Safety and Tolerability of COVID-19 Vaccines in Patients with Cancer: A Single Center Retrospective Analysis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Designand Participants
2.2. Data Collection and Study Instrument
2.3. Endpoint and Statistical Analysis
2.4. Ethics
3. Results
3.1. Demographic and Clinical Features of Participants
3.2. Safety
3.2.1. First Dose
3.2.2. Second Dose
3.2.3. Comparison between Percentages of Adverse Events in Our Cancer Population and in a Healthy Individuals’ Population
3.2.4. Risk Factors for Adverse Events
3.2.5. Effects of Previous COVID-19 on Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| COVID-19 | severe acute respiratory syndrome coronavirus 2 disease |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| ICIs | immune checkpoint inhibitors |
| mRNA | messenger RNA |
| OR | odds ratio |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SPSS | Statistical Package for Social Science |
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 26 May 2022).
- DRAFT landscape of COVID-19 candidate vaccines. Available online: https://www.who.int/docs/default-source/a-future-for-children/novel-coronavirus_landscape_covid-19.pdf?sfvrsn=4d8bd201_1 (accessed on 21 October 2021).
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer Patients in SARS-CoV-2 Infection: A Nationwide Analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- ElGohary, G.M.; Hashmi, S.; Styczynski, J.; Kharfan-Dabaja, M.A.; Alblooshi, R.M.; de la Cámara, R.; Mohmed, S.; Alshaibani, A.; Cesaro, S.; Abd El-Aziz, N.; et al. The Risk and Prognosis of COVID-19 Infection in Cancer Patients: A Systematic Review and Meta-Analysis. Hematol. Oncol. Stem Cell Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Recommendations of the National Comprehensive Cancer Network® (NCCN®) Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis. Available online: https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v4-0.pdf?sfvrsn=b483da2b_70 (accessed on 21 October 2021).
- ESMO. ESMO Statements on Vaccination against COVID-19 in People with Cancer. Available online: https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination (accessed on 26 May 2022).
- Documento AIOM CIPOMO COMU Vaccinazione COVID-19 per i Pazienti Oncologici ver 1.0. Aiom.it. Available online: https://www.aiom.it/wp-content/uploads/2020/12/20201231_Vaccino_COVID_19_AIOM_CIPOMO_COMU_1.0.pdf (accessed on 21 October 2021).
- Desai, A.; Gainor, J.F.; Hegde, A.; Schram, A.M.; Curigliano, G.; Pal, S.; Liu, S.V.; Halmos, B.; Groisberg, R.; Grande, E.; et al. COVID-19 Vaccine Guidance for Patients with Cancer Participating in Oncology Clinical Trials. Nat. Rev. Clin. Oncol. 2021, 18, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Fendler, A.; Shepherd, S.T.C.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; Del Rosario, L.; Shon, J.; et al. Cytokine Release Syndrome in a Patient with Colorectal Cancer after Vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Kaiki, Y.; Sugiyama, A.; Nagashima, S.; Kurisu, A.; Nomura, T.; Omori, K.; Akita, T.; Shigemoto, N.; Tanaka, J.; et al. Adverse Reactions to the BNT162b2 and MRNA-1273 MRNA COVID-19 Vaccines in Japan. J. Infect. Chemother. 2022, 28, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Fda.gov. Available online: https://www.fda.gov/media/73679/download (accessed on 21 October 2021).
- Clinical Spectrum. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 26 May 2022).
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of MRNA Vaccines Administered during the Initial 6 Months of the US COVID-19 Vaccination Programme: An Observational Study of Reports to the Vaccine Adverse Event Reporting System and v-Safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Perez, J.L.; Lockhart, S.P.; Hariharan, S.; Kitchin, N.; Bailey, R.; Liau, K.; Lagkadinou, E.; Türeci, Ö.; Şahin, U.; et al. Efficacy and Safety of the BNT162b2 MRNA COVID-19 Vaccine in Participants with a History of Cancer: Subgroup Analysis of a Global Phase 3 Randomized Clinical Trial. Vaccine 2022, 40, 1483–1492. [Google Scholar] [CrossRef]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, H.; Safadi, E.; Etan, T.; Vaknin, N.; Waller, M.; Croll, A.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Wasserman, A.; et al. Immunogenicity and Safety of the BNT162b2 MRNA COVID-19 Vaccine Among Actively Treated Cancer Patients. J. Natl. Cancer Inst. 2022, 114, 203–209. [Google Scholar] [CrossRef] [PubMed]
- So, A.C.P.; McGrath, H.; Ting, J.; Srikandarajah, K.; Germanou, S.; Moss, C.; Russell, B.; Monroy-Iglesias, M.; Dolly, S.; Irshad, S.; et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers 2021, 13, 3573. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, Y.; Yakushijin, K.; Ohji, G.; Hojo, W.; Sakai, H.; Takai, R.; Nose, T.; Ohata, S.; Nagatani, Y.; Koyama, T.; et al. Safety and Immunogenicity of the COVID-19 Vaccine BNT162b2 in Patients Undergoing Chemotherapy for Solid Cancer. J. Infect. Chemother. 2022, 28, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.M.; Weinberg, D.S.; Ross, E.A.; Ruth, K.; Rall, G.F.; Olszanski, A.J.; Helstrom, J.; Hall, M.J.; Judd, J.; Chen, D.Y.T.; et al. Adverse Events Reported by Patients With Cancer After Administration of a 2-Dose MRNA COVID-19 Vaccine. J. Natl. Compr. Canc. Netw. 2022, 20, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Trillo Aliaga, P.; Trapani, D.; Sandoval, J.L.; Crimini, E.; Antonarelli, G.; Vivanet, G.; Morganti, S.; Corti, C.; Tarantino, P.; Friedlaender, A.; et al. Safety of COVID-19 MRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials. Cancers 2021, 13, 5829. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First Month of COVID-19 Vaccine Safety Monitoring-United States, 14 December 2020–13 January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef] [PubMed]
- European Database of Suspected Adverse Drug Reaction Reports. Europa.eu. Available online: https://dap.ema.europa.eu/analytics/saw.dll?PortalPages (accessed on 21 October 2021).
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-Associated Changes in the Impact of Sex Steroids on Influenza Vaccine Responses in Males and Females. NPJ Vaccines 2019, 4, 1–12. [Google Scholar] [CrossRef]
- Vaxzevria Summary of Products Characteristics. Europa.eu. Available online: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf (accessed on 21 October 2021).
- d’Arminio Monforte, A.; Tavelli, A.; Perrone, P.M.; Za, A.; Razzini, K.; Tomasoni, D.; Bordoni, V.; Romanò, L.; Orfeo, N.; Marchetti, G.; et al. Association between Previous Infection with SARS CoV-2 and the Risk of Self-Reported Symptoms after MRNA BNT162b2 Vaccination: Data from 3078 Health Care Workers. EClinicalMedicine 2021, 36, 100914. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 377) |
|---|---|
| Median Age (range) | 66 (27–87) |
| Patients older than 65 years | 235 (62.3) |
| Sex—no. of patients (%) | |
| Male | 191 (50.7) |
| Female | 186 (49.3) |
| ECOG PS—no. of patients (%) | |
| 0 | 211 (56) |
| 1 | 133 (35.3) |
| 2 | 28 (7.4) |
| 3 | 5 (1.3) |
| Non oncological comorbidities—no. of patients (%) | |
| At least one condition | 258 (68.4) |
| Cardiovascular disease (includes hypertension) | 181 (48) |
| Lung disease | 28 (7.4) |
| Eye, ear, nose and throat disease | 14 (3.7) |
| Gastroenteric and epatic disease | 37 (9.8) |
| Genito-urinary disease | 37 (9.8) |
| Musculoskeletal and cutaneous disease | 21 (5.5) |
| Neurologic disease | 15 (3.9) |
| Reumatological disease | 13 (3.4) |
| Endocrinological Disease | 85 (22.5) |
| Diabetes | 43 (11.4) |
| Psychiatric disease (includes dementia) | 19 (5) |
| Obesity (Body Mass Index > 30 kg/m2) | 36 (9.5) |
| Type of malignancy—no. of patients (%) | |
| Women’s cancer (breast and gynecological) | 78 (20.7) |
| Urological cancer (renal, prostate, testicular, bladder) | 61 (16.2) |
| Skin cancers | 21 (5.6) |
| Thoracic cancers | 103 (27.3) |
| Gastrointestinal cancers | 103 (27.3) |
| Head and neck cancer | 5 (1.3) |
| Others | 6 (1.6) |
| TNM staging—no. of patients (%) | |
| III | 52 (13.79) |
| IV | 325 (86.21) |
| Treatment setting—no. of patients (%) | |
| Neoadjuvant | 17 (4.5) |
| Adjuvant | 35 (9.2) |
| First line | 213 (56.5) |
| Second line or following | 112 (29.8) |
| Anticancer treatment—no. of patients (%) | |
| Chemotherapy | 127 (33.7) |
| Target therapy | 104 (27.6) |
| Immunotherapy (includes immunotherapy combinations) | 111 (29.4) |
| Chemotherapy plus target-therapy | 35 (9.3) |
| Previous COVID-19—no. of patients (%) | |
| no | 344 (91.2) |
| yes | 33 (8.8) |
| Vaccine administered—no. of patients (%) | |
| Astrazeneca | 8 (2.1) |
| Janssen | 6 (1.6) |
| Moderna | 183 (48.5) |
| Pfizer | 180 (47.8) |
| FD | FDH | SD | SDH | p-Values | ||
|---|---|---|---|---|---|---|
| FD vs. FDH | SD vs. SDH | |||||
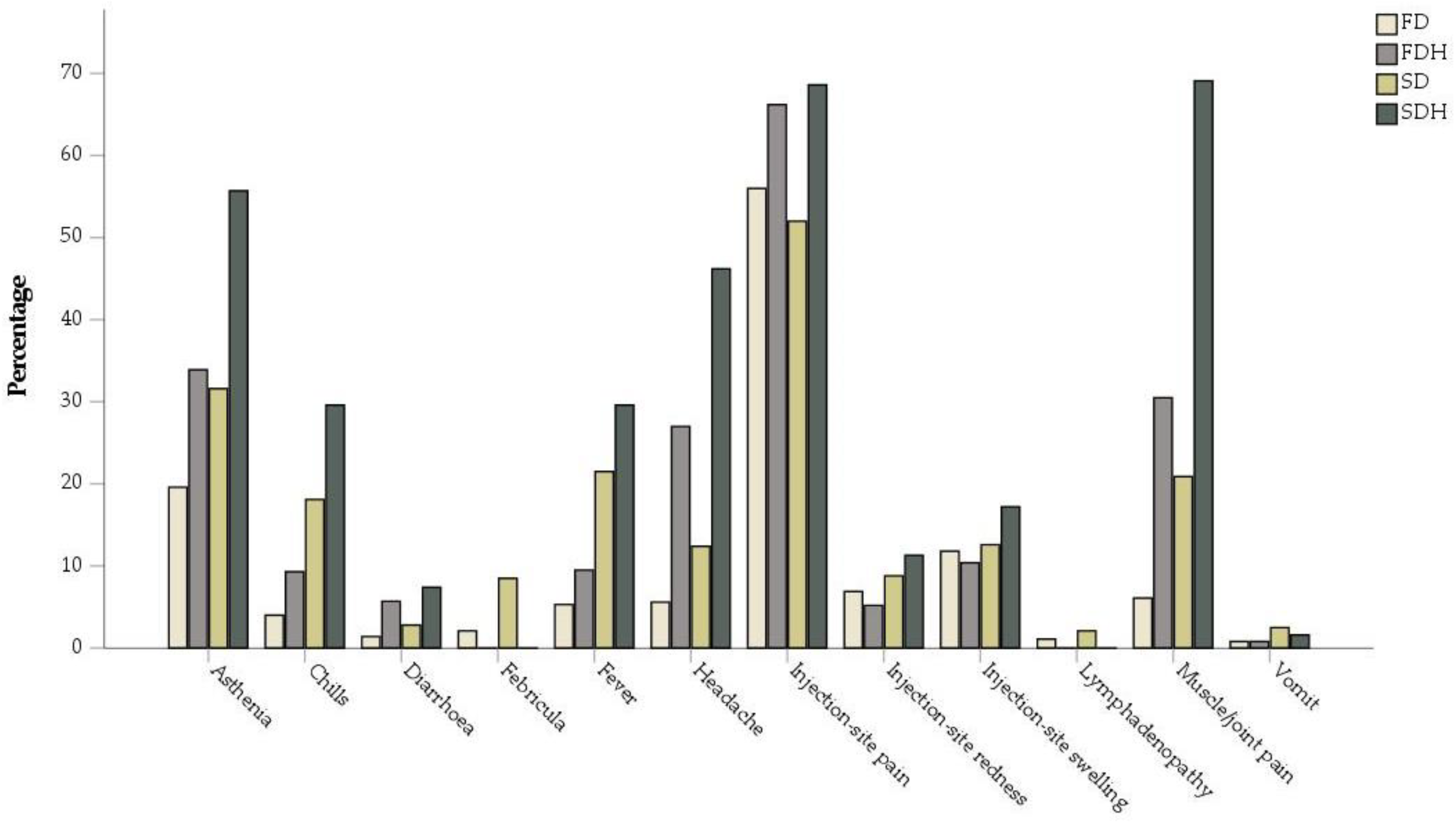
| Injection-site pain | 56 | 66.2 | 52 | 68.6 | <0.001 * | <0.001 * |
| Injection-site redness | 6.9 | 5.2 | 8.8 | 11.3 | 0.174 | 0.163 |
| Injection-site swelling | 11.8 | 10.4 | 12.6 | 17.2 | 0.424 | 0.027 * |
| Lymphadenopathy | 1.1 | not available | 2.1 | not available | / | / |
| Asthenia | 19.6 | 33.9 | 31.6 | 55.7 | <0.001 * | <0.001 * |
| Headache | 5.6 | 27 | 12.4 | 46.2 | <0.001 * | <0.001 * |
| Febricula | 2.1 | not available | 8.5 | not available | / | / |
| Fever | 5.3 | 9.5 | 21.5 | 29.6 | 0.007 * | 0.001 * |
| Chills | 4 | 9.3 | 18.1 | 29.6 | <0.001 * | <0.001 * |
| Muscle/joint pain | 6.1 | 30.5 | 20.9 | 69.1 | <0.001 * | <0.001 * |
| Vomit | 0.8 | 0.8 | 2.5 | 1.6 | 0.999 | 0.260 |
| Diarrhoea | 1.4 | 5.7 | 2.8 | 7.4 | <0.001 * | <0.001 * |
| Outcome | Factor | OR (95% CI) | p-Value |
|---|---|---|---|
| Local adverse events after the first dose | Age | 0.55 (0.32–0.94) | 0.029 * |
| Sex | 1.84 (1.17–2.89) | 0.008 * | |
| ECOG PS | 0.91 (0.64–1.32) | 0.634 | |
| Comorbidity | 0.85 (0.69–1.06) | 0.152 | |
| Setting | 0.75 (0.37–1.51) | 0.422 | |
| ICIs treatment | 0.77 (0.48–1.24) | 0.283 | |
| Vaccine | 0.39 (0.12–1.25) | 0.112 | |
| Systemic adverse events after the first dose | Age | 0.57 (0.32–1.01) | 0.055 |
| Sex | 1.61 (0.98–2.64) | 0.060 | |
| ECOG PS | 1.13 (0.75–1.72) | 0.552 | |
| Comorbidity | 0.93 (0.73–1.19) | 0.566 | |
| Setting | 0.87 (0.45–1.68) | 0.674 | |
| ICIs treatment | 0.89 (0.52–1.52) | 0.671 | |
| Vaccine | 1.69 (0.55–5.24) | 0.362 | |
| Local adverse events after the second dose | Age | 0.68 (0.34–1.16) | 0.158 |
| Sex | 1.46 (0.92–2.31) | 0.105 | |
| ECOG PS | 0.81 (0.56–1.18) | 0.281 | |
| Comorbidity | 1.07 (0.85–1.33) | 0.580 | |
| Setting | 0.79 (0.39–1.54) | 0.471 | |
| ICIs treatment | 0.95 (0.58–1.55) | 0.848 | |
| Vaccine | 0.19 (0.03–0.97) | 0.046 * | |
| Systemic adverse events after the second dose | Age | 0.95 (0.55–1.63) | 0.854 |
| Sex | 2.31 (1.45–3.69) | <0.001 * | |
| ECOG PS | 0.68 (0.46–1) | 0.050 * | |
| Comorbidity | 0.87 (0.69–1.10) | 0.249 | |
| Setting | 0.84 (0.43–1.65) | 0.613 | |
| ICIs treatment | 1.67 (1.01–2.76) | 0.047 * | |
| Vaccine | 0.22 (0.04–1.21) | 0.082 |
| Outcome | Factor | PCC | p-Value |
|---|---|---|---|
| Grade of the local adverse events after the first dose | Age | −008 | 0.179 |
| Sex | 0.18 | 0.001 * | |
| ECOG PS | −0.03 | 0.601 | |
| Comorbidity | −0.02 | 0.686 | |
| Setting | −0.09 | 0.101 | |
| ICIs treatment | −0.01 | 0909 | |
| Vaccine | −0.01 | 0.908 | |
| Grade of the systemic adverse events after the first dose | Age | −0.11 | 0.066 |
| Sex | 0.12 | 0.021 * | |
| ECOG PS | 0.05 | 0.360 | |
| Comorbidity | 0.08 | 0.163 | |
| Setting | −0.02 | 0.756 | |
| ICIs treatment | −0.03 | 0.534 | |
| Vaccine | 0.05 | 0.343 | |
| Grade of the local adverse events after the second dose | Age | −0.10 | 0105 |
| Sex | 0.16 | 0.003 * | |
| ECOG PS | −0.04 | 0.523 | |
| Comorbidity | −0.01 | 0.860 | |
| Setting | −0.09 | 0.086 | |
| ICIs treatment | −0.01 | 0.893 | |
| Vaccine | −0.12 | 0.025 * | |
| Grade of the systemic adverse events after the second dose | Age | −0.05 | 0.391 |
| Sex | 0.16 | 0.004 * | |
| ECOG PS | −0.07 | 0.253 | |
| Comorbidity | 0.02 | 0.685 | |
| Setting | −0.11 | 0.046 * | |
| ICIs treatment | 0.10 | 0.055 | |
| Vaccine | −0.10 | 0.048 * |
| Outcome | Factor | PCC | p-Value |
|---|---|---|---|
| Duration of the local adverse events after the first dose | Age | −0.06 | 0.315 |
| Sex | 0.12 | 0.022 * | |
| ECOG PS | −0.01 | 0.849 | |
| Comorbidity | −0.03 | 0.565 | |
| Setting | 0.03 | 0.557 | |
| ICIs treatment | 0.05 | 0.362 | |
| Vaccine | 0.11 | 0.035 * | |
| Duration of the systemic adverse events after the first dose | Age | −0.07 | 0.240 |
| Sex | 0.02 | 0.689 | |
| ECOG PS | 0.15 | 0.008 * | |
| Comorbidity | 0.07 | 0.182 | |
| Setting | 0.01 | 0.884 | |
| ICIs treatment | −0.02 | 0.765 | |
| Vaccine | 0.05 | 0.330 | |
| Duration of the local adverse events after the second dose | Age | −0.05 | 0.450 |
| Sex | 0.17 | 0.002 * | |
| ECOG PS | 0.04 | 0.490 | |
| Comorbidity | −0.04 | 0.495 | |
| Setting | −0.13 | 0.017 * | |
| ICIs treatment | 0.08 | 0.121 | |
| Vaccine | −0.09 | 0.079 | |
| Duration of the systemic adverse events after the second dose | Age | −0.01 | 0.831 |
| Sex | 0.17 | 0.003 * | |
| ECOG PS | 0.03 | 0.576 | |
| Comorbidity | 0.05 | 0.330 | |
| Setting | −0.06 | 0.289 | |
| ICIs treatment | 0.13 | 0.016 * | |
| Vaccine | −0.07 | 0.177 |
| Prior COVID-19 | p-Value | ||
|---|---|---|---|
| Adverse event developed | no | yes | |
| Local adverse events | 0.108 | ||
| no | 138 | 18 | |
| yes | 206 | 15 | |
| Systemic adverse events | 0.034 * | ||
| no | 257 | 19 | |
| yes | 87 | 14 | |
| Medium grade of local adverse events | 0.22 (0.25) | 0.19 (0.26) | 0.448 |
| Medium grade of systemic adverse events | 0.06 (0.14) | 0.13 (0.18) | 0.006 * |
| Medium duration of local adverse events | 0.49 (1.02) | 0.50 (1.07) | 0.947 |
| Medium duration of systemic adverse events | 0.17 (0.64) | 0.38 (0.60) | 0.062 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, A.; Manacorda, S.; Sammarco, E.; Sbrana, A.; Bazzurri, S.; Paolieri, F.; Manfredi, F.; Mercinelli, C.; Ferrari, M.; Massaro, G.; et al. Safety and Tolerability of COVID-19 Vaccines in Patients with Cancer: A Single Center Retrospective Analysis. Vaccines 2022, 10, 892. https://doi.org/10.3390/vaccines10060892
Nuzzo A, Manacorda S, Sammarco E, Sbrana A, Bazzurri S, Paolieri F, Manfredi F, Mercinelli C, Ferrari M, Massaro G, et al. Safety and Tolerability of COVID-19 Vaccines in Patients with Cancer: A Single Center Retrospective Analysis. Vaccines. 2022; 10(6):892. https://doi.org/10.3390/vaccines10060892
Chicago/Turabian StyleNuzzo, Amedeo, Simona Manacorda, Enrico Sammarco, Andrea Sbrana, Serena Bazzurri, Federico Paolieri, Fiorella Manfredi, Chiara Mercinelli, Marco Ferrari, Giulia Massaro, and et al. 2022. "Safety and Tolerability of COVID-19 Vaccines in Patients with Cancer: A Single Center Retrospective Analysis" Vaccines 10, no. 6: 892. https://doi.org/10.3390/vaccines10060892
APA StyleNuzzo, A., Manacorda, S., Sammarco, E., Sbrana, A., Bazzurri, S., Paolieri, F., Manfredi, F., Mercinelli, C., Ferrari, M., Massaro, G., Bonato, A., Salfi, A., Galli, L., Morganti, R., Antonuzzo, A., Cremolini, C., & Masi, G. (2022). Safety and Tolerability of COVID-19 Vaccines in Patients with Cancer: A Single Center Retrospective Analysis. Vaccines, 10(6), 892. https://doi.org/10.3390/vaccines10060892








