Pulmonary Embolism after Moderna Vaccination in Kidney Transplant Patients: Two Case Reports and Literature Review
Abstract
1. Introduction
2. Detailed Case Description
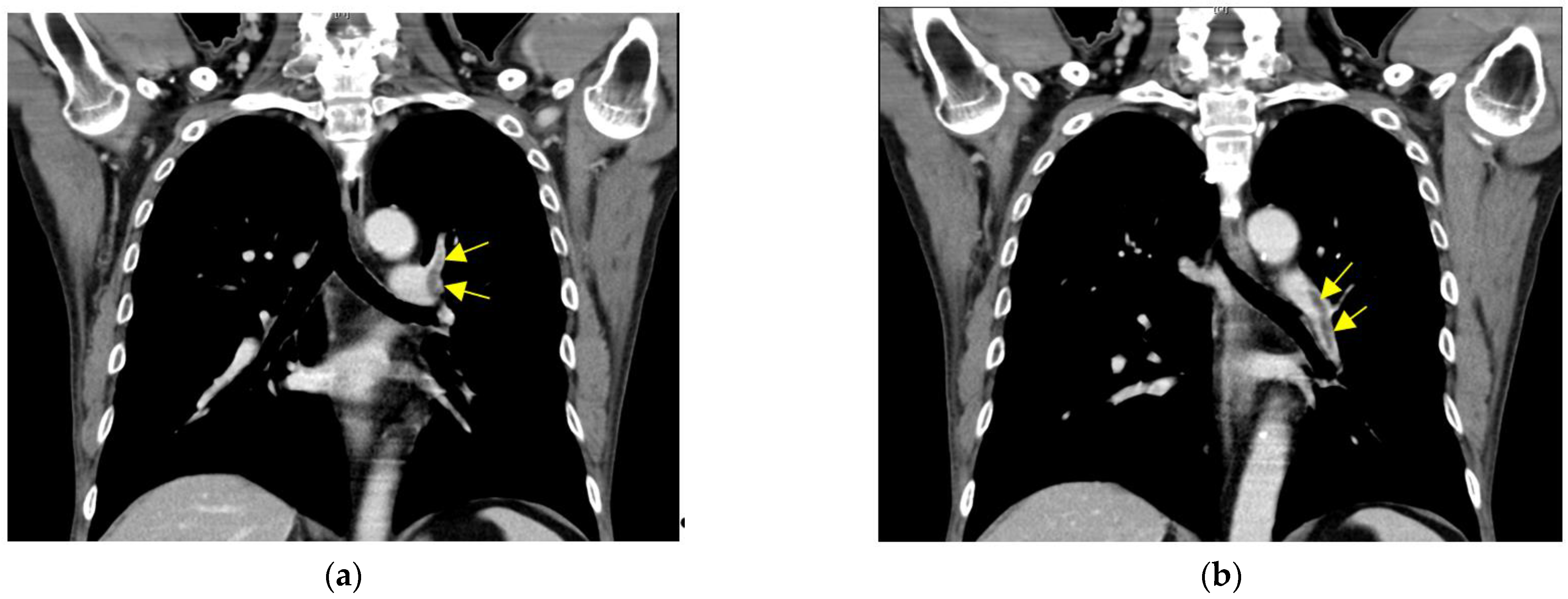
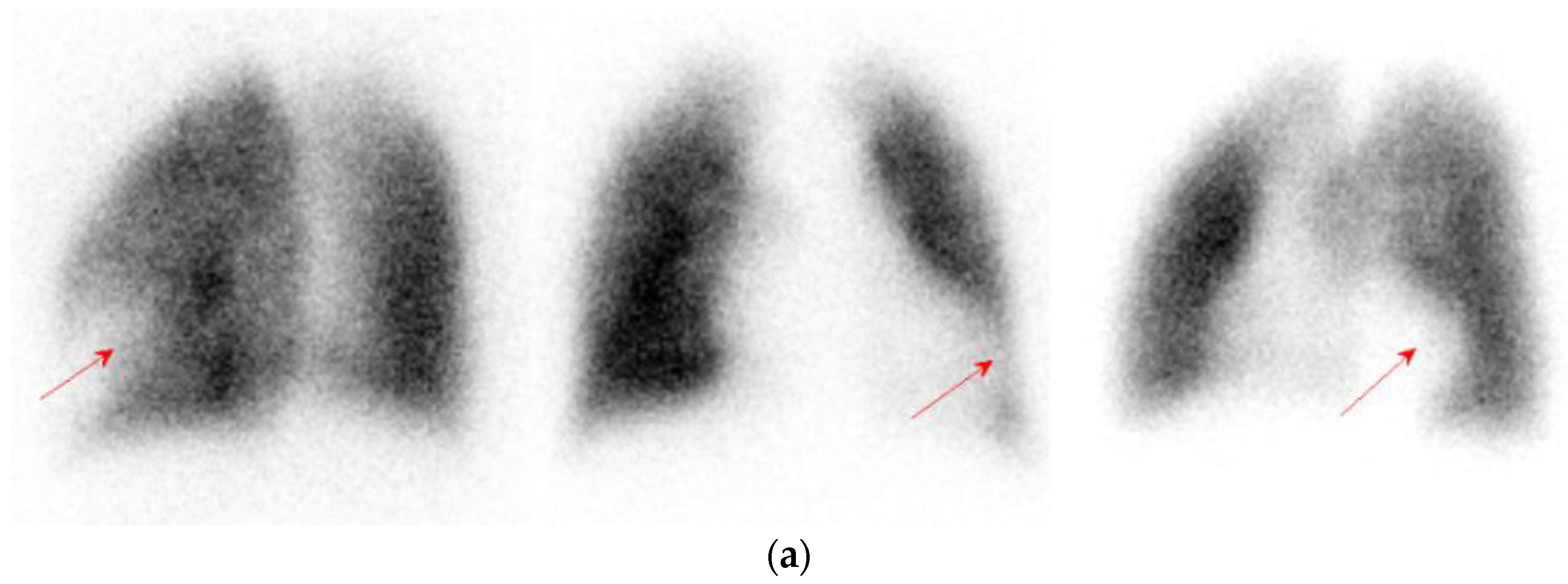
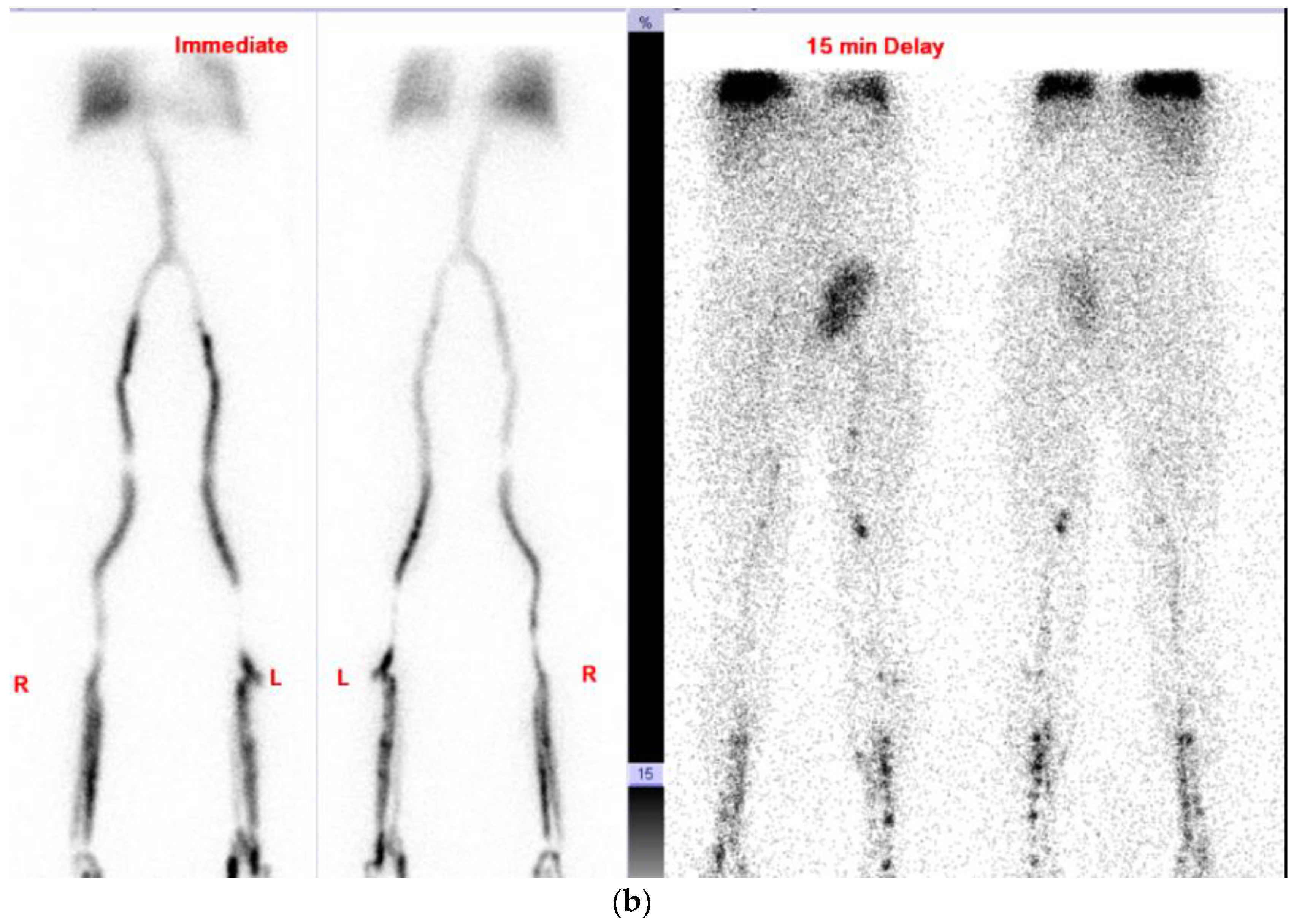
2.1. Case 1
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention: COVID-19 Vaccines for Moderately or Severely Immunocompromised People. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 12 April 2022).
- Cines, B.; James, B. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, M.G.; Murrone, A.; De Luca, L.; Roncon, L.; Di Lenarda, A.; Valente, S.; Caldarola, P.; Riccio, C.; Oliva, F.; Gulizia, M.M.; et al. COVID-19, Vaccines, and Thrombotic Events: A Narrative Review. J. Clin. Med. 2022, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. SARS-CoV-2 mRNA vaccines and the possible mechanism of vaccine-induced immune thrombotic thrombocytopenia (VITT). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e932899-1. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef] [PubMed]
- Von Hundelshausen, P.; Lorenz, R.; Siess, W.; Weber, C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Targeting pathomechanisms with Bruton tyrosine kinase inhibitors. Thromb. Haemost. 2021, 121, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Shiravi, A.A.; Ardekani, A.; Sheikhbahaei, E.; Heshmat-Ghahdarijani, K. Cardiovascular Complications of SARS-CoV-2 Vaccines: An Overview. Cardiol. Ther. 2021, 11, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, M.; Martinelli, I.; Peyvandi, F. Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: Thrombosis at unusual sites. J. Thromb. Haemost. 2021, 19, 2554–2558. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; MacLure, K.; Elkout, H.; Stewart, D. Thrombotic adverse events reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 vaccines: Comparison of occurrence and clinical outcomes in the EudraVigilance database. Vaccines 2021, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Danovitch, G.M. Handbook of Kidney Transplantation, 6th ed.; Wolter Skluwer: Philadelphia, PA, USA, 2017; pp. 100–163. [Google Scholar]
- Al-Nouri, Z.L.; Reese, J.A.; Terrell, D.R.; Vesely, S.K.; George, J.N. Drug-induced thrombotic microangiopathy: A systematic review of published reports. Blood J. Am. Soc. Hematol. 2015, 125, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.-C.; Raymond, M.-A.; Madore, F.; Fugère, J.-A.; Pâquet, M.; St-Louis, G.; Héber, M.-J. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin–sirolimus combination. Am. J. Transplant. 2004, 4, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Zaltzman, J.S. Mycophenolate mofetil causing deep venous thrombosis in a renal transplant patient with factor V Leiden. Nephrol. Dial. Transplant. 2001, 16, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transpl. 2021, 21, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Ghady, H.; Mounzer, A.; Andrew, B.; Amy, L.; Kelsey, L.; Rachel, T.; Scott, R.; Deborah, K.M.; Melissa, D.C.; Michele, D.S.; et al. Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: The COVICS study. Clin. Infect. Dis 2022. [Google Scholar] [CrossRef]
- Caillard, S.; Chavarot, N.; Bertrand, D.; Kamar, N.; Thaunat, O.; Moal, V.; Masset, C.; Hazzan, M.; Gatault, P.; Sicard, A.; et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021, 100, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.A.; Frishman, W.H. Thrombotic complications of COVID-19 infection: A review. Cardiol. Rev. 2021, 29, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Fiore, J.R.; Ciarallo, M.; Di Stefano, M.; Salvatore Sica, S.; Scarale, M.; D’Errico1, M.; Corallo, F.; Lo Caputo, S.; Margaglione, M.; Santantonio, T. Severe systemic thrombosis in a young COVID-19 patient with a rare homozygous prothrombin G20210A mutation. Le Infez. Med. 2021, 29, 259–262. [Google Scholar]
- Taiwan Centers for Disease Control. Available online: https://www.cdc.gov.tw/Category/Page/9jFXNbCe-sFK9EImRRi2Og (accessed on 17 April 2022).
- Taiwan Food and Drug Administration. Available online: https://www.fda.gov.tw/TC/siteList.aspx?sid=1571 (accessed on 18 April 2022).
- Marion, O.; Del Bello, A.; Abravanel, F.; Couat, C.; Faguer, S.; Esposito, L.; Hebral, A.L.; Izopet, J.; Kamar, N. Safety and immunogenicity of anti–SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann. Intern. Med. 2021, 174, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Olivier Marion, O.; Del Bello, A.; Abravanel, F.; Couat, C.; Faguer, S.; Esposito, L.; Hebral, A.L.; Izopet, J.; Kamar, N. Efficacy and Safety of Third Dose of the COVID-19 Vaccine among Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis. Vaccines 2022, 10, 95. [Google Scholar]

| Variable | Case 1 | Case 2 |
|---|---|---|
| Hemoglobin, g/dL | 14.7 | 16.7 |
| Platelet count, per μL | 260,000 | 239,000 |
| Creatinine, mg/dL | 1.0 | 1.24 |
| [Everolimus] *, ng/mL | / | 6.4 |
| [Sirolimus] *, ng/mL | 6.8 | / |
| [Cyclosporin] *, ng/mL | / | 64.9 |
| Troponin I, ng/mL | / | 0.039 |
| Pro-BNP, pg/mL | / | 7231.51 |
| D-dimer **, mg/L | 3.59 | 1.10 |
| Fibrinogen, mg/dL | 439.5 | 396 |
| Pulmonary embolism | Yes | Yes |
| other thrombosis | DVT *** | No |
| Symptom onset after vaccination, days | 23 (after 2nd dose) | 26 (after 3rd dose) |
| Anticoagulation therapy | Enoxaparin/Endoxaban | Dabigatran |
| Vaccines | AstraZeneca | Moderna | BioNTech |
|---|---|---|---|
| 1st Dose | 6 | 24 | 2 |
| 2nd Dose | 5 | 23 | 3 |
| 3rd Dose | / | 22 | 1 |
| Accumulated Dose | 11 | 69 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, W.-N.; Chen, C.-S.; Ho, D.-R.; Huang, Y.-C.; Lin, J.-H.; Huang, K.-T.; Liu, Y.-L. Pulmonary Embolism after Moderna Vaccination in Kidney Transplant Patients: Two Case Reports and Literature Review. Vaccines 2022, 10, 868. https://doi.org/10.3390/vaccines10060868
Chan W-N, Chen C-S, Ho D-R, Huang Y-C, Lin J-H, Huang K-T, Liu Y-L. Pulmonary Embolism after Moderna Vaccination in Kidney Transplant Patients: Two Case Reports and Literature Review. Vaccines. 2022; 10(6):868. https://doi.org/10.3390/vaccines10060868
Chicago/Turabian StyleChan, Wai-Nga, Chih-Shou Chen, Dong-Ru Ho, Yun-Ching Huang, Jian-Hui Lin, Kuo-Tsai Huang, and Yu-Liang Liu. 2022. "Pulmonary Embolism after Moderna Vaccination in Kidney Transplant Patients: Two Case Reports and Literature Review" Vaccines 10, no. 6: 868. https://doi.org/10.3390/vaccines10060868
APA StyleChan, W.-N., Chen, C.-S., Ho, D.-R., Huang, Y.-C., Lin, J.-H., Huang, K.-T., & Liu, Y.-L. (2022). Pulmonary Embolism after Moderna Vaccination in Kidney Transplant Patients: Two Case Reports and Literature Review. Vaccines, 10(6), 868. https://doi.org/10.3390/vaccines10060868






