Bacterial Ghosts of Pseudomonas aeruginosa as a Promising Candidate Vaccine and Its Application in Diabetic Rats
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents, Bacterial Strain, and Cultivation
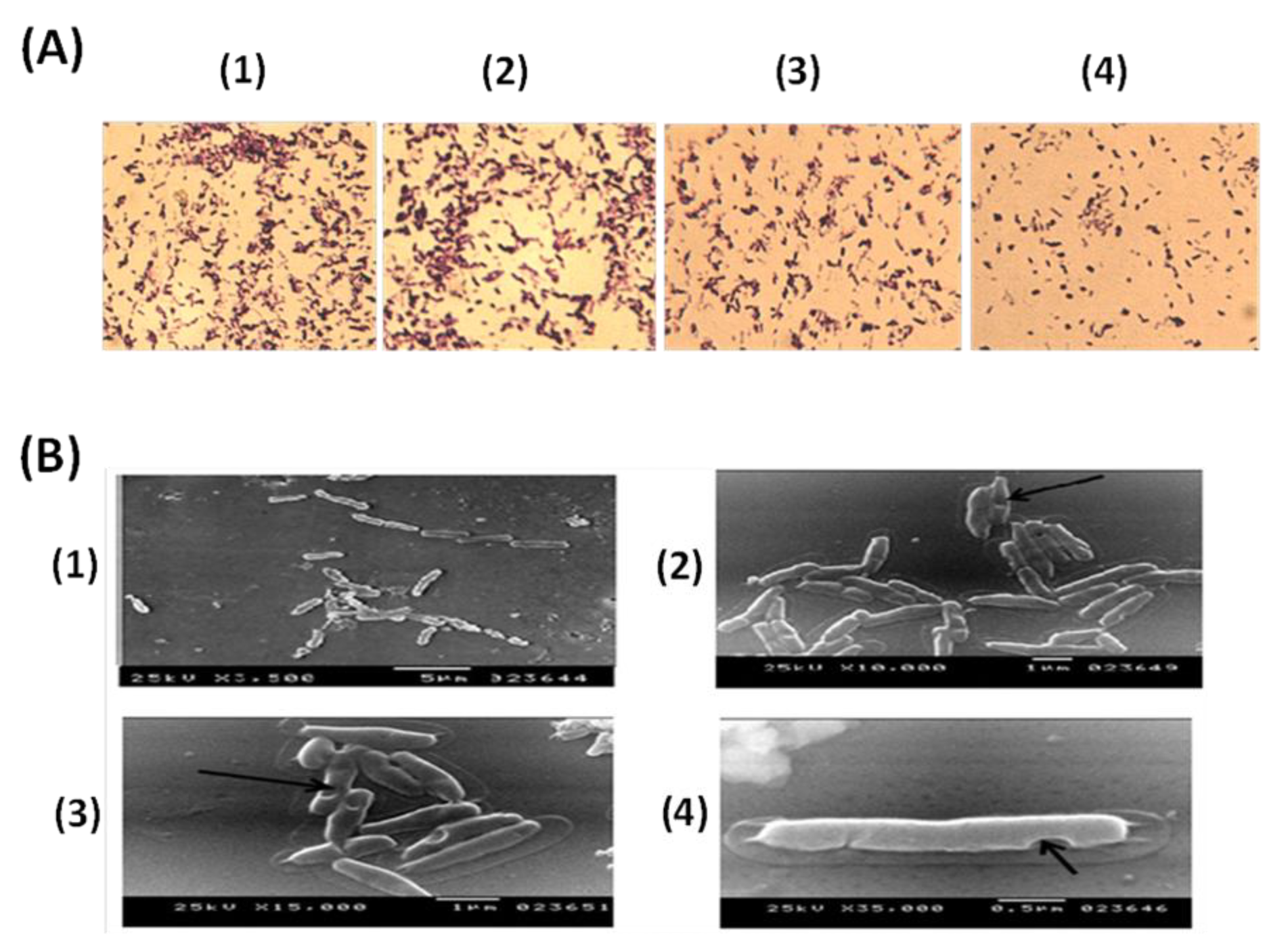
2.2. Preparation of P. aeruginosa Ghosts (PAGs)
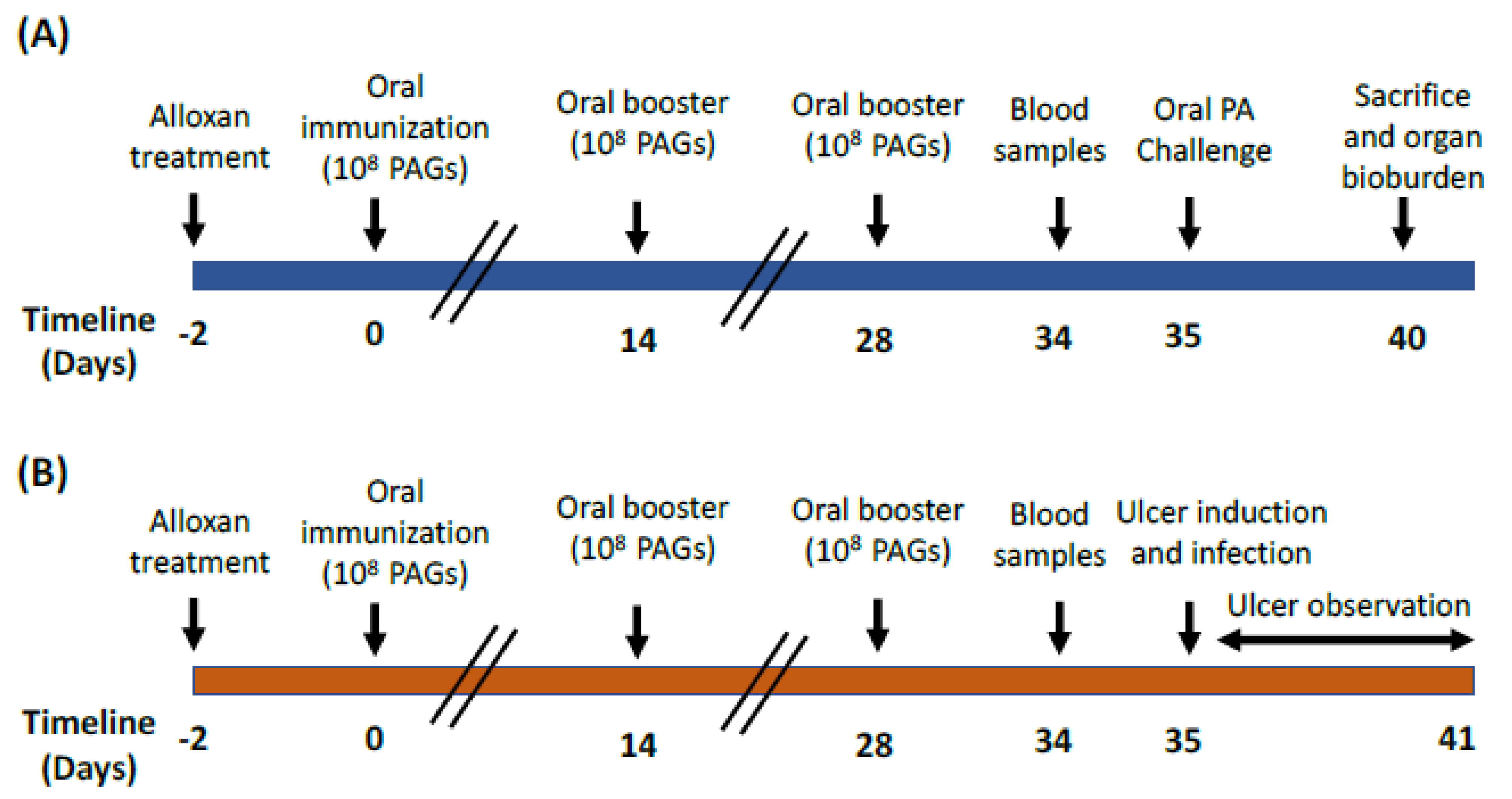
2.3. Animals and Experimental Design
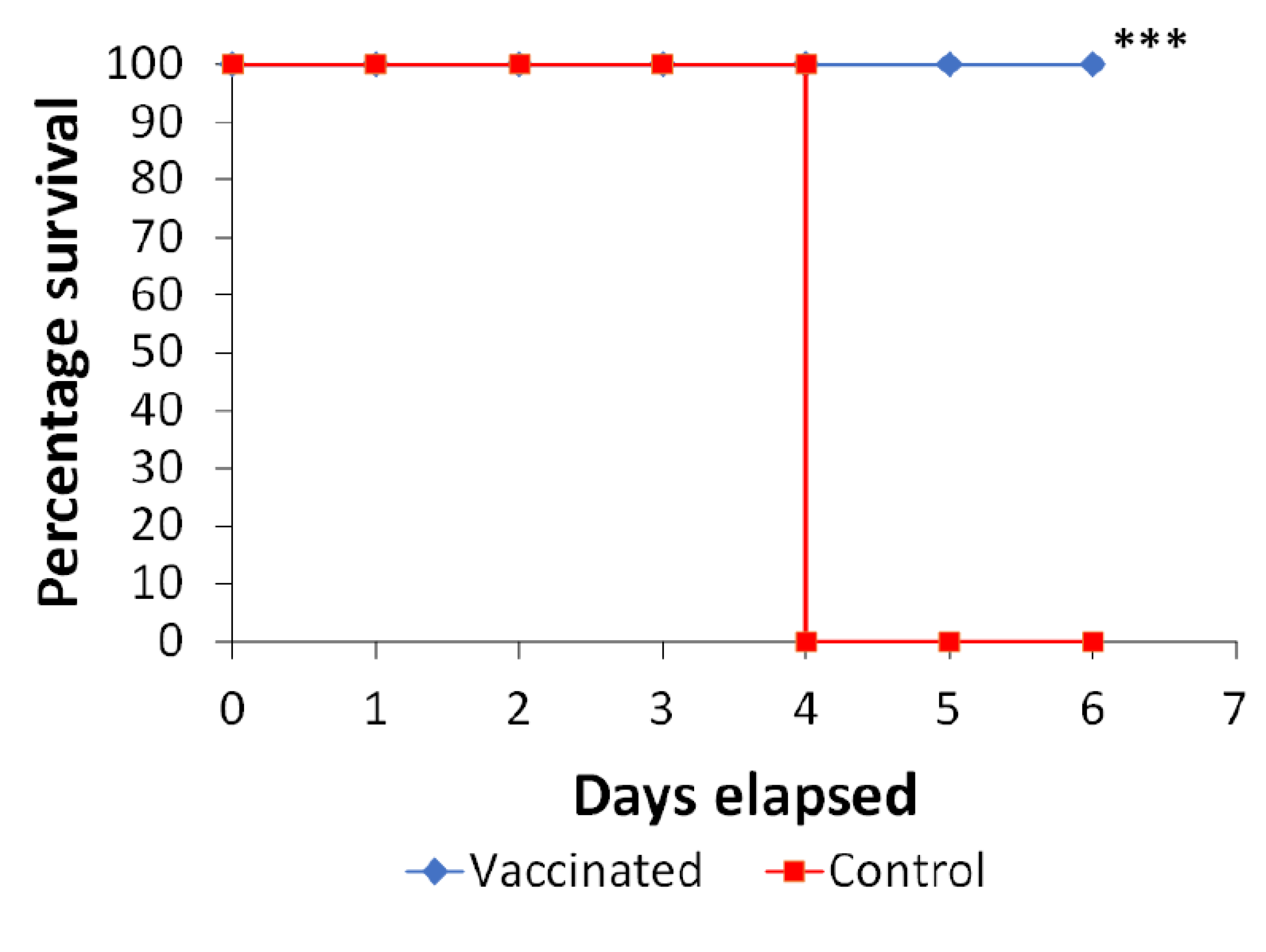
2.4. Oral Challenge with P. aeruginosa Clinical Strain
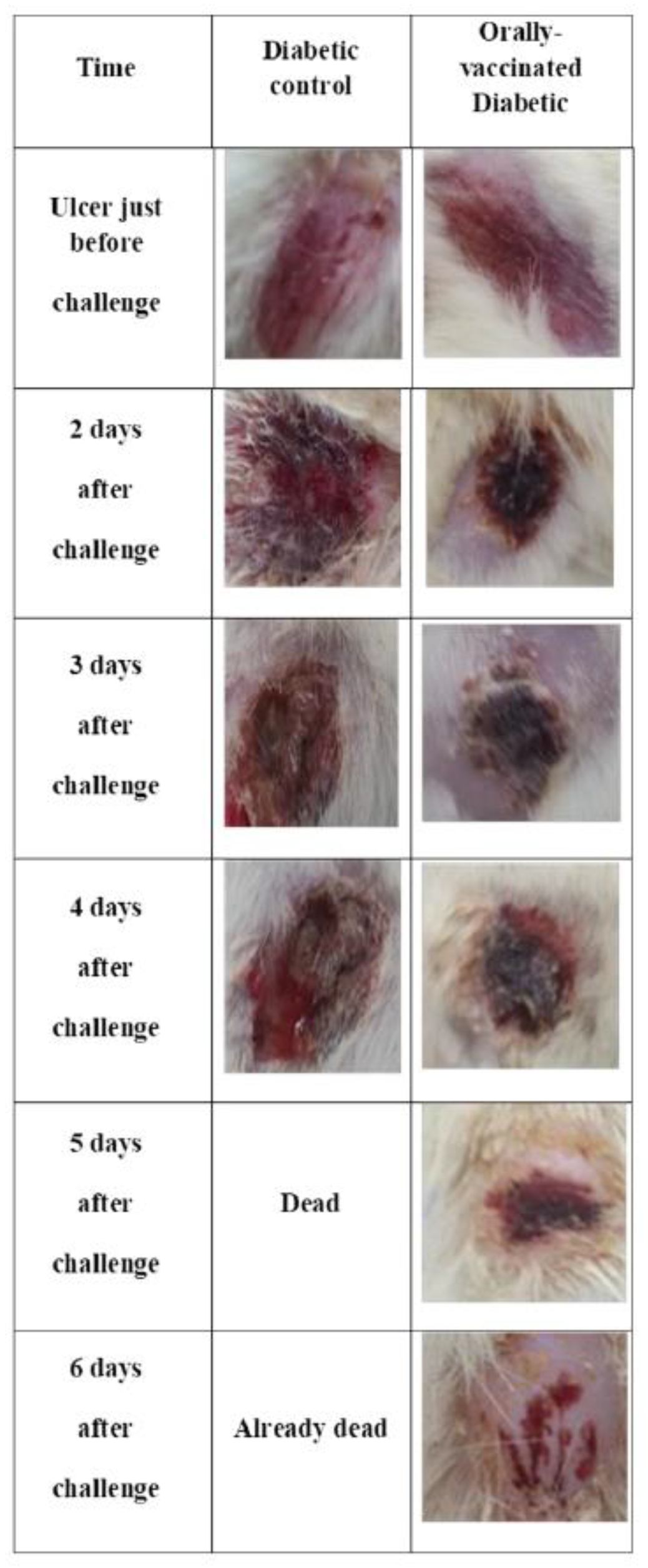
2.5. Preparation of Artificial Ulcers Followed by Topical PA Challenge
2.6. Slide Agglutination Test for Detection of P. aeruginosa-Specific Antibodies following Oral Immunization with PAGs
2.7. Evaluation of Phagocytic Activity
2.8. Nitro-Blue Tetrazolium Test (NBT)
2.9. Measurement of IFN-γ Levels
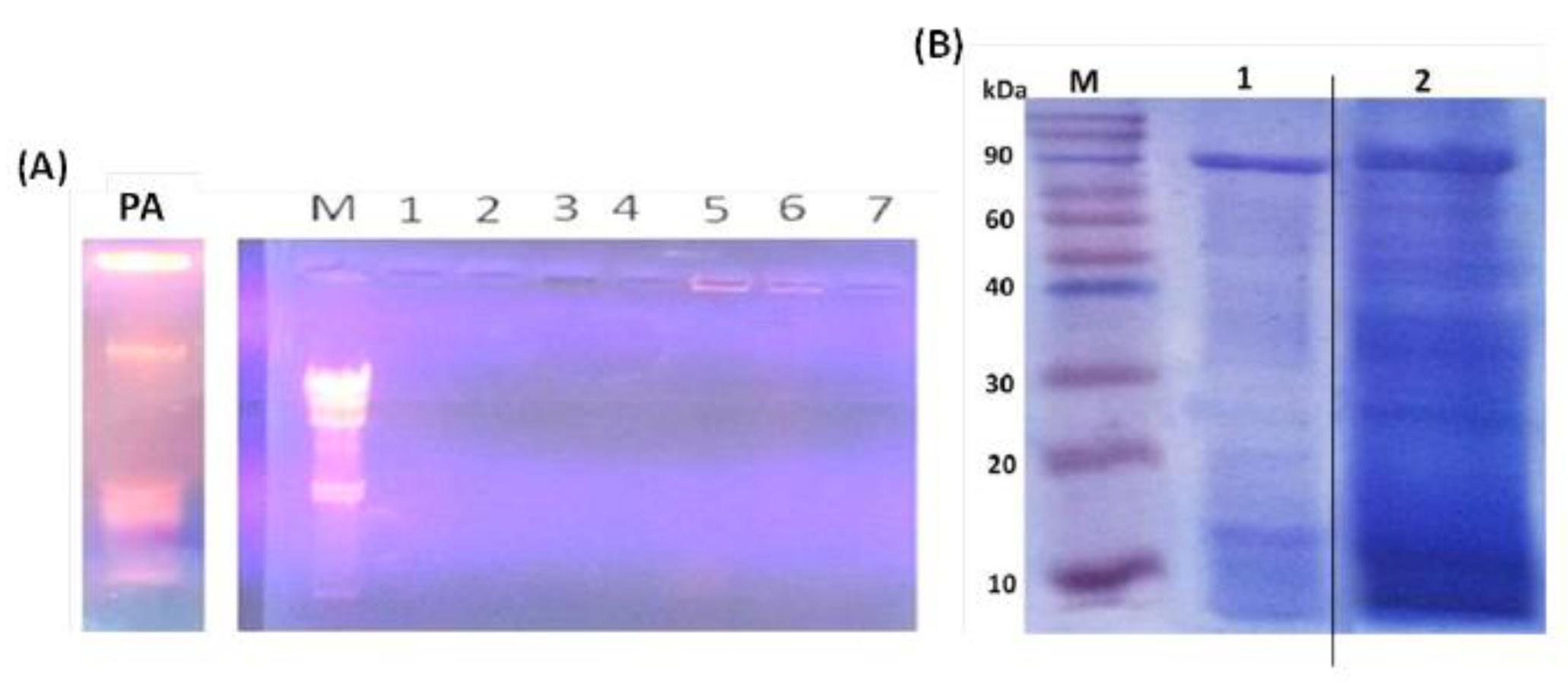
2.10. PAGs Proteins Immunoblot Analysis
2.11. Isolation of Lipopolysaccharide (LPS) and Immunoblot Analysis
2.12. Statistical Analysis of Data
3. Results
3.1. Evaluation of PAGs Quality
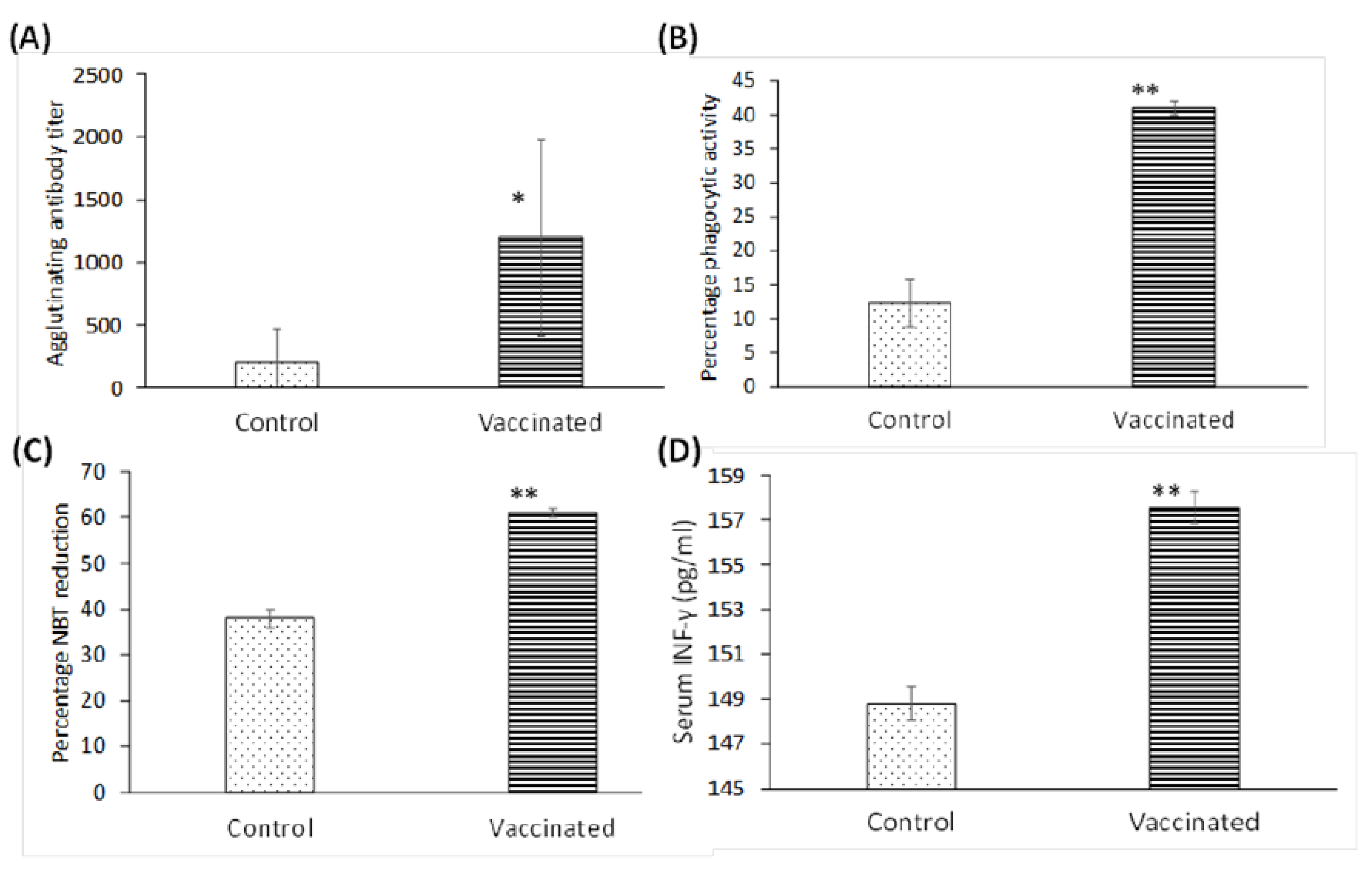
3.2. Elicitation of the Specific Immune Response following Oral Immunization with PAGs
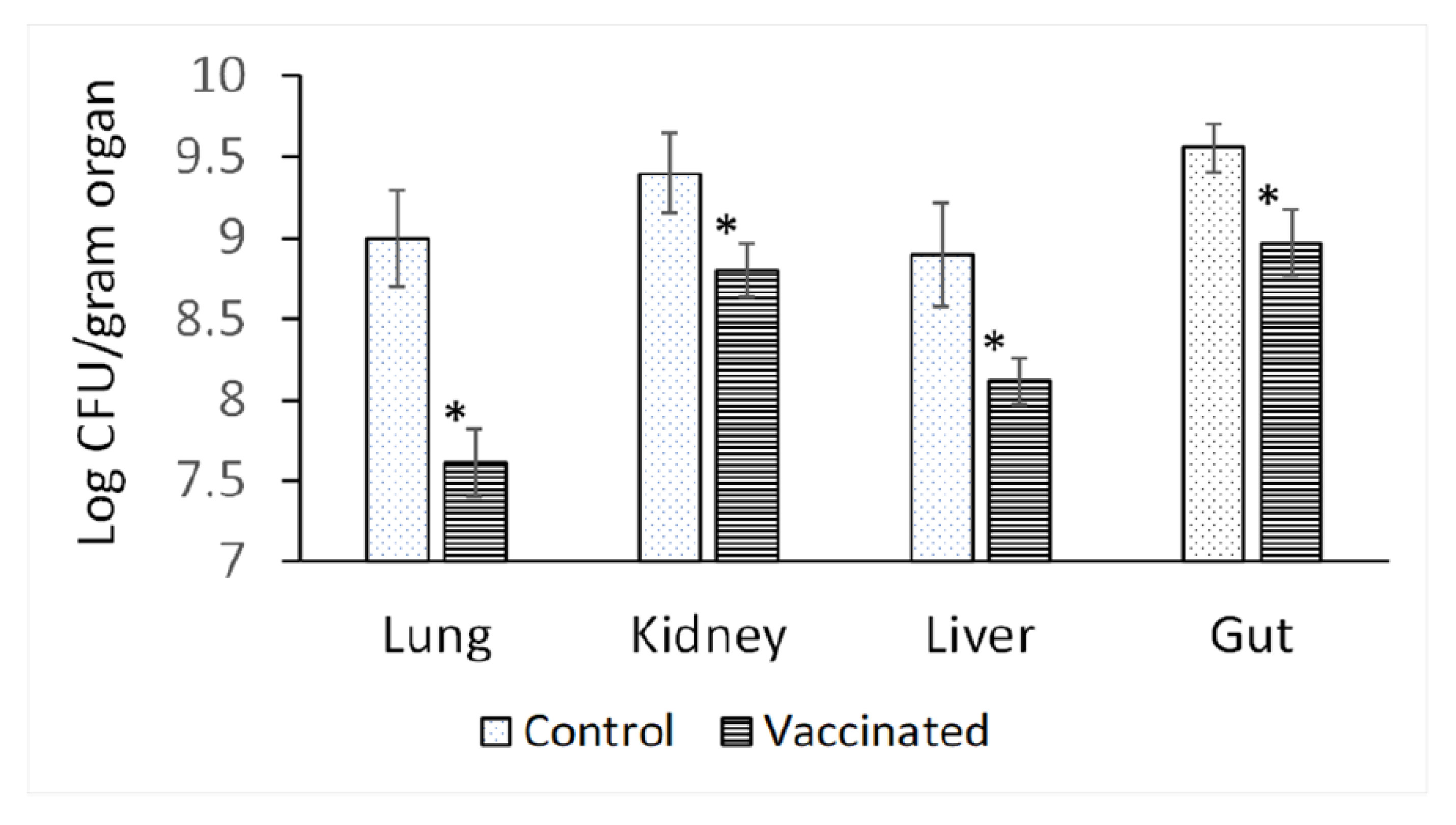
3.3. Bioburden in Different Organs of Vaccinated and Unvaccinated Rats after Challenging with P. aeruginosa
3.4. Oral Immunization with PAGs Protects against Infection of Artificial Ulcers in Diabetic Rats
3.5. PAGs-Specific Antibodies Interact with LPS and Proteins of P. aeruginosa
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BGs | Bacterial ghosts |
| CFU | Colony forming units |
| SDS-PAGE | Dodecylsulafte-polyacrylamide gel electrophoresis |
| EM | Electron microscope |
| ELISA | Enzyme-linked immunosorbent assay |
| H2O2 | Hydrogen peroxide |
| PAMPs | Immune-stimulating pathogen-associated molecular patterns |
| IFN-γ | Interferon- γ |
| LPS | Lipopolysaccharide |
| MGC | Minimum growth concentration |
| MIC | Minimum inhibitory concentration |
| MOI | Multiplicity of infection |
| PAGs | P. aeruginosa ghosts |
| PMNs | Phagocytic activity of polymorphonuclear cells |
| PA | Pseudomonas aeruginosa |
| SLRP | Sponge-like reduced protocol |
| SL | Sponge-like |
| TEM | Transmission Electron Microscope |
References
- Ruiz-Garbajosa, P.; Cantón, R. Epidemiology of antibiotic resistance in Pseudomonas aeruginosa. Implications for empiric and definitive therapy. Rev. Esp. Quimioter. 2017, 1, 8–12. [Google Scholar]
- Wahab, W.F.A. Diabetic Foot Infections with Pseudomonas: Jabir Abueliz Diabetic Center Khartoum Experience. Clin. Res. Foot Ankle 2014, s3. [Google Scholar] [CrossRef]
- Sivanmaliappan, T.S.; Sevanan, M. Antimicrobial Susceptibility Patterns ofPseudomonas aeruginosafrom Diabetes Patients with Foot Ulcers. Int. J. Microbiol. 2011, 2011, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, F.; Emami, A.; Pirbonyeh, N.; Keshavarzi, A.; Rajaee, M. A systematic review and meta-analysis on Exo-toxins prevalence in hospital acquired Pseudomonas aeruginosa isolates. Infect. Genet. Evol. 2019, 75, 104037. [Google Scholar] [CrossRef]
- LaBauve, A.E.; Wargo, M.J. Growth and Laboratory Maintenance of Pseudomonas aeruginosa. Curr. Protoc. Microbiol. 2012, 25, 6E.1.1.–6E.1.8. [Google Scholar] [CrossRef] [PubMed]
- Priebe, G.P.; Goldberg, J.B. Vaccines forPseudomonas aeruginosa: A long and winding road. Expert Rev. Vaccines 2014, 13, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Bläsi, U.; Linke, R.P.; Lubitz, W. Evidence for membrane-bound oligomerization of bacteriophage phi X174 lysis protein-E. J. Biol. Chem. 1989, 264, 4552–4558. [Google Scholar] [CrossRef]
- Witte, A.; Wanner, G.; Bläsi, U.; Halfmann, G.; Szostak, M.; Lubitz, W. Endogenous transmembrane tunnel formation mediated by phi X174 lysis protein E. J. Bacteriol. 1990, 172, 4109–4114. [Google Scholar] [CrossRef] [PubMed]
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The bacterial ghost platform system. Bioeng. Bugs 2010, 1, 326–336. [Google Scholar] [CrossRef]
- Amara, A.A.; Salem-Bekhit, M.M.; Alanazi, F.K. Sponge-Like: A New Protocol for Preparing Bacterial Ghosts. Sci. World J. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Amara, A.A.; Salem-Bekh, M.M.; Alanazi, F.K. Preparation of Bacterial Ghosts for E. coli JM109 Using “Sponge-like Reduced Protocol”. Asian J. Biol. Sci. 2013, 6, 363–369. [Google Scholar] [CrossRef][Green Version]
- Menisy MM, H.A.; Hussein, A.; Ghazy, A.A.; Sheweita, S.; Amara, A.A. Klebsiella pneumoniae Ghosts as Vaccine Using Sponge Like Reduced Protocol. Cell. Mol. Med. Open Access 2017, 3. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Newairy, A.-S.; Yousef, M.; Sheweita, S. Biochemical study on the effects of some Egyptian herbs in alloxan-induced diabetic rats. Toxicology 2002, 170, 221–228. [Google Scholar] [CrossRef]
- Hussein, M.Z.; Amara, A. Case-by-case study using antibiotic-EDTA combination to control pseudomonas aeruginosa. Pak. J. Pharm. Sci. 2006, 19, 236–243. [Google Scholar]
- Sheweita, S.; Batah, A.; Ghazy, A.; Hussein, A.; Amara, A. A new strain of Acinetobacter baumannii and characterization of its ghost as a candidate vaccine. J. Infect. Public Health 2019, 12, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Nagl, M.; Kacani, L.; Müllauer, B.; Lemberger, E.-M.; Stoiber, H.; Sprinzl, G.M.; Schennach, H.; Dierich, M.P. Phagocytosis and Killing of Bacteria by Professional Phagocytes and Dendritic Cells. Clin. Vaccine Immunol. 2002, 9, 1165–1168. [Google Scholar] [CrossRef]
- Freeman, R.; King, B. Technique for the performance of the nitro-blue tetrazolium (NBT) test. J. Clin. Pathol. 1972, 25, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Attridge, S.R.; Voss, E.; Manning, P.A. The role of toxin-coregulated pili in the pathogenesis of Vibrio cholerae O1 El Tor. Microb. Pathog. 1993, 15, 421–431. [Google Scholar] [CrossRef]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide extraction with phenol water and further applications of the procedure. In Methods Carbohydr. Chem; Whistler, R., Wolfan, M.L., Eds.; Academic Press: New York, NY, USA, 1965; pp. 83–91. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. On Behalf of the Scientific Study Committee of the Surgical Infection Society Infection in Burns. Surg. Infect. 2016, 17, 250–255. [Google Scholar] [CrossRef]
- Grimwood, K.; Kyd, J.M.; Owen, S.J.; Massa, H.M.; Cripps, A.W. Vaccination against respiratoryPseudomonas aeruginosainfection. Hum. Vaccines Immunother. 2014, 11, 14–20. [Google Scholar] [CrossRef]
- Pier, G.B.; Sidberry, H.F.; Sadoff, J.C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect. Immun. 1978, 22, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Elhosary, M.A.; Bahey-El-Din, M.; AbdelBary, A.; El Guink, N.; Aboushleib, H.M. Immunization with the ferric iron-binding periplasmic protein HitA provides protection against Pseudomonas aeruginosa in the murine infection model. Microb. Pathog. 2019, 131, 181–185. [Google Scholar] [CrossRef]
- Cripps, A.W.; Dunkley, M.L.; Clancy, R.L. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect. Immun. 1994, 62, 1427–1436. [Google Scholar] [CrossRef]
- Smith, K.; Kleynhans, L.; Warren, R.M.; Goosen, W.J.; Miller, M.A. Cell-Mediated Immunological Biomarkers and Their Diagnostic Application in Livestock and Wildlife Infected with Mycobacterium bovis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Tancrède, C.H.; Andremont, A.O. Bacterial Translocation and Gram-Negative Bacteremia in Patients with Hematological Malignancies. J. Infect. Dis. 1985, 152, 99–103. [Google Scholar] [CrossRef]
- Andremont, A.; Marang, B.; Tancrède, C.; Baume, D.; Hill, C. Antibiotic treatment and intestinal colonization by Pseudomonas aeruginosa in cancer patients. Antimicrob. Agents Chemother. 1989, 33, 1400–1402. [Google Scholar] [CrossRef]
- Toth, A.; Schödel, F.; Duchene, M.; Massarrat, K.; Blum, B.; Schmitt, A.; Domdey, H.; von Specht, B.-U. Protection of immunosuppressed mice against translocation of Pseudomonas aeruginosa from the gut by oral immunization with recombinant Pseudomonas aeruginosa outer membrane protein I expressing Salmonella dublin. Vaccine 1994, 12, 1215–1221. [Google Scholar] [CrossRef]
- Fisher, B.S.; Dambrauskas, N.; Trakhimets, O.; Andrade, D.V.; Smedley, J.; Sodora, D.L.; Sather, D.N. Oral Immunization with HIV-1 Envelope SOSIP trimers elicits systemic immune responses and cross-reactive anti-V1V2 antibodies in non-human primates. PLoS ONE 2020, 15, e0233577. [Google Scholar] [CrossRef]
- Abraham, E.; Robinson, A. Oral immunization with bacterial polysaccharide and adjuvant enhances antigen-specific pulmonary secretory antibody response and resistance to pneumonia. Vaccine 1991, 9, 757–764. [Google Scholar] [CrossRef]
- Otani, H.; Morimoto, T.; Kawahara, T. Oral ingestion of phosphorylated dextrin stimulates antibody responses in mice. J. Nutr. Sci. Vitaminol. 2007, 53, 354–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, X.; Lu, C. Mice orally vaccinated with Edwardsiella tarda ghosts are significantly protected against infection. Vaccine 2009, 27, 1571–1578. [Google Scholar] [CrossRef]
- Gerke, J.R.; Nelson, J.S. Oral vaccination and multivalent vaccine against Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 1977, 16, 76–80. [Google Scholar]
- DiGiandomenico, A.; Rao, J.; Goldberg, J.B. Oral Vaccination of BALB/c Mice with Salmonella enterica Serovar Typhimurium Expressing Pseudomonas aeruginosa O Antigen Promotes Increased Survival in an Acute Fatal Pneumonia Model. Infect. Immun. 2004, 72, 7012–7021. [Google Scholar] [CrossRef]
- Mayr, U.B.; Haller, C.; Haidinger, W.; Atrasheuskaya, A.; Bukin, E.; Lubitz, W.; Ignatyev, G. Bacterial Ghosts as an Oral Vaccine: A Single Dose of Escherichia coli O157:H7 Bacterial Ghosts Protects Mice against Lethal Challenge. Infect. Immun. 2005, 73, 4810–4817. [Google Scholar] [CrossRef]
- Roth, J.; Blatteis, C.M. Mechanisms of Fever Production and Lysis: Lessons from Experimental LPS Fever. Compr. Physiol. 2014, 4, 1563–1604. [Google Scholar] [CrossRef] [PubMed]
- Mader, H.J.; Szostak, M.P.; Hensel, A.; Lubitz, W.; Haslberger, A.G. Endotoxicity does not limit the use of bacterial ghosts as candidate vaccines. Vaccine 1997, 15, 195–202. [Google Scholar] [CrossRef]
- Arnold, H.; Bumann, D.; Felies, M.; Gewecke, B.; Sörensen, M.; Gessner, J.E.; Freihorst, J.; von Specht, B.U.; Baumann, U. Enhanced Immunogenicity in the Murine Airway Mucosa with an Attenuated Salmonella Live Vaccine Expressing OprF-OprI from Pseudomonas aeruginosa. Infect. Immun. 2004, 72, 6546–6553. [Google Scholar] [CrossRef]
- Conlan, J.W.; Cox, A.D.; KuoLee, R.; Webb, A.; Perry, M.B. Parenteral immunization with a glycoconju- gate vaccine containing the O157 antigen of Escherichia coli O157:H7 elicits a systemic humoral immune response in mice, but fails to prevent colonization by the pathogen. Can. J. Microbiol. 1999, 45, 279–286. [Google Scholar]
- Vande Walle, K.; De Zutter, L.; Cox, E. Oral infection with a Shiga toxin-negative Escherichia coli O157:H7 strain elicits humoral and cellular responses but does not protect sheep from colonisation with the homolo- gous strain. Vet. Microbiol. 2011, 148, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Spickler, A.R.; Roth, J.A. Adjuvants in veterinary vaccines: Modes of action and adverse effects. J. Vet. Intern. Med. 2003, 17, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Xian, T.H.; Sinniah, K.; Yean, C.Y.; Krishnamoorthy, V.; Bahari, M.B.; Chan, Y.Y.; Prabhakaran, G. Immunogenicity and protective efficacy of a live, oral cholera vaccine formulation stored outside-the-cold-chain for 140 days. BMC Immunol. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert Opin. Drug Deliv. 2012, 10, 131–149. [Google Scholar] [CrossRef]
- Van Der Weken, H.; Cox, E.; Devriendt, B. Advances in Oral Subunit Vaccine Design. Vaccines 2020, 9, 1. [Google Scholar] [CrossRef]

| Used Chemical Concentration | ||||
|---|---|---|---|---|
| Experimental Scheme | NaOH | CaCO3 | SDS | H2O2 |
| Scheme 1 | MGC | 1.05 µg /mL | MIC | MIC |
| Scheme 2 | MGC | 0.35 µg /mL | MIC | MGC |
| Protein and DNA Content (µg/mL) after Each Step | ||||||
|---|---|---|---|---|---|---|
| Basic Step | H2O2 Step | Ethanol Step | ||||
| Protein | DNA | Protein | DNA | Protein | DNA | |
| PAGs prepared by Scheme 1 | 1720.08 | 130.9 | 280.16 | 27.14 | 102.35 | 25.34 |
| PAGs prepared by Scheme 2 | 1430.35 | 163.17 | 154.98 | 43.54 | 67.08 | 13.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheweita, S.A.; Amara, A.A.; Gamal, H.; Ghazy, A.A.; Hussein, A.; Bahey-El-Din, M. Bacterial Ghosts of Pseudomonas aeruginosa as a Promising Candidate Vaccine and Its Application in Diabetic Rats. Vaccines 2022, 10, 910. https://doi.org/10.3390/vaccines10060910
Sheweita SA, Amara AA, Gamal H, Ghazy AA, Hussein A, Bahey-El-Din M. Bacterial Ghosts of Pseudomonas aeruginosa as a Promising Candidate Vaccine and Its Application in Diabetic Rats. Vaccines. 2022; 10(6):910. https://doi.org/10.3390/vaccines10060910
Chicago/Turabian StyleSheweita, Salah A., Amro A. Amara, Heba Gamal, Amany A. Ghazy, Ahmed Hussein, and Mohammed Bahey-El-Din. 2022. "Bacterial Ghosts of Pseudomonas aeruginosa as a Promising Candidate Vaccine and Its Application in Diabetic Rats" Vaccines 10, no. 6: 910. https://doi.org/10.3390/vaccines10060910
APA StyleSheweita, S. A., Amara, A. A., Gamal, H., Ghazy, A. A., Hussein, A., & Bahey-El-Din, M. (2022). Bacterial Ghosts of Pseudomonas aeruginosa as a Promising Candidate Vaccine and Its Application in Diabetic Rats. Vaccines, 10(6), 910. https://doi.org/10.3390/vaccines10060910






