Correlation between Anti-SARS-CoV-2 Total Antibodies and Spike Trimeric IgG after BNT162b2 Booster Immunization
Abstract
:1. Introduction
2. Materials and Methods
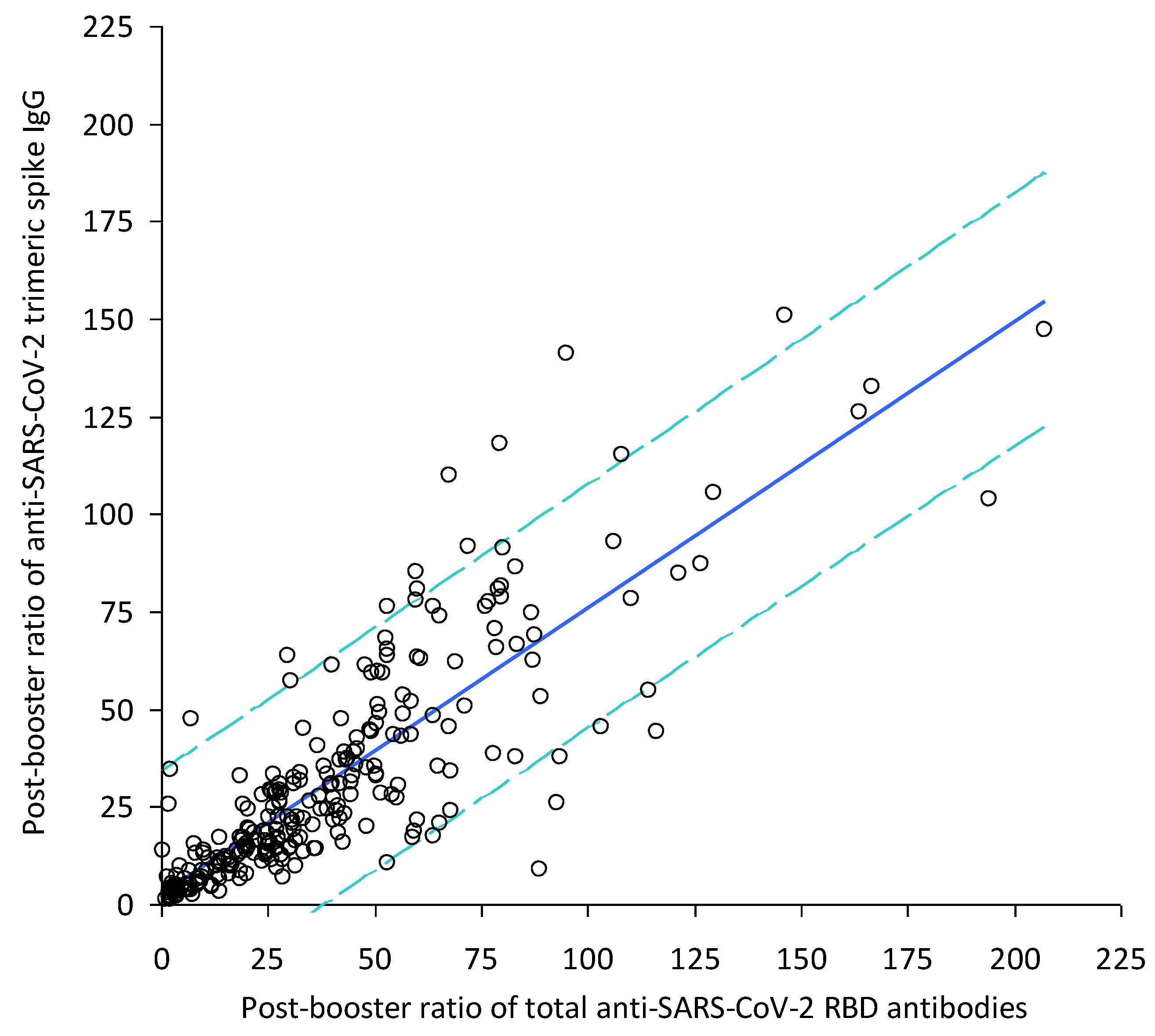
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skrzat-Klapaczyńska, A.; Bieńkowski, C.; Kowalska, J.; Paciorek, M.; Puła, J.; Krogulec, D.; Stengiel, J.; Pawełczyk, A.; Perlejewski, K.; Osuch, S.; et al. The Beneficial Effect of the COVID-19 Vaccine Booster Dose among Healthcare Workers in an Infectious Diseases Center. Vaccines 2022, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Riester, E.; Findeisen, P.; Hegel, J.K.; Kabesch, M.; Ambrosch, A.; Rank, C.M.; Pessl, F.; Laengin, T.; Niederhauser, C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J. Virol. Methods 2021, 297, 114271. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Blocki, F.A.; Bunnell, T.; Chu, E.; De La, O.A.; Grenache, D.G.; Marzucchi, G.; Montomoli, E.; Okoye, L.; Pallavicini, L.; et al. Evaluation of the automated LIAISON® SARS-CoV-2 TrimericS IgG assay for the detection of circulating antibodies. Clin. Chem. Lab. Med. 2021, 59, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvagno, G.L.; Lippi, G. Correlation between Anti-SARS-CoV-2 Total Antibodies and Spike Trimeric IgG after BNT162b2 Booster Immunization. Vaccines 2022, 10, 890. https://doi.org/10.3390/vaccines10060890
Salvagno GL, Lippi G. Correlation between Anti-SARS-CoV-2 Total Antibodies and Spike Trimeric IgG after BNT162b2 Booster Immunization. Vaccines. 2022; 10(6):890. https://doi.org/10.3390/vaccines10060890
Chicago/Turabian StyleSalvagno, Gian Luca, and Giuseppe Lippi. 2022. "Correlation between Anti-SARS-CoV-2 Total Antibodies and Spike Trimeric IgG after BNT162b2 Booster Immunization" Vaccines 10, no. 6: 890. https://doi.org/10.3390/vaccines10060890
APA StyleSalvagno, G. L., & Lippi, G. (2022). Correlation between Anti-SARS-CoV-2 Total Antibodies and Spike Trimeric IgG after BNT162b2 Booster Immunization. Vaccines, 10(6), 890. https://doi.org/10.3390/vaccines10060890






