Evaluation of the Potency of the Pertussis Vaccine in Experimental Infection Model with Bordetella pertussis: Study of the Case of the Pertussis Vaccine Used in the Expanded Vaccination Program in Algeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Vaccines
2.3. Bordetella PertussisStrain 18–323 and Growing Conditions
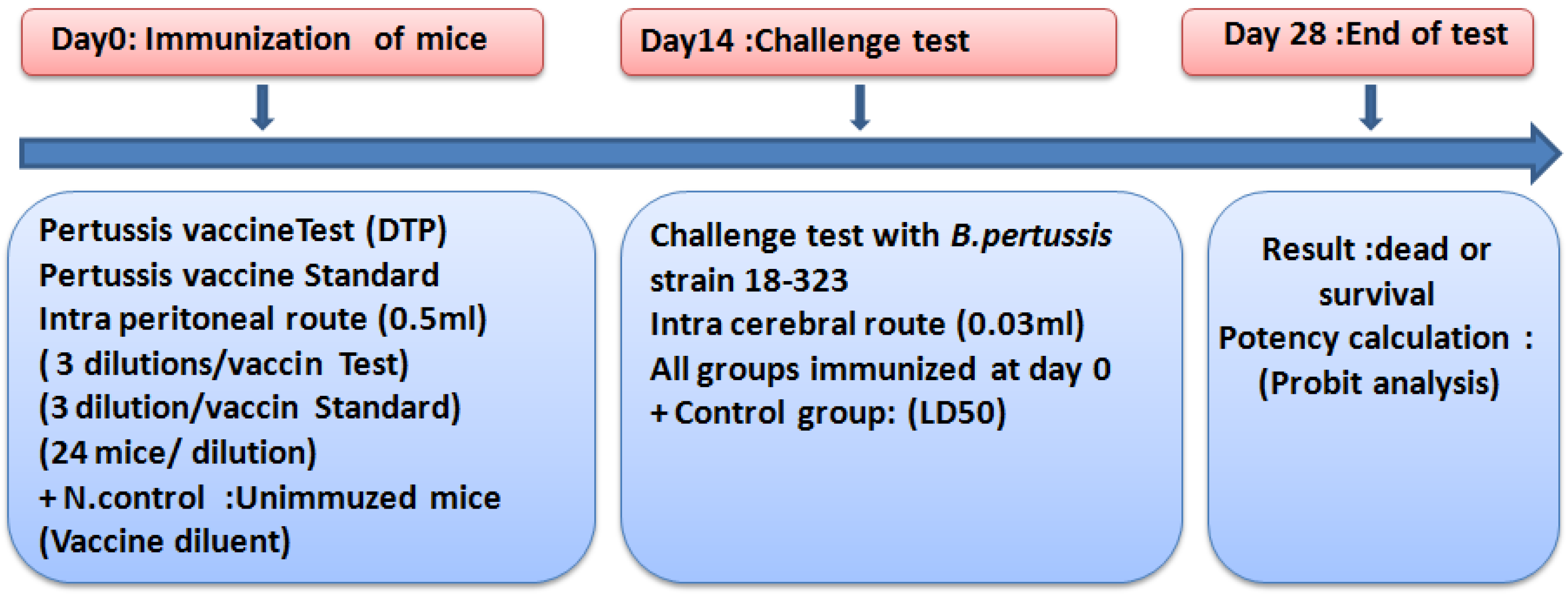
2.4. Mouse Protection Test (MPT)
2.4.1. Immunization
2.4.2. Preparation of B. pertussis 18–323 Challenge Suspension
2.4.3. Injection of Challenge Suspension of B. pertussis (Challenge Test)
2.5. Statistical Analysis
2.6. Validity Criteria
2.7. Ethical Considerations
3. Results
3.1. Identity and Viability of B. pertussis 18–323
3.2. Infection by B. pertusis Strain 18–323
3.3. Determination of the Lethal Dose of B. pertussis 18–323 (LD50) and the Number of LD50 Used in Each Trial
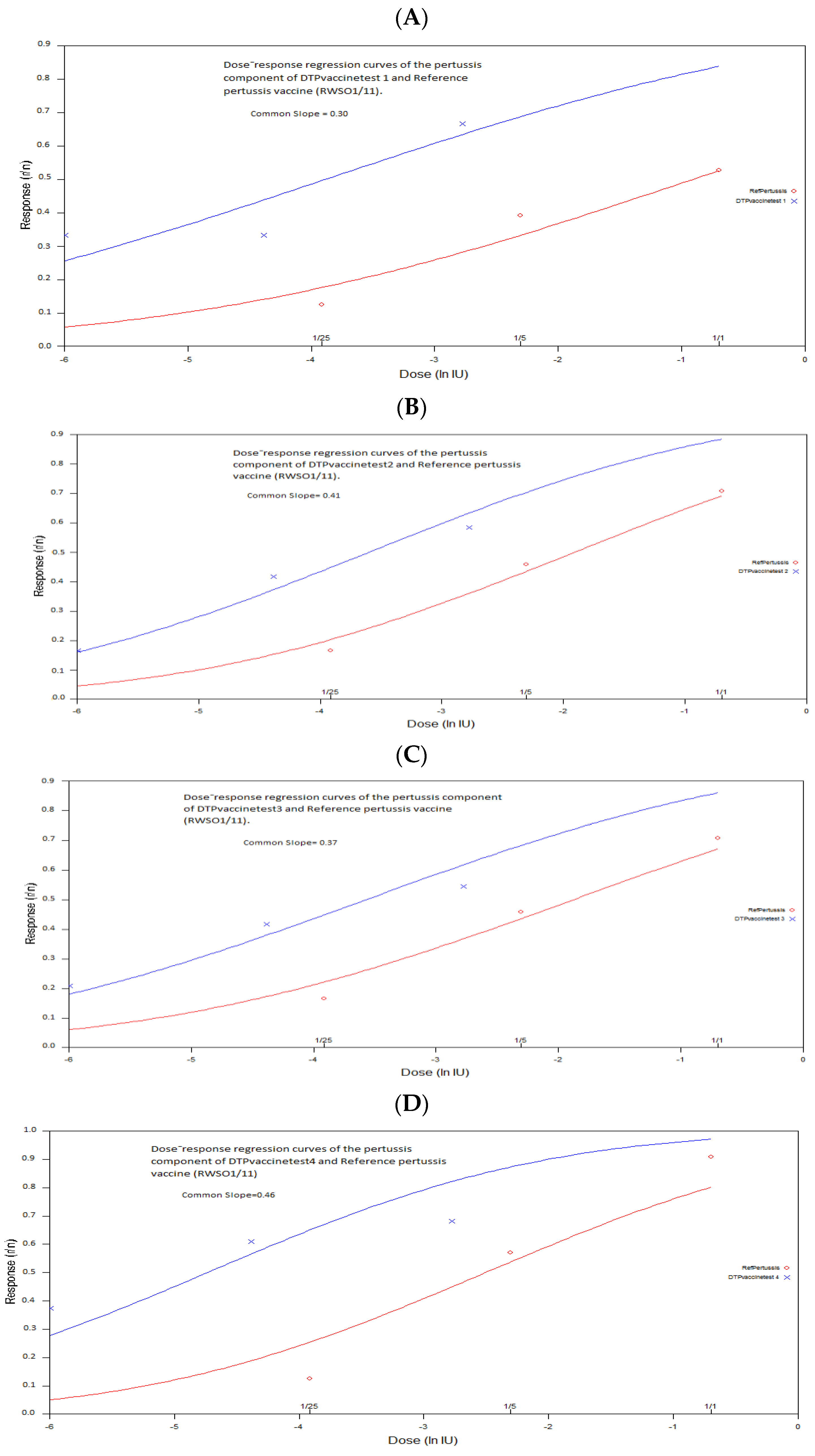
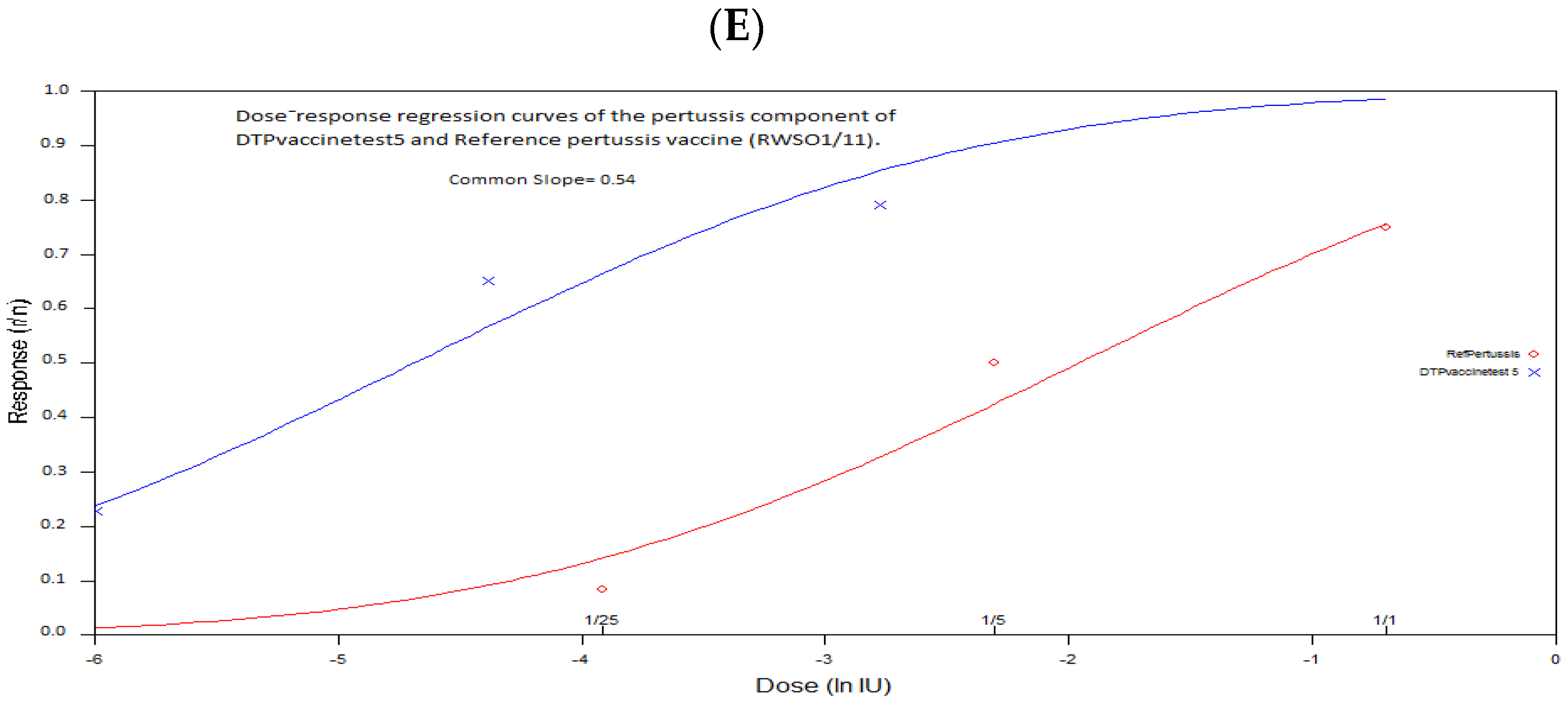
3.4. Estimation of the Relative Potency and the Protective Dose ED50 of the Pertussis Componentof the DTP Test Vaccine against B. pertussis Strain 18–323 Infection
4. Discussion
- -
- Trial 1 satisfied the validation criteria, despite a decrease in the number of mice during the immunization period by the highest dose of the interval recommended for the two vaccine preparations (standard and test vaccine). However, the analysis of variance showed that the trial was statistically validated. This was explained by the WHO recommendation that in some cases, the immunized group with the highest dose of the standard vaccine should be monitored for signs of abnormalities in gait and posture.That 50% of the mice in this group should show clinical signs [15]. In addition, this situation can also be explained by accidents or technical errors that can occur in the first trials in this type of dosage, such as the use of an incorrect injection technique by an inexperienced staff member, or incorrect storage of a product [20].
- -
- Trials 2 and 3 met all of the validity criteria for the test, except that the lower limit of the 95% confidence interval was less than 2 IU/human dose, which is an important criterion in the potency specification. This result rendered the trial invalid.
- -
- Trial 4 did not satisfy all of the criteria. However, analysis of variance showed that the dose-response regression curves for the test and standard vaccines deviated significantly from parallelism (p < 0.05) (Table 3). This result led to a fundamental invalidity of the trial explained by the preparation of the dilutions of the two vaccines used Although, the calculated relative potency and its lower bound were within recommended specifications and the joint slope of the dose-response regression showed no significant difference in the preparations of each vaccine (standard and test vaccine). Indeed, whatever the situation, the parallelism of the dose-response regression curves of the two vaccine preparations takes a very important consideration in the validity of the trial and shows the importance of the preparation of the dilutions of the vaccine to be tested, taking into account the titer value of the standard vaccine used.
- -
- Trial 5 met all validity criteria and the statistical analysis showed a relative potency greater than 4 IU/human dose with the lower limit of the 95% confidence limits greater than 2 IU/human dose. The dose-response regression was highly significant (p = 0.00) for both the standard and test vaccines, and the analysis showed no significant deviation from linearity and parallelism. Indeed, the range of doses chosen for the standard and test vaccines gave responses within the linear range, and the dilutions of both vaccine preparations were performed correctly, which explained the absence of significant deviation from linearity and parallelism.
- -
- The relative potency of the tested pertussis vaccine (DTP vaccine test) was calculated from the two valid estimates of relative potency from Trial n°1 and Trial n°5 that satisfied the relative potency specifications and test validity criteria. The two estimates were homogeneous (p > 0.05). This showed that the estimated relative potency is approximately normally distributed with a mean defined by the estimation of the log potency and the weight (given by the inverse of the variance of the log potency). This information can be used to determine the probability of particular values for subsequent estimates [20]. However, more several tests are necessary for further validation of the potency assessment of whole-cell pertussis vaccines.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kilgore, P.E.; Salim, A.M.; Zervos, M.J.; Schmitt, H.J. Pertussis: Microbiology, disease, treatment, and prevention. Clin. Microbiol. Rev. 2016, 29, 449–486. [Google Scholar] [CrossRef]
- Dias, W.; Raw, I. Pertussis: Disease, Control and Challenges; BoD–Books on Demand: Norderstedt, Germany, 2018. [Google Scholar]
- Stefanelli, P. Pertussis: Identification, prevention and control. In Pertussis Infection and Vaccines; Springer: Berlin/Heidelberg, Germany, 2019; pp. 127–136. [Google Scholar]
- Lazri, M.; Caro, V.; Brun, D.; Njamkepo, E.; Ouraghi, R. La coqueluche en Algérie: Diagnostic direct et indirect de l’infection, sensibilité aux antibiotiques des souches identifiées. L’institut Pasteur D’algérie 2008, 66, 29. [Google Scholar]
- Cantarelli, V.V.; Hoffmann, E.R.; Fitarelli, D.B.; Comerlato, L.; Baungarten, C.C. Pertussis: A re-emerging or under diagnosed infectious disease? Braz. J. Infect. Dis. 2013, 17, 385. [Google Scholar] [CrossRef]
- Fullen, A.R.; Yount, K.S.; Dubey, P.; Deora, R. Whoop! There it is: The surprising resurgence of pertussis. PLoS Pathog. 2020, 16, e1008625. [Google Scholar] [CrossRef]
- Guiso, N. Pertussis vaccination and whooping cough: And now what? Expert Rev. Vaccines 2014, 13, 1163–1165. [Google Scholar] [CrossRef]
- Kapil, P.; Merkel, T.J. Pertussis vaccines and protective immunity. Curr. Opin. Immunol. 2019, 59, 72–78. [Google Scholar] [CrossRef]
- Benamrouche, N.; Maamar, H.T.; Chemli, S.; Senouci, H.; Rahal, K. Immune responses to vaccine-preventable diseases among toddlers and preschool children after primary immunization and first booster in Northwestern Algiers, Algeria. Heliyon 2018, 4, e00664. [Google Scholar] [CrossRef]
- WHO. Pertussis Vaccines: WHO Position Paper—August 2015; Weekly Epidemiological Record = Relevé Épidémiologique Hebdomadaire; World Health Organization: Geneva, Switzerland, 2015; pp. 433–458.
- Guiso, N.; Meade, B.D.; von König, C.H.W. Pertussis vaccines: The first hundred years. Vaccine 2020, 38, 1271–1276. [Google Scholar] [CrossRef]
- WHO. Recommendations for Diphtheria, Tetanus, Pertussis and Combined Vaccines; WHO Expert Committee on Biological Standardization, Fifty-Fourth Report; World Health Organization: Geneva, Switzerland, 2005.
- Locht, C. Will we have new pertussis vaccines? Vaccine 2018, 36, 5460–5469. [Google Scholar] [CrossRef]
- Benamrouche, N.; Maamar, H.T.; Lazri, M.; Hasnaoui, S.; Radoui, A.; Lafer, O.; Boukari, R.; Kaddache, C.; Arrada, Z.; Rahal, K. Pertussis in north-central and northwestern regions of Algeria. J. Infect. Dev. Ctries. 2016, 10, 1191–1199. [Google Scholar] [CrossRef]
- WHO. Recommendations for Whole-Cell Pertussis Vaccine; Technical Report Series No. 941, Annex 6; World Health Organization: Geneva, Switzerland, 2007.
- Dewan, K.K.; Linz, B.; DeRocco, S.E.; Harvill, E.T. Acellular pertussis vaccine components: Today and tomorrow. Vaccines 2020, 8, 217. [Google Scholar] [CrossRef]
- Wilkinson, K.; Righolt, C.H.; Elliott, L.J.; Fanella, S.; Mahmud, S.M. Pertussis vaccine effectiveness and duration of protection–A systematic review and meta-analysis. Vaccine 2021, 39, 3120–3130. [Google Scholar] [CrossRef]
- WHO. WHO Expert Committee on Biological Standardization: Sixty-Fifth Report; World Health Organization: Geneva, Switzerland, 2015; Volume 993.
- Kendrick, P.L.; Eldering, G.; Dixon, M.K.; Misner, J. Mouse protection tests in the study of pertussis vaccine: A comparative series using the intracerebral route for challenge. Am. J. Public Health Nations Health 1947, 37, 803–810. [Google Scholar] [CrossRef]
- WHO. Manual for Quality Control of Diphtheria, Tetanus and Pertussis Vaccines; World Health Organization: Geneva, Switzerland, 2013.
- WHO. Control of Whole Cell Pertussis Vaccine; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1990.
- Jansen, D.L.; Meade, B.D. Preparation and Standardization of US Standard Pertussis Vaccine, Lot No. 11. Biologicals 1996, 24, 363–370. [Google Scholar] [CrossRef]
- Benamrouche, N.; Lazri, M.; Mahrane, S.; Ouraghi, R.; Touati, D.; Rahal, K. Diagnostic Biologique de la Coqueluche. Ministère de la St. de la Popul. et de la Réforme Hosp. Algérie 2013. [Google Scholar]
- Xing, D.; Markey, K.; Gaines Das, R.; Feavers, I. Whole-cell pertussis vaccine potency assays: The Kendrick test and alternative assays. Expert Rev. Vaccines 2014, 13, 1175–1182. [Google Scholar] [CrossRef]
- Van der Ark, A.; Van Straaten-van de Kappelle, I.; Hendriksen, C.; Van de Donk, H. Pertussis serological potency test as an alternatively to the intracerebral mouse protection test. Dev. Biol. Stand. 1996, 86, 271–281. [Google Scholar]
- Von Hunolstein, C.; Gomez Miguel, M.J.; Pezzella, C.; Scopetti, F.; Behr-Gross, M.E.; Halder, M.; Hoffmann, S.; Levels, L.; van der Gun, J.; Hendriksen, C. Evaluation of two serological methods for potency testing of whole cell pertussis vaccines. Pharmeuropa Bio 2008, 1, 7–18. [Google Scholar]
- Mohammadbagher, D.; Noofeli, M.; Karimi, G. Comparative Assessment of the Whole-cell Pertussis Vaccine Potency Using Serological and Intracerebral Mouse Protection Methods. Arch. Razi Inst. 2019, 74, 103–109. [Google Scholar]
- Matos, D.C.S.; Marcovistz, R.; Silva, A.M.V.D.; Quintilio, W.; Georgini, R.A. Comparative analysis of the intracerebral mouse protection test and serological method for potency assays of pertussis component in DTP vaccine. Braz. J. Microbiol. 2012, 43, 429–431. [Google Scholar] [CrossRef][Green Version]
- Queenan, A.M.; Fernandez, J.; Shang, W.; Wiertsema, S.; van den Dobbelsteen, G.P.; Poolman, J. The mouse intranasal challenge model for potency testing of whole-cell pertussis vaccines. Expert Rev. Vaccines 2014, 13, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jadhav, S.S.; Gairola, S.; Deobagkar, D.D. Intranasal Mice Model to Study the role of Bordetella pertussis antigens in Immunity. J. Sci. Ind. Res. 2019, 78, 699–702. [Google Scholar]
- Xing, D.K.; Canthaboo, C.; Corbel, M.J. Nitric oxide induction in murine macrophages and spleen cells by whole-cell Bordetella pertussis vaccine. Vaccine 1998, 16, 16–23. [Google Scholar] [CrossRef]
- Canthaboo, C.; Xing, D.; Corbel, M. Development of a nitric oxide induction assay as a potential replacement for the intracerebral mouse protection test for potency assay of pertussis whole cell vaccines. Dev. Biol. Stand. 1999, 101, 95–103. [Google Scholar]
- Gupta, R.K.; Sharma, S.B.; Ahuja, S.; Saxena, S.N. Evaluation of repeated immunoassays (mouse intracerebral potency tests) of the second International Standard of Pertussis Vaccine. J. Immunoass. Immunochem. 1987, 8, 309–318. [Google Scholar] [CrossRef]
- Preston, N.W. Potency tests for pertussis vaccines: Doubtful value of intracerebral challenge test in mice. J. Pathol. Bacteriol. 1966, 91, 173–179. [Google Scholar] [CrossRef]
- Xing, D.; Das, R.G.; O’Neill, T.; Corbel, M.; Dellepiane, N.; Milstien, J. Laboratory testing of whole cell pertussis vaccine: A WHO proficiency study using the Kendrick test. Vaccine 2001, 20, 342–351. [Google Scholar] [CrossRef]
- Verch, T.; Trausch, J.J.; Shank-Retzlaff, M. Principles of vaccine potency assays. Bioanalysis 2018, 10, 163–180. [Google Scholar] [CrossRef]
- Redhead, K.; Das, R.E.G. A collaborative assay of the proposed third British reference preparation for pertussis vaccine and of the relative potencies of the second international standard and the second British reference preparation for pertussis vaccine. Biologicals 1991, 19, 107–111. [Google Scholar] [CrossRef]
- Seagroatt, V.; Sheffield, F. A collaborative assay of the proposed second International Standard for Pertussis Vaccine and of the proposed first British Standard for Pertussis Vaccine. J. Biol. Stand. 1981, 9, 351–365. [Google Scholar] [CrossRef]
- Yoshioka, M.; Takatsu, K.; Kawahira, M. Studies on the protection test of pertussis vaccine. Immunization period and protectivity. Jpn. J. Microbiol. 1965, 9, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lacey, B. Antigenic modulation of Bordetella pertussis. Epidemiol. Infect. 1960, 58, 57–93. [Google Scholar]
- Cameron, J. Problems Associated with the Control Testing of Pertussis Vaccine.I n Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1976; pp. 57–80. [Google Scholar]
- Standfast, A. Some factors influencing the virulence for mice of Bordetella pertussis by the intracerebral route. Immunology 1958, 1, 123. [Google Scholar] [PubMed]
- Weiss, A.A.; Falkow, S. Genetic analysis of phase change in Bordetella pertussis. Infect. Immun. 1984, 43, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Melton, A.R.; Weiss, A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J. Bacteriol. 1989, 171, 6206–6212. [Google Scholar] [CrossRef]
- Stainer, D.; Scholte, M. A simple chemically defined medium for the production of phase I Bordetella pertussis. Microbiology 1970, 63, 211–220. [Google Scholar] [CrossRef]
- Gaines-Das, R.; Horiuchi, Y.; Zhang, S.M.; Newland, P.; Kim, Y.; Corbel, M.; Xing, D. Modified intra-cerebral challenge assay for acellular pertussis vaccines: Comparisons among whole cell and acellular vaccines. Vaccine 2009, 27, 6824–6832. [Google Scholar] [CrossRef]
- Xing, D.; Gaines Das, R.E.; Newland, P.; Corbel, M.J.; Unit, B.; World Health Organization. WHO Expert Committee on Biological Standardization. In Study Report: International Collaborative Study to: Evaluate a Candidate Replacement International Standard for Whole Cell Pertussis Vaccine Code 94/532; (No. WHO/BS/06.2036); World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Das, R.G.; Newland, P.; Asokanathan, C.; Douglas-Bardsley, A.; Corbel, M.; Xing, D. Evaluation of fourth international standard for whole cell pertussis vaccine. Biologicals 2010, 38, 644–651. [Google Scholar] [CrossRef]
| Trial N° | Dose B.p (µo/0.03 mL) | NB Dead Mice (Control Group) | Viability B.p Nb Colonies (M) (BG Medium) | ViabilityB.p CFU Counting/ Challenge Dose | LD50 (95% IC) | NB LD50/ Challenge Dose | Slope of Curve p ≤ 0.05 | R2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 10,000 | 10/10 | 13 | 2.43 × 103 | 318.87 (144.95–690.10) | 313.65 | 0.84 | 1 |
| 2000 | 10/10 | |||||||
| 400 | 10/4 | |||||||
| 80 | 10/2 | |||||||
| C.Diluent | 10/10 | |||||||
| 2 | 10,000 | 10/10 | 18 | 3.37 × 103 | 269.3 (71.19–631.54) | 371.32 | 1.2 | 0.88 |
| 2000 | 10/8 | |||||||
| 400 | 10/7 | |||||||
| 80 | 10/2 | |||||||
| C.Diluent | 10/10 | |||||||
| 3 | 10,000 | 10/9 | 15 | 2.81 × 103 | 202.17 (7.25–662.39) | 494.61 | 0.82 | 0.92 |
| 2000 | 10/8 | |||||||
| 400 | 10/7 | |||||||
| 80 | 10/3 | |||||||
| C.Diluent | 10/10 | |||||||
| 4 | 10,000 | 10/10 | 33 | 6.18 × 103 | 463.20 (190.54–1018.43) | 215.88 | 1.52 | 0.98 |
| 2000 | 10/8 | |||||||
| 400 | 10/5 | |||||||
| 80 | 10/1 | |||||||
| C.Diluent | 10/10 | |||||||
| 5 | 10,000 | 10/10 | 24 | 4.50 × 103 | 441.06 (161.54–104530) | 226.72 | 1.2 | 0.97 |
| 2000 | 10/8 | |||||||
| 400 | 10/4 | |||||||
| 80 | 10/2 | |||||||
| C.Diluent | 10/10 | |||||||
| Mean 95% CI | 338.92 (200.34–477.50) | 324.43 (182.04–466.83) | ||||||
| SD | 111.6 | 114.68 | ||||||
| Dilution | Number of Mice after Immunization | Percentage of Immunization (a) (%) | Number of Surviving Immune Mice after the Challenge Test | Percentage of Survival (b) after the Challenge Test (%) | ||
|---|---|---|---|---|---|---|
| Trial 1 | Standard Vaccine P * 0.5 IU/dose | 1:1 | 19/24 | 76.16 | 10/19 | 70.96 |
| 1:5 | 23/24 | 95.83 | 9/23 | 34.28 | ||
| 1:25 | 24/24 | 100 | 3/24 | 6.38 | ||
| DTP vaccine test 1 | 1:8 | 18/24 | 75 | 12/18 | 82.35 | |
| 1:40 | 24/24 | 100 | 8/24 | 42.1 | ||
| 1:200 | 24/24 | 100 | 8/24 | 17.39 | ||
| N.C | V.S | 10/10 | 09/10 ** | |||
| Trial 2 | Standard Vaccine P * 0.5 IU/dose | 1:1 | 24/24 | 100 | 17/24 | 82.05 |
| 1:5 | 24/24 | 100 | 11/24 | 42.85 | ||
| 1:25 | 24/24 | 100 | 4/24 | 9.09 | ||
| DTP vaccine test 2 | 1:8 | 24/24 | 100 | 14/24 | 73.68 | |
| 1:40 | 24/24 | 100 | 10/24 | 36.84 | ||
| 1:200 | 24/24 | 100 | 4/24 | 8.33 | ||
| N.C | V.S | 10/10 | 10/10 | |||
| Trial 3 | Standard Vaccine P * 0.5 IU/dose | 1:1 | 24/24 | 100 | 17/24 | 82.05 |
| 1:5 | 24/24 | 100 | 11/24 | 42.85 | ||
| 1:25 | 24/24 | 100 | 4/24 | 9.09 | ||
| DTP vaccine test 3 | 1:8 | 22/24 | 91.66 | 12/22 | 69.23 | |
| 1:40 | 24/24 | 100 | 10/24 | 36.58 | ||
| 1:200 | 24/24 | 100 | 5/24 | 10 | ||
| NC | V.S | 10/10 | 10/10 | |||
| Trial 4 | Standard Vaccine P * 0.5 IU/dose | 1:1 | 22/24 | 91.66 | 20/22 | 94.59 |
| 1:5 | 21/24 | 87.5 | 12/21 | 57.69 | ||
| 1:25 | 24/24 | 100 | 3/24 | 17.64 | ||
| DTP vaccine test 4 | 1:8 | 22/24 | 9.66 | 15/22 | 84.44 | |
| 1:40 | 23/24 | 95.83 | 14/23 | 58.97 | ||
| 1:200 | 24/24 | 100 | 9/24 | 22.5 | ||
| N.C | V.S | 10/10 | 10/10 | |||
| Trial 5 | Standard Vaccine P * 0.5 IU/dose | 1:1 | 24/24 | 100 | 18/24 | 84.21 |
| 1:5 | 24/24 | 100 | 12/24 | 43.75 | ||
| 1:25 | 24/24 | 100 | 2/24 | 4.76 | ||
| DTP vaccine test 5 | 1:8 | 24/24 | 100 | 19/24 | 88.63 | |
| 1:40 | 23/24 | 95.83 | 15/23 | 60.6 | ||
| 1:200 | 22/24 | 91.66 | 5/22 | 14.28 | ||
| N.C | V.S | 10/10 | 10/10 | |||
| Trial N° | P0TENCY (IU/Human Dose) 95% FL (p ≤ 0.05) | Number of ED50/Human Dose | ED50 (IU/Human Dose) 95% FI (p ≤ 0.05) | Common Slope B | Linearity (p ≥ 0.05) | Parallelism (p ≥ 0.05) |
|---|---|---|---|---|---|---|
| 1 | 9.77 (2.15–93.70) | 24.28 | 0.40 (0.44–1.35) | 0.30 | No significant deviation p = 0.32 | No significant deviation p = 0.52 |
| 2 | 2.71 (0.79–8.18) | 18.24 | 0.14 (0.11–0.21) | 0.41 | No significant deviation p = 0.83 | No significant deviation p = 0.53 |
| 3 | 2.720 (0.66–9.21) | 17.75 | 0.15 (0.12–0.22) | 0.37 | No significant deviation p = 0.87 | No significant deviation p = 0.29 |
| 4 | 4.65 (1.67–13.46) | 56.97 | 0.08 (0.02–0.10) | 0.46 | No significant deviation p = 0.75 | significant deviation p = 0.006(˂0.05) No Parallelism |
| 5 | 7.672 (3.31–19.96) | 54.47 | 0.14 (0.11–0.18) | 0.54 | No significant deviation p = 0.33 | No significant deviation p = 0.48 |
| Relative Potency | |||
|---|---|---|---|
| Lower Limit | Estimate | Upper Limit | |
| DTP vaccine test 1 | 2.15 | 9.77 | 93.70 |
| DTP vaccine test 5 | 3.31 | 7.67 | 19.96 |
| Weighted combination * | |||
| potency | 3.56 | 8.02 | 18.05 |
| Estimate in percent | 44.4% | 100% | 225.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahar djebbar, K.; Allouache, M.; Kezzal, S.; Benguerguoura, F.; TouilBoukoffa, C.; Zidi, I.; Raache, R.; Ouzari, H.-I. Evaluation of the Potency of the Pertussis Vaccine in Experimental Infection Model with Bordetella pertussis: Study of the Case of the Pertussis Vaccine Used in the Expanded Vaccination Program in Algeria. Vaccines 2022, 10, 906. https://doi.org/10.3390/vaccines10060906
Tahar djebbar K, Allouache M, Kezzal S, Benguerguoura F, TouilBoukoffa C, Zidi I, Raache R, Ouzari H-I. Evaluation of the Potency of the Pertussis Vaccine in Experimental Infection Model with Bordetella pertussis: Study of the Case of the Pertussis Vaccine Used in the Expanded Vaccination Program in Algeria. Vaccines. 2022; 10(6):906. https://doi.org/10.3390/vaccines10060906
Chicago/Turabian StyleTahar djebbar, Khedidja, Mounia Allouache, Salim Kezzal, Fouzia Benguerguoura, Chafia TouilBoukoffa, Ines Zidi, Rachida Raache, and Hadda-Imene Ouzari. 2022. "Evaluation of the Potency of the Pertussis Vaccine in Experimental Infection Model with Bordetella pertussis: Study of the Case of the Pertussis Vaccine Used in the Expanded Vaccination Program in Algeria" Vaccines 10, no. 6: 906. https://doi.org/10.3390/vaccines10060906
APA StyleTahar djebbar, K., Allouache, M., Kezzal, S., Benguerguoura, F., TouilBoukoffa, C., Zidi, I., Raache, R., & Ouzari, H.-I. (2022). Evaluation of the Potency of the Pertussis Vaccine in Experimental Infection Model with Bordetella pertussis: Study of the Case of the Pertussis Vaccine Used in the Expanded Vaccination Program in Algeria. Vaccines, 10(6), 906. https://doi.org/10.3390/vaccines10060906






