Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile
Abstract
:1. Introduction
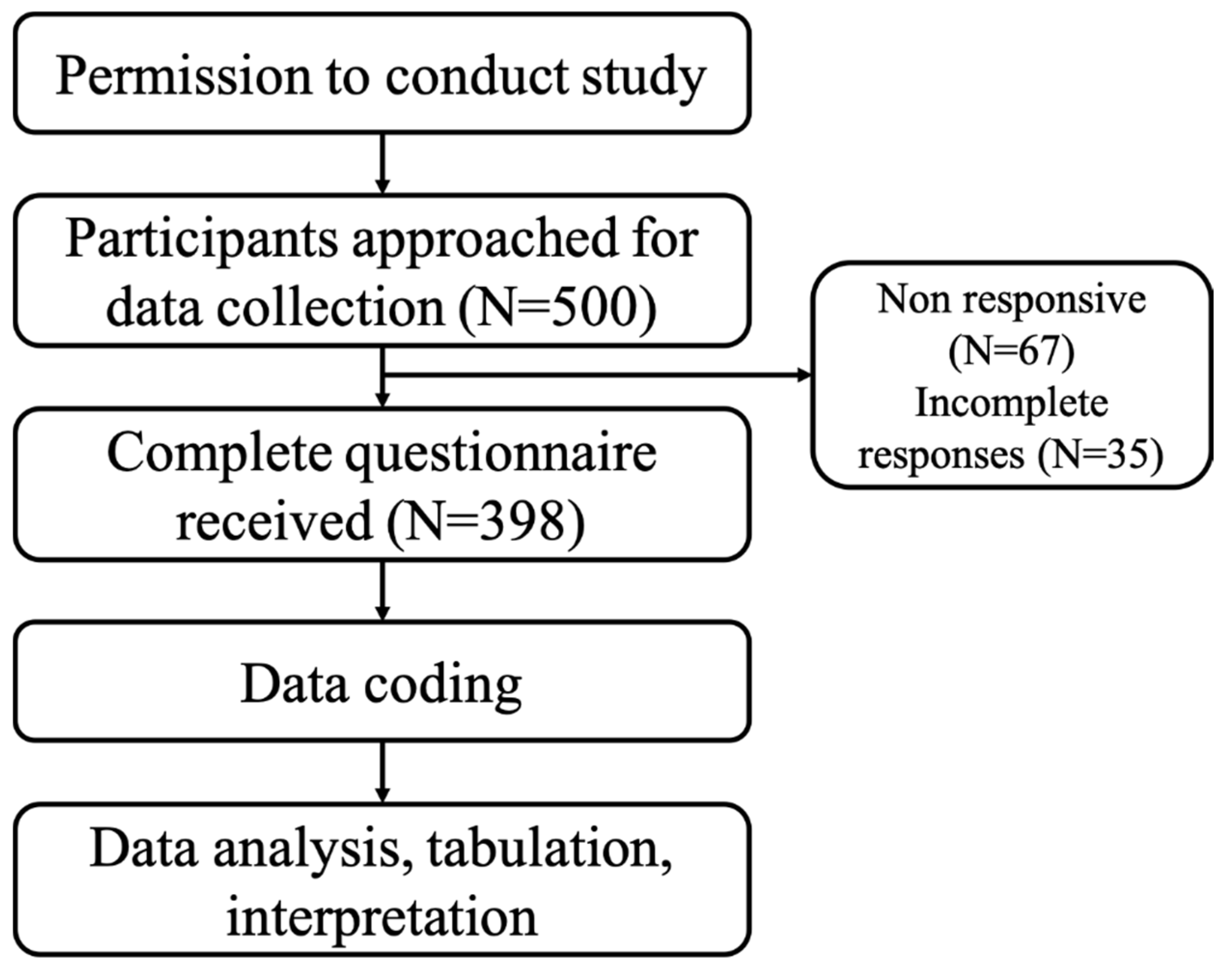
2. Methodology
2.1. Ethics Statement
2.2. Study Design and Location
2.3. Study Population
2.4. Validation and Reliability of Study Instrument
2.5. Components of Study Instrument
2.6. Data Collection
2.7. Sample Size
2.8. Statistical Analysis
3. Results
3.1. Demographic Profile
3.2. Side Effects Profile
3.3. Association of Side Effects among Types of Vaccine
3.4. Association of Individual Side Effects across Demographics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pradhan, D.; Biswasroy, P.; Naik, P.K.; Ghosh, G.; Rath, G. A review of current interventions for COVID-19 prevention. Arch. Med. Res. 2020, 51, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Misbah, S.; Ahmad, A.; Butt, M.H.; Khan, Y.H.; Alotaibi, N.H.; Mallhi, T.H. A systematic analysis of studies on corona virus disease 19 (COVID-19) from viral emergence to treatment. J. Coll. Physicians Surg. Pak. 2020, 30, 9–18. [Google Scholar] [PubMed]
- Ferrante, L.; Duczmal, L.H.; Capanema, E.; Steinmetz, W.A.C.; Almeida, A.C.L.; Leão, J.; Vassão, R.C.; Fearnside, P.M.; Tupinambás, U. Dynamics of COVID-19 in Amazonia: A history of government denialism and the risk of a third wave. Prev. Med. Rep. 2022, 26, 101752. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Subedi, S.; Davis, J.J.; Hodjat, P.; Walley, D.R.; Kinskey, J.C.; Saavedra, M.O.; Pruitt, L. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am. J. Pathol. 2022, 192, 320–331. [Google Scholar] [CrossRef]
- DeFrancesco, L. Whither COVID-19 vaccines? Nat. Biotechnol. 2020, 38, 1132–1145. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and safety of COVID-19 vaccines: A systematic review and meta-analysis of randomized clinical trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; Spector, T.D. COVID-19 Vaccine Waning and Effectiveness and Side Effects of Boosters: A Prospective Community Study From the ZOE COVID Study. Lancet Infect. Dis. 2022, 3980542. [Google Scholar] [CrossRef]
- Shrestha, S.; Khatri, J.; Shakya, S.; Danekhu, K.; Khatiwada, A.P.; Sah, R.; Kc, B.; Paudyal, V.; Khanal, S.; Rodriguez-Morales, A.J. Adverse events related to COVID-19 vaccines: The need to strengthen pharmacovigilance monitoring systems. Drugs Ther. Perspect. 2021, 37, 376–382. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Luchini, S. Addressing COVID-19 vaccine hesitancy: Is official communication the key? Lancet Public Health 2021, 6, e353–e354. [Google Scholar] [CrossRef]
- VIPER Group COVID19 Vaccine Tracker Team. Vaccination Rates, Approvals & Trials by Country. Available online: https://covid19.trackvaccines.org/trials-vaccines-by-country/ (accessed on 12 April 2022).
- Kwok, H.F. Review of COVID-19 vaccine clinical trials-A puzzle with missing pieces. Int. J. Biol. Sci. 2021, 17, 1461. [Google Scholar] [CrossRef]
- Ahsan, W.; Syed, N.K.; Alsraeya, A.A.; Alhazmi, H.A.; Najmi, A.; Al Bratty, M.; Javed, S.; Makeen, H.A.; Meraya, A.M.; Albarraq, A.A. Post-vaccination survey for monitoring the side effects associated with COVID-19 vaccines among healthcare professionals of Jazan province, Saudi Arabia. Saudi Med. J. 2021, 42, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Alamer, E.; Alhazmi, A.; Qasir, N.A.; Alamer, R.; Areeshi, H.; Gohal, G.; Qadri, M.; Hashem, A.M.; Algaissi, A. Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12–18 Years in Saudi Arabia. Vaccines 2021, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, A.; Alkattan, A.; Radwan, N.; Elzohri, M.; Alzaher, A.; Ibrahim, M.; Alsalameen, E.; Alsultan, A.; Alhabib, D.; Alshelwah, A. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia. Drugs Ther. Perspect. 2022, 38, 84–92. [Google Scholar] [CrossRef]
- Alhazmi, A.; Alamer, E.; Daws, D.; Hakami, M.; Darraj, M.; Abdelwahab, S.; Maghfuri, A.; Algaissi, A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines 2021, 9, 674. [Google Scholar] [CrossRef]
- Al Bahrani, S.; Albarrak, A.; Alghamdi, O.A.; Alghamdi, M.A.; Hakami, F.H.; Al Abaadi, A.K.; Alkhrashi, S.A.; Alghamdi, M.Y.; Almershad, M.M.; Alenazi, M.M. Safety and reactogenicity of the ChAdOx1 (AZD1222) COVID-19 vaccine in Saudi Arabia. Int. J. Infect. Dis. 2021, 110, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.A.; Alkazemi, A.; Alissa, A.; Alghamdi, I.; Alwarafi, G.; Waggas, H.A. Adverse Events following AstraZeneca COVID-19 Vaccine in Saudi Arabia: A Cross-Sectional Study among Healthcare and Nonhealthcare Workers. Intervirology 2022, 65, 104–109. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.; Almukadi, H.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: A retrospective cross-sectional study. Int. J. Gen. Med. 2021, 14, 1389. [Google Scholar] [CrossRef]
- OpenEpi. Sample Size for Frequency in a Population. Available online: http://www.openepi.com/SampleSize/SSPropor.htm (accessed on 17 May 2022).
- Mallhi, T.H.; Khan, A.H.; Sarriff, A.; Adnan, A.S.; Khan, Y.H. Determinants of mortality and prolonged hospital stay among dengue patients attending tertiary care hospital: A cross-sectional retrospective analysis. BMJ Open 2017, 7, e016805. [Google Scholar] [CrossRef]
- Butt, M.H.; Ahmad, A.; Misbah, S.; Mallhi, T.H.; Khan, Y.H.; Muhammad, K.; Iqbal, Z. Ensuring the Quality and Appropriate Use of Hand Sanitizers During the COVID-19 Pandemic: Suggestions and Recommendations With the Role of the Pharmacist. Disaster Med. Public Health Prep. 2021, 1–2. [Google Scholar] [CrossRef]
- Mallhi, T.H.; Khan, Y.H.; Butt, M.H.; Liaqat, A.; Abid, A.; Ahmad, A.; Misbah, S. Risks of zoonotic transmission of COVID-19 during Eid-Ul-Adha in Pakistan. Disaster Med. Public Health Prep. 2020, 14, e40–e41. [Google Scholar] [CrossRef]
- Saleem, R.T.; Butt, M.H.; Ahmad, A.; Amin, M.; Amir, A.; Ahsan, A.; Fayyaz, F.; Saleem, R.; Riaz, T.; Waheed, U. Practices and Attitude of Self-medication during COVID-19 Pandemic in University Students with Interventional Role of Pharmacist: A Regional Analysis. Lat. Am. J. Pharm. 2021, 40, 1946–1953. [Google Scholar]
- Jentsch, P.C.; Anand, M.; Bauch, C.T. Prioritising COVID-19 vaccination in changing social and epidemiological landscapes: A mathematical modelling study. Lancet Infect. Dis. 2021, 21, 1097–1106. [Google Scholar] [CrossRef]
- Medeiros, K.S.; Costa, A.P.F.; Sarmento, A.C.A.; Freitas, C.L.; Gonçalves, A.K. Side effects of COVID-19 vaccines: A systematic review and meta-analysis protocol of randomised trials. BMJ Open 2022, 12, e050278. [Google Scholar] [CrossRef]
- Khan, Y.H.; Mallhi, T.H.; Alotaibi, N.H.; Alzarea, A.I.; Alanazi, A.S.; Tanveer, N.; Hashmi, F.K. Threat of COVID-19 vaccine hesitancy in Pakistan: The need for measures to neutralize misleading narratives. Am. J. Trop. Med. Hyg. 2020, 103, 603. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Saudi Arabia Situation on COVID-19. Available online: https://covid19.who.int/region/emro/country/sa (accessed on 14 April 2022).
- Harry, A.M.; Edet, C.K.; Ekanem, N.E.; Kemdirim, C.J.; Uduak, A.E. Adverse Events Following COVID-19 Vaccination in Rivers State, Nigeria: A Cross-Sectional Study. Niger. Postgrad. Med. J. 2022, 29, 89. [Google Scholar] [PubMed]
- Rahman, M.; Masum, M.; Ullah, H.; Wajed, S.; Talukder, A. A comprehensive review on COVID-19 vaccines: Development, effectiveness, adverse effects, distribution and challenges. Virusdisease 2022, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.M.M.; Al-Hatamleh, M.A.; Olaimat, A.N.; Mohamud, R.; Fawaz, M.; Kateeb, E.T.; Alkhairy, O.K.; Tayyem, R.; Lounis, M.; Al-Raeei, M. Reported Adverse Effects and Attitudes among Arab Populations Following COVID-19 Vaccination: A Large-Scale Multinational Study Implementing Machine Learning Tools in Predicting Post-Vaccination Adverse Effects Based on Predisposing Factors. Vaccines 2022, 10, 366. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Haas, J.W.; Bender, F.L.; Ballou, S.; Kelley, J.M.; Wilhelm, M.; Miller, F.G.; Rief, W.; Kaptchuk, T.J. Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2143955. [Google Scholar] [CrossRef]
- Lo, S.P.; Hsieh, T.-C.; Pastuszak, A.W.; Hotaling, J.M.; Patel, D.P. Effects of SARS-CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: Where do we stand? Int. J. Impot. Res. 2021, 34, 138–144. [Google Scholar] [CrossRef]
- Lifshitz, D.; Haas, J.; Lebovitz, O.; Raviv, G.; Orvieto, R.; Aizer, A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod. BioMedicine Online 2022, 44, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Stromme, M.; Moyassari, S.; Chadha, A.S.; Tartaglia, M.C.; Szoeke, C.; Ferretti, M.T. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp. Clin. Trials 2022, 115, 106700. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Chen, S.; Morris, K.; Chui, K.; Allen, J.D. Mental health symptoms and association with COVID-19 vaccination receipt and intention to vaccinate among adults, United States. Prev. Med. 2022, 154, 106905. [Google Scholar] [CrossRef] [PubMed]

| Variables | Frequency (N) | Percentage (%) |
|---|---|---|
| Age | ||
| 18–35 years | 177 | 44.5% |
| 36–50 years | 177 | 44.5% |
| >50 years | 44 | 11.0% |
| Gender | ||
| Male | 235 | 59.0% |
| Female | 163 | 41.0% |
| Geographic location | ||
| Central Region | 197 | 49.5% |
| Northern Region | 122 | 30.7% |
| Eastern Region | 21 | 5.3% |
| Southern Region | 6 | 1.5% |
| Western Region | 52 | 13.1% |
| Occupation | ||
| Students | 46 | 11.6% |
| Private sector employees | 51 | 12.8% |
| Government sector employees | 230 | 57.8% |
| Retired | 27 | 6.8% |
| Own Business | 44 | 11.0% |
| Occupational field | ||
| Medical | 157 | 39.4% |
| Non-medical | 241 | 60.6% |
| Average monthly income | ||
| <5 thousand SAR | 108 | 27.1% |
| 5–15 thousand SAR | 141 | 35.4% |
| 15–20 thousand SAR | 71 | 17.8% |
| >20 thousand SAR | 78 | 19.6% |
| Marital status | ||
| Married | 276 | 69.3% |
| Single | 107 | 26.9% |
| Divorced | 15 | 3.8% |
| Nationality | ||
| Saudi | 382 | 96.0% |
| Non-Saudi | 16 | 4.0% |
| Highest Certificate Obtained | ||
| High school | 48 | 12.1% |
| Bachelor’s degree | 222 | 55.8% |
| Masters | 38 | 9.5% |
| Ph.D. | 60 | 15.1% |
| Post-secondary diploma | 30 | 7.5% |
| Type of vaccine receive | ||
| Pfizer | 224 | 56.3% |
| AstraZeneca | 174 | 43.7% |
| Second dose received | ||
| Yes | 90 | 22.6% |
| No | 308 | 77.4% |
| Time recommended for 2nd dose | ||
| 3 weeks | 224 | 56.3% |
| 3 months | 174 | 43.7% |
| Variables | N | Mean ± S.D. | p-Value |
|---|---|---|---|
| Age | 0.181 | ||
| 18–35 years | 177 | 3.59 ± 2.13 | |
| 36–50 years | 177 | 3.19 ± 2.21 | |
| 51–65 years | 44 | 3.14 ± 2.28 | |
| Gender | <0.001 | ||
| Male | 235 | 2.89 ± 1.95 | |
| Female | 163 | 4.04 ± 2.33 | |
| Geographic location | 0.390 | ||
| Central | 197 | 3.15 ± 2.24, | |
| Northern | 122 | 3.57 ± 2.26 | |
| Eastern | 21 | 3.33 ± 1.77 | |
| Southern | 6 | 3.83 ± 1.6 | |
| Western | 52 | 3.63 ± 2 | |
| Occupation | <0.001 | ||
| Student | 46 | 3.35 ± 1.98 | |
| Private sector employee | 51 | 3.92 ± 2.28 | |
| Government sector employee | 230 | 3.2 ± 2.08 | |
| Retired | 27 | 2.19 ± 1.88 | |
| Own Business | 44 | 4.32 ± 2.57 | |
| Occupational field | 0.205 | ||
| Medical | 157 | 3.22 ± 2.06 | |
| Non-medical | 241 | 3.46 ± 2.27 | |
| Average monthly income | 0.062 | ||
| <5 thousand SAR | 108 | 3.7 ± 2.31 | |
| 5–15 thousand SAR | 141 | 3.45 ± 2.09 | |
| 15–20 thousand SAR | 71 | 2.85 ± 1.93, | |
| >20 thousand SAR | 78 | 3.19 ± 2.35, | |
| Marital Status | 0.006 | ||
| Married | 276 | 3.17 ± 2.12 | |
| Single | 107 | 3.67 ± 2.21 | |
| Divorced | 15 | 4.73 ± 2.71 | |
| Nationality | 0.009 | ||
| Saudi | 382 | 3.3 ± 2.18 | |
| Non-Saudi | 16 | 4.75 ± 1.95 | |
| Highest Certificate Obtained | 0.718 | ||
| High school | 48 | 3.35 ± 2.06 | |
| Bachelor’s degree | 222 | 3.37 ± 2.23 | |
| Masters | 38 | 2.92 ± 2.12 | |
| Ph.D. | 60 | 3.57 ± 2.36 | |
| Post-secondary diploma | 30 | 3.43 ± 1.85 | |
| Type of vaccine receive | <0.001 | ||
| Pfizer | 224 | 2.74 ± 2.07 | |
| AstraZeneca | 174 | 4.16 ± 2.08 | |
| Side Effect | Total (398) | Pfizer (N = 224) | AstraZeneca (N = 174) | p-Value | R2 |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Pain at the injection site | 339 (85.2%) | 203 (90.6%) | 136 (78.20%) | 0.001 | 0.030 |
| Redness at the injection site | 73(18.3%) | 34 (15.2%) | 39 (22.40%) | 0.064 | 0.009 |
| Swelling at the injection site | 106 (26.7%) | 64 (28.6%) | 42 (24.10%) | 0.321 | 0.002 |
| Fever | 169 (42.5%) | 51 (22.8%) | 118 (67.80%) | <0.001 | 0.204 |
| Bone or joint pain | 215 (38.4%) | 86 (38.4%) | 129 (74.10%) | <0.001 | 0.127 |
| Fatigue | 246 (54.0%) | 100 (44.6%) | 146 (83.90%) | <0.001 | 0.161 |
| Loss of appetite | 67 (16.8%) | 25 (11.2%) | 42 (24.10%) | 0.001 | 0.030 |
| Headache | 43 (10.8%) | 16 (7.1%) | 27 (15.50%) | 0.008 | 0.018 |
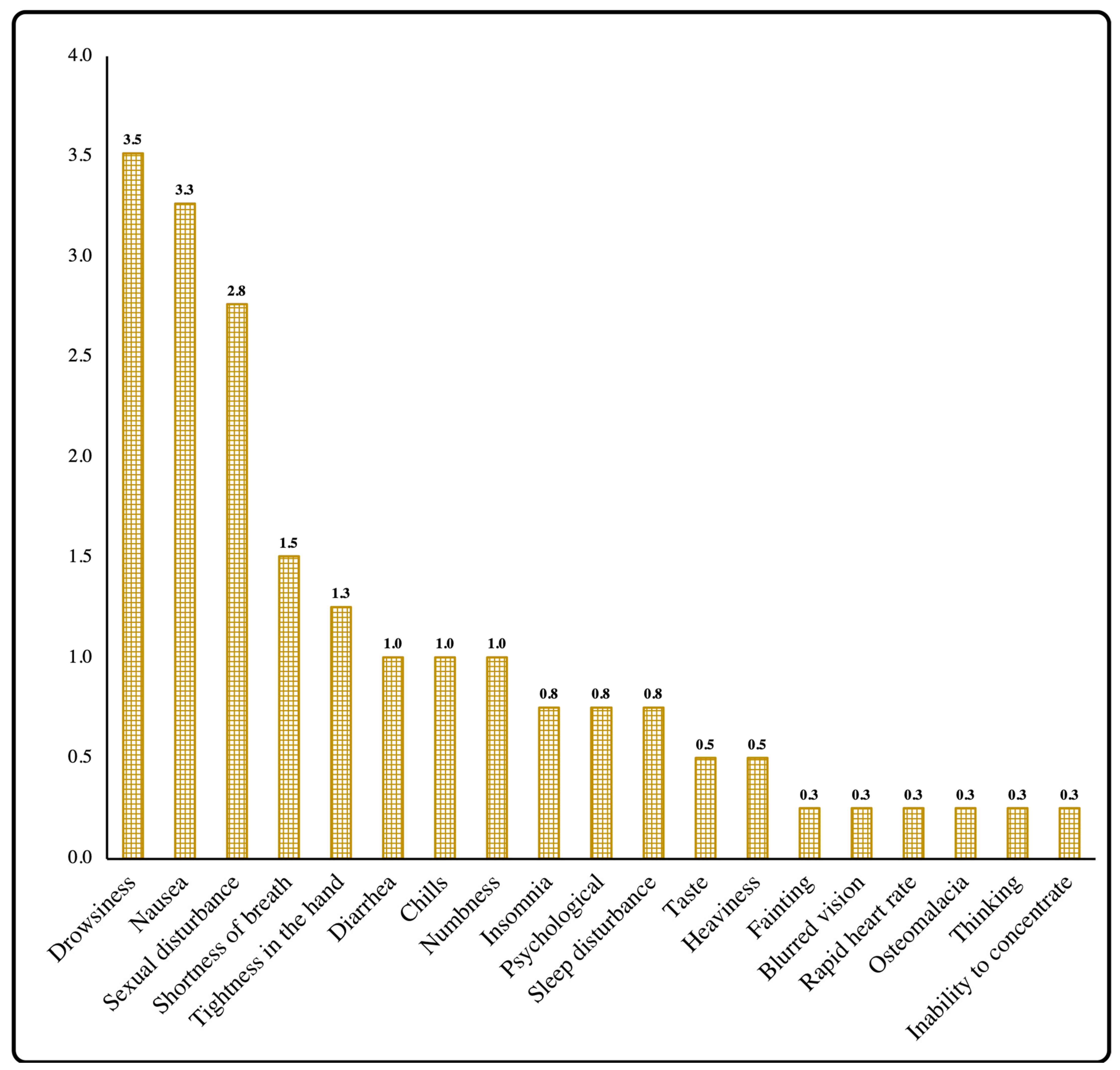
| Sexual disturbance | 11 (2.8%) | 10 (4.5%) | 1 (0.60%) | 0.019 | 0.014 |
| Drowsiness | 14 (3.5%) | 4 (1.8%) | 10 (5.70%) | 0.033 | 0.011 |
| Nausea | 13 (3.3%) | 7 (3.1%) | 6 (3.40%) | 0.857 | 0.000 |
| Shortness of breath | 6 (1.5%) | 3 (1.3%) | 3 (1.70%) | 0.775 | 0.000 |
| Diarrhea | 4 (1.0%) | 2 (0.9%) | 2 (1.10%) | 1.000 | 0.000 |
| Chills | 4 (1.0%) | 2 (0.9%) | 2 (1.10%) | 1.000 | 0.000 |
| Insomnia | 3 (0.8%) | 2 (0.9%) | 1 (0.60%) | 1.000 | 0.000 |
| Tightness in the hand | 5 (1.3%) | 1 (0.4%) | 4 (2.30%) | 0.173 | 0.007 |
| Numbness | 4 (1.0%) | 2 (0.9%) | 2 (1.10%) | 1.000 | 0.000 |
| Psychological | 3 (0.8%) | 1 (0.4%) | 2 (1.10%) | 0.583 | 0.002 |
| Taste | 2 (0.5%) | 0 (0.0%) | 2 (1.10%) | 0.191 | 0.007 |
| Heaviness | 2 (0.5%) | 0 (0.0%) | 2 (1.10%) | 0.191 | 0.007 |
| Sleep disturbance | 3 (0.8%) | 0 (0.0%) | 3 (1.70%) | 0.083 | 0.010 |
| Fainting | 1 (0.3%) | 0 (0.0%) | 1 (0.60%) | 0.437 | 0.003 |
| Blurred vision | 1 (0.3%) | 0 (0.0%) | 1 (0.60%) | 0.437 | 0.003 |
| Rapid heart rate | 1 (0.3%) | 1 (0.4%) | 0 (0.00%) | 1.000 | 0.002 |
| Osteomalacia | 1 (0.3%) | 0 (0.0%) | 1 (0.60%) | 0.437 | 0.003 |
| Thinking | 1 (0.3%) | 0 (0.0%) | 1 (0.60%) | 0.437 | 0.003 |
| Inability to concentrate | 1 (0.3%) | 0 (0.0%) | 1 (0.60%) | 0.437 | 0.003 |
| Variables | Pain | Fatigue | Joint Pain | Fever | Redness | Swelling | Loss of Appetite | Headache | Drowsiness | Nausea | Sexual Disturbance | Shortness of Breath |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Gender | ||||||||||||
| Male | 191 (56.3%) | 132 (53.7%) | 114 (53%) | 87 (51.5%) | 31 (42.5%) | 49 (46.2%) | 19 (28.4%) | 16 (37.2%) | 6 (42.9%) | 6 (46.1%) | 11 (100%) | 1 (16.6%) |
| Female | 148 (43.7%) | 114 (46.3%) | 101 (47%) | 82 (48.5%) | 42 (57.5%) | 57 (53.8%) | 48 (71.6%) | 27 (62.8%) | 8 (57.1%) | 7 (53.9%) | 0 (0%) | 5 (83.4%) |
| p-value | 0.009 * | 0.005 * | 0.008 * | 0.008 * | 0.001 * | 0.002 * | <0.001 * | 0.002 * | 0.21 | 0.337 | 0.005 * | 0.033 * |
| Geographic location | ||||||||||||
| Central | 179 (52.8%) | 103 (41.9%) | 94 (43.7%) | 65 (38.5%) | 35 (47.9%) | 46 (43.4%) | 36 (53.7%) | 19 (44.2%) | 6 (42.9%) | 7 (53.9%) | 9 (81.8%) | 5 (83.3%) |
| Northern | 91 (26.8%) | 84 (34.1%) | 72 (33.5%) | 65 (38.5%) | 29 (39.7%) | 40 (37.7%) | 22 (32.8%) | 11 (25.6%) | 4 (28.6%) | 4 (30.8%) | 2 (18.2%) | 0 (0%) |
| Eastern | 18 (5.3%) | 17 (6.9%) | 12 (5.6%) | 10 (5.9%) | 2 (2.7%) | 4 (3.8%) | 1 (1.5%) | 2 (4.7%) | 2 (14.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Southern | 5 (1.5%) | 5 (2%) | 6 (2.8%) | 4 (2.4%) | 1 (1.4%) | 2 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Western | 46 (13.6%) | 37 (15%) | 31 (14.4%) | 25 (14.8%) | 6 (8.2%) | 14 (13.2%) | 8 (11.9%) | 11 (25.6%) | 2 (14.3%) | 2 (15.4%) | 0 (0%) | 1 (16.7%) |
| p-value | 0.003 * | 0.002 * | 0.037 * | 0.004 * | 0.272 | 0.378 | 0.42 | 0.125 | 0.624 | 0.907 | 0.267 | 0.441 |
| Age | ||||||||||||
| 18–35 years | 155 (45.7%) | 124 (50.4%) | 104 (48.4%) | 87 (51.5%) | 36 (49.3%) | 46 (43.4%) | 35 (52.2%) | 17 (39.5%) | 7 (50%) | 4 (30.8%) | 3 (27.3%) | 2 (33.3%) |
| 36–50 years | 150 (44.2%) | 100 (40.7%) | 89 (41.4%) | 65 (38.5%) | 28 (38.4%) | 44 (41.5%) | 28 (41.8%) | 19 (44.2%) | 6 (42.9%) | 8 (61.6%) | 8 (72.7%) | 4 (66.7%) |
| 51–65 years | 34 (10%) | 22 (8.9%) | 22 (10.2%) | 17 (10.1%) | 10 (13.7%) | 16 (11.1%) | 4 (6%) | 7 (16.3%) | 1 (7.1%) | 1 (7.7%) | 1 (9.1%) | 0 (0%) |
| p-value | <0.001 * | <0.001 * | 0.038 * | 0.008 * | 0.622 | 0.151 | 0.298 | 0.259 | 0.928 | 0.627 | 0.258 | 0.678 |
| Occupation | ||||||||||||
| Student | 39 (11.5%) | 30 (12.2%) | 25 (11.6%) | 22 (13%) | 5 (6.8%) | 9 (8.5%) | 11 (16.4%) | 6 (14%) | 2 (14.3%) | 0 (0%) | 0 (0%) | 1 (16.7%) |
| Private sector employees | 43 (12.7%) | 38 (15.4%) | 37 (17.2%) | 25 (14.8%) | 11 (15.1%) | 15 (14.2%) | 10 (14.9%) | 7 (16.3%) | 2 (14.3%) | 4 (30.8%) | 0 (0%) | 1 (16.7%) |
| Government sector employees | 199 (58.7%) | 135 (54.9%) | 114 (53%) | 92 (54.4%) | 39 (53.4%) | 59 (55.7%) | 31 (46.3%) | 22 (51.2%) | 6 (42.9%) | 7 (53.9%) | 11 (100%) | 4 (66.7%) |
| Retired | 17 (5%) | 9 (3.7%) | 12 (5.6%) | 5 (3%) | 2 (2.7%) | 6 (5.7%) | 1 (1.5%) | 3 (7%) | 1 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Own Business | 41 (12.1%) | 34 (13.8%) | 27 (12.6%) | 25 (14.8%) | 16 (21.9%) | 17 (16%) | 14 (20.9%) | 5 (11.6%) | 3 (21.4%) | 2 (15.4%) | 0 (0%) | 0 (0%) |
| p-value | 0.001 * | 0.001 * | 0.028 * | 0.017 * | 0.007 * | 0.287 | 0.007 * | 0.898 | 0.719 | 0.191 | 0.082 | 0.848 |
| Profession | ||||||||||||
| Medical | 141 (41.6%) | 94 (38.2%) | 79 (36.7%) | 57 (33.7%) | 23 (31.5%) | 39 (36.8%) | 23 (34.3%) | 16 (37.2%) | 4 (28.6%) | 7 (53.9%) | 3 (27.3%) | 2 (33.3%) |
| non-medical | 198 (58.4%) | 152 (61.8%) | 136 (63.3%) | 112 (66.3%) | 50 (68.5%) | 67 (63.2%) | 44 (65.7%) | 27 (62.8%) | 10 (71.4%) | 6 (46.2%) | 8 (72.7%) | 4 (66.7%) |
| p-value | 0.036 * | 0.521 | 0.232 | 0.045 * | 0.125 | 0.514 | 0.347 | 0.751 | 0.397 | 0.28 | 0.402 | 0.758 |
| Monthly income | ||||||||||||
| <5000 SAR | 93 (27.4%) | 75 (30.5%) | 65 (30.2%) | 51 (30.2%) | 25 (34.2%) | 29 (27.4%) | 28 (41.8%) | 12 (27.9%) | 5 (35.7%) | 4 (30.8%) | 0 (0%) | 4 (66.7%) |
| 5000–15,000 SAR | 120 (35.4%) | 88 (35.8%) | 82 (38.1%) | 69 (40.8%) | 24 (32.9%) | 35 (33%) | 25 (37.3%) | 19 (44.2%) | 6 (42.9%) | 2 (15.4%) | 3 (27.3%) | 1 (16.7%) |
| 15,000–20,000 SAR | 57 (16.8%) | 41 (16.7%) | 36 (16.7%) | 25 (14.8%) | 10 (13.7%) | 15 (14.2%) | 6 (9%) | 6 (14%) | 1 (7.1%) | 3 (23.1%) | 0 (0%) | 0 (0%) |
| >20,000 SAR | 69 (20.4%) | 42 (17.1%) | 32 (14.9%) | 24 (14.2%) | 14 (19.2%) | 27 (25.5%) | 8 (11.9%) | 6 (14%) | 2 (14.3%) | 4 (30.8%) | 8 (72.7%) | 1 (16.7%) |
| p-value | 0.554 | 0.152 | 0.041 * | 0.025 * | 0.441 | 0.274 | 0.006 * | 0.523 | 0.624 | 0.449 | <0.001 * | 0.156 |
| Marital status | ||||||||||||
| Married | 233 (68.7%) | 159 (64.6%) | 142 (66%) | 105 (62.1%) | 45 (61.6%) | 72 (67.9%) | 36 (53.7%) | 27 (62.8%) | 9 (64.3%) | 9 (69.2%) | 11 (100%) | 4 (66.7%) |
| Single | 95 (28%) | 74 (30.1%) | 62 (28.8%) | 53 (31.4%) | 24 (32.9%) | 29 (27.4%) | 26 (38.8%) | 11 (25.6%) | 5 (35.7%) | 3 (23.1%) | 0 (0%) | 2 (33.3%) |
| Divorced | 11 (3.2%) | 13 (5.3%) | 11 (5.1%) | 11 (6.5%) | 4 (5.5%) | 5 (4.7%) | 5 (7.5%) | 5 (11.6%) | 0 (0%) | 1 (7.7%) | 0 (0%) | 0 (0%) |
| p-value | 0.241 | 0.035 * | 0.23 | 0.012 * | 0.29 | 0.13 | 0.008 * | 0.012 * | 0.796 | 0.814 | 0.172 | 0.954 |
| Nationality | ||||||||||||
| Saudi | 326 (96.2%) | 231 (93.9%) | 201 (93.5%) | 156 (92.3%) | 70 (95.9%) | 102 (96.2%) | 60 (89.6%) | 40 (93%) | 12 (85.71%) | 13 (100%) | 11 (100%) | 5 (83.3%) |
| non-Saudi | 13 (3.8%) | 15 (6.1%) | 14 (6.5%) | 13 (7.7%) | 3 (4.1%) | 4 (3.8%) | 7 (10.4%) | 3 (7%) | 2 (14.29%) | 0 (0%) | 0 (0%) | 1 (16.7%) |
| p-value | 0.652 | 0.007 * | 0.006 * | 0.001 * | 0.966 | 0.88 | 0.003 * | 0.296 | 0.047 * | 0.453 | 0.491 | 0.112 |
| Education | ||||||||||||
| High school | 39 (11.5%) | 34 (13.8%) | 28 (13%) | 27 (16%) | 6 (8.2%) | 7 (6.6%) | 11 (16.4%) | 5 (11.6%) | 2 (14.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Bachelor’s degree | 191 (56.3%) | 132 (53.7%) | 118 (54.9%) | 90 (53.3%) | 43 (58.9%) | 65 (61.3%) | 42 (62.7%) | 24 (55.8%) | 7 (50.0%) | 7 (53.8%) | 1 (9.1%) | 5 (83.3%) |
| Masters | 28 (8.3%) | 22 (8.9%) | 19 (8.8%) | 13 (7.7%) | 7 (9.6%) | 8 (7.5%) | 3 (4.5%) | 5 (11.6%) | 1 (7.14%) | 2 (15.4%) | 0 (0%) | 0 (0%) |
| Ph.D. | 53 (15.6%) | 42 (17.1%) | 30 (14%) | 27 (16%) | 14 (19.2%) | 16 (15.1%) | 8 (11.9%) | 5 (11.6%) | 2 (14.29%) | 3 (23.1%) | 7 (63.6%) | 1 (16.7%) |
| Post-secondary diploma | 28 (8.3%) | 16 (6.5%) | 20 (9.3%) | 12 (7.1%) | 3 (4.1%) | 10 (9.4%) | 3 (4.5%) | 4 (9.3%) | 2 (14.29%) | 1 (7.7%) | 3 (27.3%) | 0 (0%) |
| p-value | 0.321 | 0.395 | 0.554 | 0.236 | 0.487 | 0.291 | 0.339 | 0.961 | 0.489 | 0.719 | <0.001 * | 0.727 |
| Vaccine | ||||||||||||
| Pfizer | 203 (59.9%) | 100 (40.7%) | 86 (40%) | 51 (30.2%) | 34 (46.6%) | 64 (60.4%) | 25 (37.3%) | 16 (37.2%) | 4 (28.57%) | 7 (53.9%) | 10 (90.9%) | 3 (50%) |
| AstraZeneca | 136 (40.1%) | 146 (59.3%) | 129 (60%) | 118 (69.8%) | 39 (53.4%) | 42 (39.6%) | 42 (62.7%) | 27 (62.8%) | 10 (71.43%) | 6 (46.2%) | 1 (9.1%) | 3 (50%) |
| p-value | 0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.064 | 0.321 | 0.001 * | 0.008 * | 0.033 * | 0.857 | 0.019 * | 0.755 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzarea, A.I.; Khan, Y.H.; Alatawi, A.D.; Alanazi, A.S.; Alzarea, S.I.; Butt, M.H.; Almalki, Z.S.; Alahmari, A.K.; Mallhi, T.H. Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile. Vaccines 2022, 10, 924. https://doi.org/10.3390/vaccines10060924
Alzarea AI, Khan YH, Alatawi AD, Alanazi AS, Alzarea SI, Butt MH, Almalki ZS, Alahmari AK, Mallhi TH. Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile. Vaccines. 2022; 10(6):924. https://doi.org/10.3390/vaccines10060924
Chicago/Turabian StyleAlzarea, Abdulaziz Ibrahim, Yusra Habib Khan, Ahmed D. Alatawi, Abdullah Salah Alanazi, Sami I. Alzarea, Muhammad Hammad Butt, Ziyad Saeed Almalki, Abdullah K. Alahmari, and Tauqeer Hussain Mallhi. 2022. "Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile" Vaccines 10, no. 6: 924. https://doi.org/10.3390/vaccines10060924
APA StyleAlzarea, A. I., Khan, Y. H., Alatawi, A. D., Alanazi, A. S., Alzarea, S. I., Butt, M. H., Almalki, Z. S., Alahmari, A. K., & Mallhi, T. H. (2022). Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile. Vaccines, 10(6), 924. https://doi.org/10.3390/vaccines10060924








