 0.16 | 80%–120% | N | |
| 60–64 | 60.09 | 0.28 | 80%–120% | N | |
| ≥65 | 60.24 | 0.18 | 80%–120% | N | |
| Complicated outpatient c | 1–6 | 128.44 | 0.51 | 80%–120% | N |
| 7–12 | 119.06 | 0.46 | 80%–120% | N | |
| 13–18 | 115.95 | 0.40 | 80%–120% | N | |
| 19–49 | 118.32 | 0.17 | 80%–120% | N | |
| 50–59 | 119.38 | 0.32 | 80%–120% | N | |
| 60–64 | 113.63 | 0.53 | 80%–120% | N | |
| ≥65 | 108.11 | 0.32 | 80%–120% | N | |
| Uncomplicated hospitalization c | 1–6 | 525.19 | 2.07 | 80%–120% | N |
| 7–12 | 563.54 | 2.17 | 80%–120% | N | |
| 13–18 | 590.60 | 2.02 | 80%–120% | N | |
| 19–49 | 669.92 | 0.96 | 80%–120% | N | |
| 50–59 | 883.46 | 2.34 | 80%–120% | N | |
| 60–64 | 1027.54 | 4.81 | 80%–120% | N | |
| ≥65 | 1018.42 | 3.01 | 80%–120% | N | |
| Complicated hospitalization c | 1–6 | 830.34 | 3.27 | 80%–120% | N |
| 7–12 | 773.68 | 2.98 | 80%–120% | N | |
| 13–18 | 663.93 | 2.28 | 80%–120% | N | |
| 19–49 | 950.77 | 1.36 | 80%–120% | N | |
| 50–59 | 956.59 | 2.53 | 80%–120% | N | |
| 60–64 | 1648.05 | 7.71 | 80%–120% | N | |
| ≥65 | 1198.25 | 3.54 | 80%–120% | N | |
| Anti-influenza agents d | 1–6 | 8.08 | – | – | H |
| 7–12 | 14.50 | – | – | H | |
| ≥13 | 14.65 | – | – | H | |
| Rapid antigen testc | All | 24.69 | 0.15 | 15.31–31 | [49] |
| Direct nonmedical cost (USD) | |||||
| Nursingc | All | 36.41 | 1.15 | 80%–120% | [15] |
| Transportation for outpatient c | All | 3.11 | 0.03 | 3.04–3.18 | [15] |
| Transportation for hospitalization c | All | 14.65 | 0.58 | 13.30–15.99 | [15] |
| Indirect cost | |||||
| Cost per working day lost (USD) e | 19–49 | 74.87 | – | 80%–120% | S |
| 50–59 | 92.41 | – | 80%–120% | S | |
| 60–64 | 51.00 | – | 80%–120% | S | |
| Duration of treatment (days) | |||||
| Uncomplicated outpatient d | All | 3.8 | – | – | [41,42] |
| Complicated outpatient d | All | 5 | – | – | Assumption |
| Hospitalization without complication c | 1–6 | 5.9 | 0.015 | – | N |
| 7–12 | 5.3 | 0.034 | – | N | |
| 13–18 | 5.5 | 0.033 | – | N | |
| 19–49 | 7.3 | 0.019 | – | N | |
| 50–59 | 9.4 | 0.020 | – | N | |
| 60–64 | 9.6 | 0.056 | – | N | |
| ≥65 | 9.2 | 0.019 | – | N | |
| Hospitalization with complication c | 1–6 | 6.8 | 0.064 | – | N |
| 7–12 | 5.9 | 0.325 | – | N | |
| 13–18 | 5.4 | 0.120 | – | N | |
| 19–49 | 8.1 | 0.127 | – | N | |
| 50–59 | 8.5 | 0.140 | – | N | |
| 60–64 | 12.3 | 0.151 | – | N | |
| ≥65 | 8.8 | 0.140 | – | N |
| Status quo | Proposed Policy | Difference | |
|---|---|---|---|
| Coverage | |||
| Number of people vaccinated | 22,281,539 | 27,940,972 | 5,659,433 |
| Influenza outcomes | |||
| Total influenza cases | 3,587,330 | 3,245,781 | −341,549 |
| Total complications | 277,063 | 258,735 | −18,328 |
| Total hospitalizations | 313,803 | 292,613 | −21,190 |
| Total influenza-associated deaths | 2094 | 1975 | −119 |
| Costs (USD) | |||
| Vaccinations | 616,940,439 | 716,297,174 | 99,356,735 |
| Outpatient visits | 435,557,031 | 402,499,966 | −33,057,065 |
| Hospitalizations | 262,794,280 | 242,647,477 | −20,146,803 |
| Direct non-healthcare costs | 247,350,909 | 231,521,717 | −15,829,192 |
| Productivity costs | 687,038,979 | 589,043,080 | −97,995,899 |
| Health effects | |||
| Total quality-adjusted life years | 50,017,614 | 50,028,303 | 10,689 |
| Strategy | Age Group | Vaccination Coverage (%) | Indirect Effects | Costs (Million USD) | QALYs | ICER (USD/QALY) | ||
|---|---|---|---|---|---|---|---|---|
| Societal | Healthcare | Societal | Healthcare | |||||
| Status quo | – | – | Yes | 2250 | 1315 | 50,017,614 | – | – |
| Scenario 1 | 50–64 | 80 | Yes | 2182 | 1361 | 50,028,303 | dominant | 4318 |
| Scenario 2 | 50–64 | 60 | Yes | 2207 | 1328 | 50,023,090 | dominant | 2295 |
| Scenario 3 | 60–64 | 80 | Yes | 2199 | 1302 | 50,019,438 | dominant | dominant |
| Scenario 4 | – | – | No | 2250 | 1315 | 50,017,614 | – | – |
| Scenario 5 | 50–64 | 80 | No | 2211 | 1375 | 50,026,855 | dominant | 6465 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, G.; Kang, D.; Cheong, H.J.; Choi, S.-E. Cost-Effectiveness of Extending the National Influenza Vaccination Program in South Korea: Does Vaccination of Older Adults Provide Health Benefits to the Entire Population? Vaccines 2022, 10, 932. https://doi.org/10.3390/vaccines10060932
Shin G, Kang D, Cheong HJ, Choi S-E. Cost-Effectiveness of Extending the National Influenza Vaccination Program in South Korea: Does Vaccination of Older Adults Provide Health Benefits to the Entire Population? Vaccines. 2022; 10(6):932. https://doi.org/10.3390/vaccines10060932
Chicago/Turabian StyleShin, Gyeongseon, Daewon Kang, Hee Jin Cheong, and Sang-Eun Choi. 2022. "Cost-Effectiveness of Extending the National Influenza Vaccination Program in South Korea: Does Vaccination of Older Adults Provide Health Benefits to the Entire Population?" Vaccines 10, no. 6: 932. https://doi.org/10.3390/vaccines10060932
APA StyleShin, G., Kang, D., Cheong, H. J., & Choi, S.-E. (2022). Cost-Effectiveness of Extending the National Influenza Vaccination Program in South Korea: Does Vaccination of Older Adults Provide Health Benefits to the Entire Population? Vaccines, 10(6), 932. https://doi.org/10.3390/vaccines10060932






