Abstract
Mass vaccination is the most effective strategy against the spread of the COVID-19 pandemic. However, concerns about the vaccine’s safety and effectiveness remain a huge obstacle to vaccine acceptance. The aim of the present study was to explore different COVID-19 vaccine outcomes, including the development of adverse events and/or COVID-19 infection following COVID-19 vaccination. A cross-sectional study was conducted by distributing an online survey targeting staff and students at the British university in Egypt. A total of 637 participants fully completed the survey. Of these, 609 (95.6%) participants received the COVID-19 vaccine. Only 12.6% of the total vaccinated participants reported COVID-19 infection after vaccination. Of these, only 2.8% reported having severe symptoms while 9.9% reported having no or mild symptoms. The most common side effects reported after the first vs. second dose were headache (36.3% vs. 14.6%), tiredness and fatigue (26.9% vs. 10.7), and fever (25.6% vs. 6.7%). In conclusion, the present study explored different COVID-19 vaccine outcomes where the overall incidence of side effects is higher after the first dose than after the second dose. There is a relationship between COVID-19 vaccines’ side effects and gastrointestinal disorders, gender, and the type of COVID-19 vaccine. Post-vaccination symptoms were more frequently reported in women compared to men and more frequent with viral vector vaccines compared to other types. The effectiveness of different types of COVID-19 vaccines was confirmed by the lower incidence rate of post-vaccination COVID-19 infection.
1. Introduction
COVID-19 disease is one of the leading causes of death in the world [1]. Because this disease is contagious and has been anticipated to have a high fatality rate, disruptions and dangers to daily life are severe [2]. However, a number of preventive measures, including social distancing, quarantining, face-covering, ventilation of indoor spaces, hygienic practices, thorough screening tests, and execution of government legislation, have lessened the health impact [3]. The most reliable means to ensure control over this pandemic is to develop effective vaccines to control its spread [4]. Different types of COVID-19 vaccines have been approved and are being used all over the world, including those of Pfizer BioNTech, AstraZeneca, Moderna, Johnson & Johnson, Sinopharm, Sinovac, Sputnik V, and COVAXIN Vaccines [5,6]. Between December 2020 and January 2022, Egypt has received around 60 million vaccine doses from different countries within the national COVID-19 immunisation program. By the end of 2021, more than 55 million vaccine doses had been administered, assuming that each person requires two doses, which would be enough to vaccinate approximately 35.5% of the country’s population [7].
Due to the urgency of the pandemic, the quick development of COVID-19 vaccines and technological advancements have led to various rumours [8], which cause vaccine hesitancy. Also, it is believed that the population’s decision on refusing or delaying vaccination is mainly due to a lack of knowledge about the relative benefit-to-risk ratio [9,10,11].
Various post-vaccination side effects have been reported and believed to resolve within a few days, which include injection site pain or redness or swelling, fatigue, headache, chills, fever, muscle and joint pain, nausea, and swollen lymph nodes [9,10]. Vaccine reactions and side effects are distinct from one another in terms of physical symptoms. Redness, swelling, or discomfort at the injection site, as well as systemic symptoms such as fever or myalgia, can all be signs of reactogenicity, which is produced by an excessive inflammatory response to vaccination. In addition, females subjected to COVID-19 vaccines may experience menstrual abnormalities [12]. The occurrence of side effects is considered one of the main causes of vaccine hesitancy among people, which represents a huge obstacle to the success of vaccination strategies [13].
During the COVID-19 pandemic, mental distress may result in vaccination avoidance and refusal [14]. People who received vaccinations reported an improvement in social skills, which may be related to better mental health. However, a number of psychological variables (such as anxiety) are thought to play a role in the occurrence and severity of vaccine-related adverse effects [15]. The aim of the present study was to determine the short-term physical and psychological side effects of different COVID-19 vaccines reported by workers and students at the British University in Egypt, in addition to the incidence of post-vaccination COVID-19 infection.
2. Materials & Methods
2.1. Study Design and Setting
An anonymous online cross-sectional survey was designed on Survey Monkey and a link was sent to participants via institutional email. It was carried out from 1 January 2022 to 31 March 2022, among administrative staff, teaching staff, researchers, university employees, and students at the British University in Egypt.
2.2. Study Sample, Sampling, and Sample Size Calculation
The eligible study population comprised the entire university population (about 11,000 people), including administrative, teaching, and research staff, researchers, university employees, and students. The sample size was calculated by using the online sample size calculator RaoSoft®. Based on an estimated population of 11,000 workers and students and on an anticipated response of 50%, the minimum required sample size was 372 participants with a confidence level of 95% and a 5% margin of error.
2.3. Ethical Considerations
In accordance with the institution’s established standards, all ethical criteria were followed. Anonymity was ensured by not collecting any personal data (such as names or other identifiers). During the research process, all of the necessary ethical considerations were taken into account. All of the participants were fully informed of the study’s purpose and scope before they agreed to participate in the survey. A notification on the Survey Monkey platform informed the participants of the estimated completion time (5 min). To prevent anyone from taking the survey more than once, we recorded each respondent’s IP address, and no more than one response was allowed per IP address (ensured by the Survey Monkey response feature). Prior to participating, all participants were given assurances of complete anonymity and confidentiality. As a final reminder, all participants were informed that their participation in the survey was entirely voluntary.
2.4. Data Collection
Pre-validated questionnaires [16,17] were modified after a comprehensive literature search. The initial draft was sent to a group of experts, chosen according to their experience and expertise in related fields, to appraise the questions in terms of relativity, simplicity, and importance. A pilot study was conducted on 10 subjects, to test the questionnaire’s validity. Following a group discussion, the questionnaire was completed. The data from the pilot study was removed from the final analysis. The questionnaire was composed of two main categories. The first section covered the background data such as gender, level of education, age, and any previously reported COVID-19 infection. The second part of the questionnaire was designed to collect information on data related to COVID-19 vaccination status. Questions were targeted at identifying the type of vaccine received, the number of doses, side effects, and onset time (i.e., after the first or second dose). A list of side effects was provided to participants to choose from, which included flu-like symptoms, pain at the site of injection, dyspnoea, headache, and tachycardia, in addition to mentioning if they experienced other side effects not listed. Precautionary actions were taken to protect privacy and freedom of terminating the survey at any time during the study. In the pilot sample, the knowledge questionnaire’s Cronbach’s alpha coefficient was 0.84, indicating very reliable internal consistency [18].
Moreover, participants were asked about their self-reported mental suffering after vaccination, as indicated by the four-item Patient Health Questionnaire (PHQ-4). The four-item Patient Health Questionnaire is used to assess mental distress (PHQ-4) [19]. Two questions assess depressive symptoms, while the other two assess anxious symptoms. Each item is rated on a scale of 0 to 4, and the responses are added up to form an index ranging from 0 to 16, with higher values indicating greater degrees of mental discomfort. Earlier research has established the PHQ-4′s validity and reliability [20].
2.5. Statistical Analysis
All statistical tests were executed by the Statistical Package for the Social Sciences (SPSS) version 23.0 (SPSS Inc., Chicago, IL, USA, 2020). The normality of data was evaluated using the Shapiro–Wilk test with a significance level p-value < 0.05. In the pilot study, Cronbach’s alpha coefficient test was used to measure internal consistency. Primarily, descriptive statistics were used to present and summarise the categorical variables like gender, age categories, profession, work experience, region, medical anamneses, COVID-19-related anamneses, and individual side effects by frequency (n) and percentage (%). A Chi-squared test (χ2) was used to estimate the association between different demographic and medical characteristics and different types of COVID-19 vaccines.
Binary logistic regression analysis was carried out to find the odds ratio for the occurrence of post-vaccination symptoms. All inferential tests were carried out assuming a confidence level (CI) of 95% and a p-value of < 0.05.
3. Results
3.1. Demographic and Clinical Characteristics of the Participants
A total of 637 participants fully completed the survey. Of these, 609 (95.6%) participants received the COVID-19 vaccine, which indicates a high vaccination rate. The most common reasons for receiving the vaccine were self and others’ protection and vaccination being required for work/university/other activities. On the other hand, the most commonly reported reasons for not receiving the vaccine were having concerns about vaccine safety, having recently recovered from COVID-19, and difficulty scheduling or getting an appointment. More than half of the non-vaccinated participants, however, reported that they planned to receive the vaccine.
Different types of vaccines were received by the vaccinated participants where 307 (50.4%) participants received inactivated vaccine (37.8% Sinovac and 12.6% Sinopharm), 240 (39.4%) participants received viral vector vaccine (27.6% AstraZeneca/Oxford, 10.8% Johnson & Johnson and 1% Sputnik V), and 62 (10.2%) participants received mRNA vaccine (9% Pfizer-BioNTech and 1.1% Moderna). A total of 609 vaccinated participants were included in the downstream analyses where 395 (64.9%) were females and 214 (35.1%) were males; 447 (73.4%) were aged between 18 to 29 years and 140 (22.9%) were aged between 30 to 59 years; the educational level of 365 (59.9%) was undergraduate, 88 (14.4%) had a bachelor’s degree and 156 (25.6%) had a postgraduate degree; 389 (63.9%) were students, 151 (24.8%) were academic staff and 69 (11.3%) were administrative and support staff; 562 (92.3%) were residents of Cairo and 47 (7.7%) were from other governorates, 109 (17.9%) were smokers, 132 (21.7%) received a flu shot this year, 68 (11.2%) reported suffering from an allergy to some types of foods or medicines, 266 (43.7%) reported at least one non-communicable disease, and 114 (18.7%) reported taking medications. There was a significant association between the type of vaccine received and the participants’ age (p-value < 0.001), educational level (p-value < 0.001), employment status (p-value < 0.001), and place of residence (p-value = 0.01) (Table 1).

Table 1.
Demographic and clinical characteristics of the participants according to type of vaccine (n = 609).
3.2. COVID-19-Related Anamneses
The majority of vaccinated participants (82.1%) received two doses. In addition, 2.1% of the participants received more than two doses (booster doses). Two hundred and fourteen participants (35.1%) reported having a previous COVID-19 infection before vaccination, compared to only 77 (12.6%) who reported having COVID-19 infection after vaccination. Of those who reported post-vaccination COVID-19 infection, only 17 (2.8%) reported having severe symptoms while 60 (9.9%) reported having no or mild symptoms, suggesting significant vaccine effectiveness. Regarding symptoms following vaccination, 379 participants (62.2%) reported having symptoms, with the majority being mild to moderate symptoms. Almost half of the participants reported an onset of symptoms within 12 h and a duration ranging from less than a day up to 3 days. The majority of the participants’ responses to relieving the vaccination symptoms were resting at home and taking painkillers. There were significant associations between the type of vaccine received and the number of vaccine doses received (p-value < 0.001), occurrence of symptoms (p-value < 0.001), onset of symptoms (p-value < 0.001), duration of symptoms (p-value = 0.046) as well as response to post-vaccination symptoms (p-value < 0.001) (Table 2).

Table 2.
COVID-19-related anamneses of the participants according to type of vaccine (n = 609).
3.3. Safety and Effectiveness of COVID-19 Vaccines
3.3.1. Reported COVID-19 Vaccines Side Effects after First and Second Dose
The most common side effects reported after the first and second dose were headache (36.3% vs. 14.6%), tiredness and fatigue (26.9% vs. 10.7%), fever (25.6% vs. 6.7%), pain or swelling at the injection site (22.5% vs. 11%), muscle pain (21.7% vs. 8.9%), excessive sleepiness/laziness (21.3% vs. 9.5%), and dizziness (17.4% vs. 6.7%), which indicates lower incidence rates of side effects following the second dose compared to the first dose (Figure 1). Interestingly, menstrual problems in females were reported in 42 (10.6% of the female participants) and 29 (7.3% of the female participants) after the first and second dose, respectively. In addition, a case of male impotence following a Johnson & Johnson single dose was reported. Also, three cases of thrombocytopenia (two with AstraZeneca/Oxford vaccine and one with Sinovac vaccine) and one case of thrombosis with AstraZeneca/Oxford vaccine were reported after the second dose. Regarding side effects following the first dose, there was a significant association between the type of vaccine received and the following side effects: sore or dry throat (p-value = 0.003), chills and shiver (p-value < 0.001), clogged nose (p-value = 0.014), cold, numbness and tingling in limbs (p-value < 0.001), cough (p-value = 0.001), decreased sleep quality (p-value < 0.001), diarrhoea (p-value = 0.003), faster or irregular heartbeats (p-value = 0.014), runny nose (p-value < 0.001), dizziness (p-value < 0.001), fever (p-value < 0.001), haziness or lack-of-clarity in the eyesight (p-value = 0.001), headache (p-value < 0.001), joints pain (p-value < 0.001), muscle pain (p-value < 0.001), nausea (p-value < 0.001), over sleepiness or laziness (p-value < 0.001), pain or swelling at the injection site (p-value < 0.001), tiredness and fatigue (p-value < 0.001) as well as body sweating for no reason (p-value < 0.001). All of these side effects were most common with viral vector vaccines, especially the AstraZeneca/Oxford vaccine.
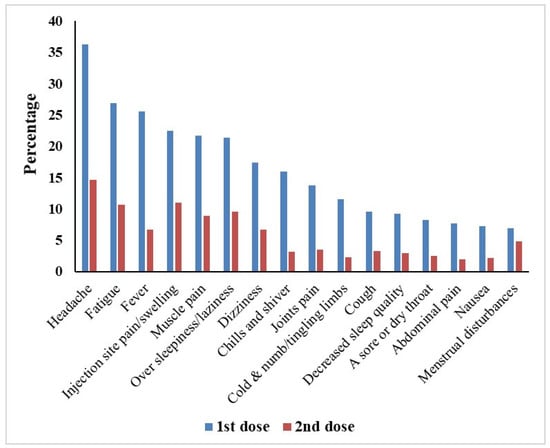
Figure 1.
Most common COVID-19 vaccine side effects reported by the participants after the first and second vaccine dose (n = 609).
On the other hand, regarding side effects following the second dose, there was a significant association between the type of vaccine received and diarrhoea (p-value = 0.002), dizziness (p-value = 0.021), fever (p -value = 0.005), headache (p-value < 0.001), muscle pain (p-value < 0.001), over sleepiness or laziness (p-value = 0.025), pain or swelling at the injection site (p-value < 0.001), tiredness and fatigue (p-value = 0.027), vomiting (p-value = 0.026) as well as menstrual disturbance in females (p-value = 0.039). The detailed incidence rates of the various COVID-19 vaccine-associated side effects stratified by the type of COVID-19 vaccine received are shown in (Table 3 and Table 4).

Table 3.
COVID-19 vaccine side effects reported by the participants after the first dose of different vaccine types (n = 609).

Table 4.
COVID-19 vaccine side effects reported by the participants after the second dose of different vaccine types (n = 609).
Moreover, the present study reported that the occurrence of side effects was higher after the first dose than after the second dose. The mean of the ratio between the occurrence of side effects between the first dose and the second dose for all the side effects was 3.3 ± 0.2 (95% confidence interval [CI], 2.8 to 3.7). Interestingly, it was observed that the ratio of the occurrence of menstrual disturbance, particularly as a side effect, was 1.4 between the first and second dose, which differs significantly from the ratio between all the other side effects.
3.3.2. Factors Associated with Post-Vaccination Symptoms and COVID-19 Infection
The association of post-vaccination symptoms and COVID-19 infection with various demographic and clinical characteristics was assessed as shown in Table 5. Regarding post-vaccination symptoms, there was a significant association between the development of symptoms and gender (p-value < 0.001), number of vaccine doses (p-value = 0.011), and type of vaccine received (p-value < 0.001) where symptoms were more likely to occur in females, those who received a single dose of the vaccine, and those who received viral vector vaccines, especially the AstraZeneca/Oxford vaccine. Regarding post-vaccination COVID-19 infection, there was a significant association between COVID-19 infection after vaccination and previous COVID-19 infection before vaccination (p-value = 0.005) where COVID-19 infection after vaccination was more likely to occur in those who had previous COVID-19 infection before vaccination. Interestingly, the incidence of COVID-19 infection after vaccination was significantly lower and there was no significant difference among vaccine types in terms of post-vaccination COVID-19 infection, suggesting a similar level of protection against COVID-19 infection.

Table 5.
Factors associated with post-vaccination symptoms and COVID-19 infection among the participants (n = 609).
3.3.3. Risk Factors for Occurrence of Symptoms after of Vaccination
A binary logistic regression analysis was performed where the occurrence of symptoms after vaccination was used as the dependent variable and different patients’ characteristics as prognostic variables. There was a significant association between the occurrence of symptoms after vaccination and gastrointestinal disorders, gender, and type of vaccine. The risk of the occurrence of symptoms after vaccination increased by ~4.5 times in patients suffering from gastrointestinal disorders (95% confidence interval [CI], 1.274 to 16.218). For males, the occurrence of symptoms after vaccination decreased by 0.3 times more than for females (95% confidence interval [CI], 0.237 to 0.506). Moreover, for participants who received Johnson & Johnson vaccine and AstraZeneca, the occurrence of post-vaccination symptoms increased by 5.9 and 5.1 times more than for participants who received Sinovac (95% confidence interval [CI], 2.852 to 12.473; 3.159 to 8.376 respectively) (Table 6).

Table 6.
Odds Ratio for the occurrence of post-vaccination symptoms.
3.4. Mental Health Symptoms and Association with COVID-19 Vaccination
The PHQ-4 was used to determine whether or not participants had suffered from mental discomfort. As shown in Table 7, there is no statistical variation in the mental health status across COVID-19 vaccine types (p-value > 0.05) and the mean of the subjects’ PHQ-4 scores was 2.1 ± 3.1. Approximately 16.1% of the sample fulfilled the PHQ-4 criteria for mild mental distress, 9.2% for moderate mental distress, and 6% for severe mental distress. Additionally, and as shown by the PHQ-4 subscales, the mean of the individuals’ anxiety ratings was 0.8 ± 1.5 and the mean of the depression score was 1.2 ± 1.7. A score of three or above on each subscale of anxiety and depression is deemed positive for screening purposes; however, the individuals’ scores were within the normal range, suggesting neither anxiety nor depression. PHQ-4 scores did not differ significantly by the type of vaccine.

Table 7.
Mental health symptoms and association with COVID-19 vaccination receipt among participants (n = 609).
4. Discussion
4.1. Principal Findings and Previous Studies
In the present study, our research group studied the short-term side effects following the administration of different types of COVID-19 vaccines administered in Egypt. A total of 637 participants from the British University in Egypt fully completed the survey. Of these, 609 (95.6%) participants received COVID-19 and 28 participants did not receive the vaccine due to negative safety thoughts. More than half of the non-vaccinated participants, however, reported that they plan to receive the vaccine, which further indicates the increasing willingness to receive vaccination. Females represented a total of 67.9% of the unvaccinated participants. In a study that investigated COVID-19 vaccine hesitancy and related fears and anxiety, males reported higher willingness than females on average, but the gender difference was not significant [21].
It was obvious in our study that the overall incidence of side effects is higher after the first dose than after the second dose. Headache (36.3% vs. 14.6%), tiredness and fatigue (26.9% vs. 10.7), fever (25.6% vs. 6.7%), pain or swelling at the injection site (22.5% vs. 11%), muscle pain (21.7% vs. 8.9%), over sleepiness/laziness (21.3% vs. 9.5%), and dizziness (17.4% vs. 6.7%) were the most common side effects reported after the first vs. second dose. That was in harmony with a previous study that confirmed that the side effects were most common after the first dose [22]. The occurrence of side effects after the first dose was almost three times higher than after the second dose. That was confirmed by a previous study that reported that the side-effect severity was greater after the first dose of Sinopharm and AstraZeneca than after the second dose, but in contrast, the side-effect severity was greater after the second dose of the Pfizer vaccine than after the first dose [23]. On the other hand, another study showed a significant increase in the number of subjects who were suffering side effects after receiving the second dose of the vaccine compared to those who reported side effects after the first dose [24].
Speaking precisely, menstrual problems in females were reported in 42 participants (10.6% of the female participants) and 29 participants (7.3% of the female participants) after the first and second dose, respectively. This finding is similar to previous studies that showed that approximately 5% of females of reproductive age reported menstrual abnormalities [25]. Menstrual abnormalities were reported to be short-term and ranged from cycle and menstrual length changes to differences in menstrual associated symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding [26]. This may be attributed to immune activation and the inflammatory response [27,28]. Also, it was observed that the occurrence of menstrual disturbance was higher after the first dose than the second dose by 1.4 times. Although a previous study confirmed that the occurrence of menstrual irregularities was higher after the second dose, those abnormalities were found to self-resolve within two months [29]. Moreover, the present study reported that the ratio between the occurrence of menstrual disturbance after the first and second dose is significantly lower than the ratio between all the other side effects after the first and second dose.
With respect to the vaccine type related side effects, we reported a significant association between the type of the vaccine and some of the side effects after the first dose, such as abdominal pain, chills, decreased sleep quality, faster or irregular heartbeats, fever, haziness or lack-of-clarity in your eyes, headache, joint pain, muscle pain, nausea, over sleepiness or laziness, pain or swelling at the injection site, tiredness and fatigue, and body sweating. The highest side effect incidence was reported after the AstraZeneca vaccine. Similar findings were reported in a prospective observational study conducted in the UK. Adverse events were considerably greater in people who received one dosage of the AstraZeneca vaccine when comparing systemic effects following one dose of each vaccination [30].
Following the second dose, three female cases of thrombocytopenia (two with AstraZeneca/Oxford vaccine and one with Sinovac vaccine) and one case of thrombosis with AstraZeneca/Oxford vaccine were reported. These findings are highly consistent with the results reported in phase III clinical trials and vaccine fact sheets, and they are mostly reported for those who received the second dose [31,32]. Also, there was a significant association between the type of vaccine received and headache as well as muscle pain after the second dose administration, which were more common with Moderna and Sputnik V vaccines, respectively. Similar findings were reported by others who found that mRNA vaccines have a higher incidence of second dose side effects (Moderna vs. Pfizer) [33].
The majority of the side effects were mild to moderate and usually resolved within a few days of vaccination, as reported in many trials [24,31,32,33]. In the current study, most of the participants reported mild to moderate symptoms, which is concomitant with the results of Elgendy et al.’s study, as most of the participants felt mild or no symptoms after vaccination [23]. Almost half of the participants indicated that their symptoms began within 12 h and lasted from less than a day to three days. Also, most of the participants in the previously mentioned Egyptian study reported that the side effects appeared on the first day of the Sinopharm, AstraZeneca, and Pfizer vaccines and endured no more than three days [23].
There was an association between post-vaccination symptoms and the type of vaccine. Therefore, a binary logistic regression analysis was carried out to find out the risk factors for these post-vaccination symptoms. Being female, suffering from gastrointestinal diseases, and receiving either AstraZeneca or Johnson & Johnson increases the risk of the occurrence of post-vaccination symptoms. Furthermore, as shown in our results, there was a significant association between the different side effects such as abdominal pain, chills and shivers, decreased sleep quality, faster or irregular heartbeats, dyspnoea, fever, haziness or lack-of-clarity in your eyes, headache, joint pain, muscle pain, nausea, oversleeping or laziness, pain or swelling at the injection site, tiredness and fatigue, and body sweating for no reason and receiving the viral vector vaccines, especially the AstraZeneca/Oxford vaccine. Interestingly, previous studies reported that the viral vector-based vaccine was associated with more frequent systemic side effects [5,6,34].
There was a significant association between post-vaccination symptom development and gender. Around seventy-three percent of the participants who had post-vaccination symptoms were females, whereas 27.2% were males. That could explain the high hesitancy of females in receiving the vaccine. Regardless of whether vaccinated women had much greater rates of adverse effects, it was still very low for both genders. In the limited trials that have been conducted, the reduced propensity to immunise females did not appear to translate into lower vaccination rates [35,36]. Moreover, there was a significant association between gender, the number of vaccine doses, and the type of vaccine received, with symptoms occurring more frequently in females, those who received a single dose of the vaccine, and those who received viral vector vaccines, particularly the AstraZeneca/Oxford vaccine. Conspicuously, the thrombosis and thrombocytopenia side effects were reported with the AstraZeneca vaccine. Yet, there was no causal relationship discovered to relate incidents of thrombotic adverse effects to the AstraZeneca vaccination [37]. A previous COVID 19 infection before vaccination was significantly associated with post-COVID-19 infection, as infection was more likely to occur in those who had previous COVID-19 infection before vaccination. This could be explained by the fact that these participants were more vulnerable to infection. Controversially, a previous study reported that the naturally infected populations were less likely to be re-infected by COVID-19 than infection-free and vaccinated individuals. Although re-infected people did not develop severe disease, a significant percentage of naturally infected or vaccinated people were re-infected by the emerging variants [38]. More research is required to address this issue.
In order to study the effectiveness of COVID-19 vaccines in terms of post-vaccination infection in the current study, there was no significant difference among vaccine types in terms of post-vaccination COVID-19 infection, implying a similar level of protection against COVID-19 infection. A previous study reported that COVID-19 inactivated vaccines, adenovirus-vectored vaccines, and mRNA vaccines’ have 60%, 65%, and 90% efficacy respectively against asymptomatic, symptomatic COVID-19 infection, COVID-19 hospitalization, severe or critical hospitalization, as well as mortality [39]. Meanwhile, this outcome needs more studies to be confirmed. More research is needed to understand the vaccination profiles for immunocompromised patients, organ transplant recipients, and other comorbidities [40].
Because the COVID-19 vaccination influences mental health both directly and indirectly [41], we investigated the effect of the COVID-19 vaccine on mental health among the study participants. We claim that vaccination type is not related to the level of mental distress experienced by patients. Aside from that, many participants were afraid of becoming infected and subsequent death during the pandemic. In addition, feelings of security and decreased mortality were a result of COVID-19 vaccination for those who were vaccinated. Our findings are consistent with a previous study which reported that individuals experienced low levels of discomfort following their initial dose of the vaccine [42].
4.2. Strengths and Limitations
The current study’s diversity in participants’ age groups, educational backgrounds, and clinical characteristics allowed the assessment of the association between different sociodemographic characteristics and safety as well as the effectiveness of different types of COVID-19 vaccines. Also, the comparison of side effects of seven COVID-19 vaccines of different types including inactivated, viral vector, and mRNA vaccines adds significant value to the present study. Furthermore, differences in mental health status across types of COVID-19 vaccines were studied in the Egyptian population for the first time. The main limitations of the present study are related to self-reported data and information about COVID-19 vaccine side effects. In addition, the present study monitored short-term post-vaccination side effects; therefore, future studies of longer periods and a larger number of participants need to be carried out to assess the long-term side effects of different COVID-19 vaccines and to assess the post-marketing surveillance of COVID-19 vaccines.
5. Conclusions
The present study explored different COVID-19 vaccine outcomes, including the development of adverse events and/or COVID-19 infection following COVID-19 vaccination. The overall incidence of side effects is higher after the first dose than after the second dose. Headache, tiredness and fatigue, fever, pain or swelling at the injection site, muscle pain, excessive sleepiness or laziness, and dizziness, as well as the occurrence of menstrual problems in females after COVID-19 vaccination, were the most common side effects reported after the first and second doses. COVID-19 vaccination is not related to the level of mental distress among the participants. Moreover, the present study reported the relationship between COVID-19 vaccines’ side effects and gastrointestinal disorders, gender, and the type of COVID-19 vaccine. In addition, it reported that the post-vaccination symptoms are more frequently reported in women compared to men and more frequent with viral vector vaccines compared to other types. The present study revealed the effectiveness of different vaccine types administered in Egypt based on the significantly low post-vaccination COVID-19 infection reported. We believe that our data could be of value to helping governmental organisations in Egypt with the likelihood of different post-vaccination side effects on the basis of age, sex, occupation, chronic diseases, and the type of vaccine being administered.
Author Contributions
Conceptualization, M.S.H., O.A.B. and M.M.E.; Data curation, M.S.H., R.T., N.S.E.B., M.S., A.S.E., O.A.B. and M.M.E.; Formal analysis, M.S.H., R.T., N.S.E.B., M.S., A.S.E., O.A.B. and M.M.E.; Methodology, M.S.H., R.T., N.S.E.B., M.S., A.S.E., O.A.B. and M.M.E.; Supervision, M.S.H., O.A.B. and M.M.E.; Writing—original draft, M.S.H., R.T., N.S.E.B., M.S., A.S.E., O.A.B. and M.M.E.; Writing—review & editing, M.S.H., R.T., N.S.E.B., M.S., A.S.E., O.A.B. and M.M.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Committee of Ethics at the Faculty of Pharmacy in the British University in Egypt approved the conduction of the present study according to its guidelines (Ref: CL-2203).
Informed Consent Statement
Each participant provided a digital informed consent with no personal information provided to protect against any retrospective identification of the participants.
Data Availability Statement
Not applicable.
Acknowledgments
The researchers acknowledge the staff, workers and students at the British University in Egypt who took part in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verikios, G. The dynamic effects of infectious disease outbreaks: The case of pandemic influenza and human coronavirus. Socio-Economic Plan. Sci. 2020, 71, 100898. [Google Scholar] [CrossRef] [PubMed]
- Rume, T.; Islam, S.M.D.-U. Environmental effects of COVID-19 pandemic and potential strategies of sustainability. Heliyon 2020, 6, e04965. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-19) Advice for the Public; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 20 April 2022).
- Hale, T.; Angrist, N. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Egypt Receives Second Shipment of 1.77 Million COVID-19 Vaccines through the COVAX Facility; World Health Organization: Geneva, Switzerland, 2022; Available online: http://www.emro.who.int/media/news/egypt-receives-second-shipment-of-177-million-covid-19-vaccines-through-the-covax-facility.html (accessed on 27 March 2022).
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef]
- Reuters. Daily Reported Trends; Reuters: London, UK, 2022; Available online: https://graphics.reuters.com/world-coronavirus-tracker-and-maps/countries-and-territories/egypt/ (accessed on 27 March 2022).
- Ball, P. The lightning-fast quest for COVID vaccines-and what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef]
- Davis, T.C.; Fredrickson, D.D.; Arnold, C.L.; Cross, J.T.; Humiston, S.G.; Green, K.W.; Bocchini, J.A. Childhood vaccine risk/benefit communication in private practice office settings: A national survey. Pediatrics 2001, 107, e17. [Google Scholar] [CrossRef] [Green Version]
- Gust, D.; Woodruff, R.; Kennedy, A.; Brown, C.; Sheedy, K.; Hibbs, B. Parental perceptions surrounding risks and benefits of immunization. Semin. Pediatr. Infect. Dis. 2003, 14, 207–212. [Google Scholar] [CrossRef]
- Bond, L.; Nolan, T.; Pattison, P.; Carlin, J. Vaccine preventable diseases and immunisations: A qualitative study of mothers’ perceptions of severity, susceptibility, benefits and barriers. Aust. N. Z. J. Public Health 1998, 22, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Muhaidat, N.; Alshrouf, M.A.; Azzam, M.I.; Karam, A.M.; Al-Nazer, M.W.; Al-Ani, A. Menstrual Symptoms After COVID-19 Vaccine: A Cross-Sectional Investigation in the MENA Region. Int. J. Women’s Health 2022, 14, 395–404. [Google Scholar] [CrossRef]
- Alhazmi, A.; Alamer, E.; Daws, D.; Hakami, M.; Darraj, M.; Abdelwahab, S.; Maghfuri, A.; Algaissi, A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines 2021, 9, 674. [Google Scholar] [CrossRef]
- Suhas, S. COVID 19 vaccination of persons with schizophrenia in India-Need for imperative action! Schizophr. Res. 2021, 231, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.P.; Jaswal, S. COVID vaccination and mental health: An Indian perspective. Asian J. Psychiatry 2022, 67, 102950. [Google Scholar] [CrossRef] [PubMed]
- Djanas, D.; Yusirwan; Martini, R.D.; Rahmadian; Putra, H.; Zanir, A.; Syahrial; Nindrea, R.D. Survey data of COVID-19 vaccine side effects among hospital staff in a national referral hospital in Indonesia. Data Brief 2021, 36, 107098. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.M.; Al-Hatamleh, M.A.I. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects. Vaccines 2021, 9, 556. [Google Scholar] [CrossRef]
- Taber, K.S. The Use of Cronbach’s Alpha When Developing and Reporting Research Instruments in Science Education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. An ultra-brief screening scale for anxiety and depression: The PHQ-4. Psychosomatics 2009, 50, 613–621. [Google Scholar]
- Löwe, B.; Wahl, I.; Rose, M.; Spitzer, C.; Glaesmer, H.; Wingenfeld, K.; Schneider, A.; Brähler, E. A 4-item measure of depression and anxiety: Validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J. Affect. Disord. 2010, 122, 86–95. [Google Scholar] [CrossRef]
- Bendau, A.; Plag, J.; Petzold, M.B.; Ströhle, A. COVID-19 vaccine hesitancy and related fears and anxiety. Int. Immunopharmacol. 2021, 97, 107724. [Google Scholar] [CrossRef]
- Abu-Hammad, O.; Alduraidi, H. Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines 2021, 9, 577. [Google Scholar] [CrossRef]
- Elgendy, M.O.; El-Gendy, A.O.; Mahmoud, S.; Mohammed, T.Y.; Abdelrahim, M.E.A.; Sayed, A.M. Side Effects and Efficacy of COVID-19 Vaccines among the Egyptian Population. Vaccines 2022, 10, 109. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Harakeh, S. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [PubMed]
- Dar-Odeh, N.; Abu-Hammad, O.; Qasem, F.; Jambi, S.; Alhodhodi, A.; Othman, A.; Abu-Hammad, A.; Al-Shorman, H.; Ryalat, S.; Abu-Hammad, S. Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum. Vaccines Immunother. 2022, 18, 2039017. [Google Scholar] [CrossRef] [PubMed]
- Brumfiel, G. Why Reports of Menstrual Changes after COVID Vaccine Are Tough to Study; Gynecology & Obstetrics: Cary, NC, USA, 2021. [Google Scholar]
- Li, K.; Chen, G.; Hou, H.; Liao, Q.; Chen, J.; Bai, H.; Lee, S.; Wang, C.; Li, H.; Cheng, L.; et al. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod. Biomed. Online 2021, 42, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Delamuta, L.C.; Monteleone, P.A.A. Coronavirus Disease 2019 and Human Reproduction: A Changing Perspective. Clinics 2021, 76, e3032. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Veronesi, G. Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey. Open Med. 2022, 17, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef]
- Sreepadmanabh, M.; Sahu, A.K.; Chande, A. COVID-19: Advances in diagnostic tools, treatment strategies, and vaccine development. J. Biosci. 2020, 45, 1–20. [Google Scholar] [CrossRef]
- Bae, S.; Lee, Y.W. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J. Korean Med. Sci. 2021, 36, e115. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Mohamed Hussein, A.A.R.; Ibrahim, I.H.; Mahmoud, I.A.; Amary, M.; Sayad, R. To what extent AstraZeneca ChAdOx1 nCoV-19 vaccine is safe and effective? Rapid systematic review. Egypt. J. Bronchol. 2022, 16, 6. [Google Scholar] [CrossRef]
- Rahman, S.; Rahman, M.M.; Miah, M.; Begum, M.N.; Sarmin, M.; Mahfuz, M.; Hossain, M.E.; Rahman, M.Z.; Chisti, M.J.; Ahmed, T.; et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci. Rep. 2022, 12, 1438. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef]
- Asghar, N.; Mumtaz, H. Safety, efficacy, and immunogenicity of COVID-19 vaccines; a systematic review. Immunol. Med. 2022, 1–13. [Google Scholar] [CrossRef]
- Pandey, K.; Thurman, M.; Johnson, S.D.; Acharya, A.; Johnston, M.; Klug, E.A.; Olwenyi, O.A.; Rajaiah, R.; Byrareddy, S.N. Mental Health Issues During and After COVID-19 Vaccine Era. Brain Res. Bull. 2021, 176, 161–173. [Google Scholar] [CrossRef]
- Perez-Arce, F.; Angrisani, M.; Bennett, D.; Darling, J.; Kapteyn, A.; Thomas, K. COVID-19 vaccines and mental distress. PLoS ONE 2021, 16, e0256406. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).