Heterologous Prime-Boost Immunization with DNA Vaccine and Modified Recombinant Proteins Enhances Immune Response against Trueperella pyogenes in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Growth Conditions
2.2. Optimization of Recombinant Protein Expression in E. coli Rosetta
2.3. Mice Models
2.4. IgG Antibody Detection
2.5. MTT Assay
2.6. Cytokine Profiling
2.7. Challenge Experiments
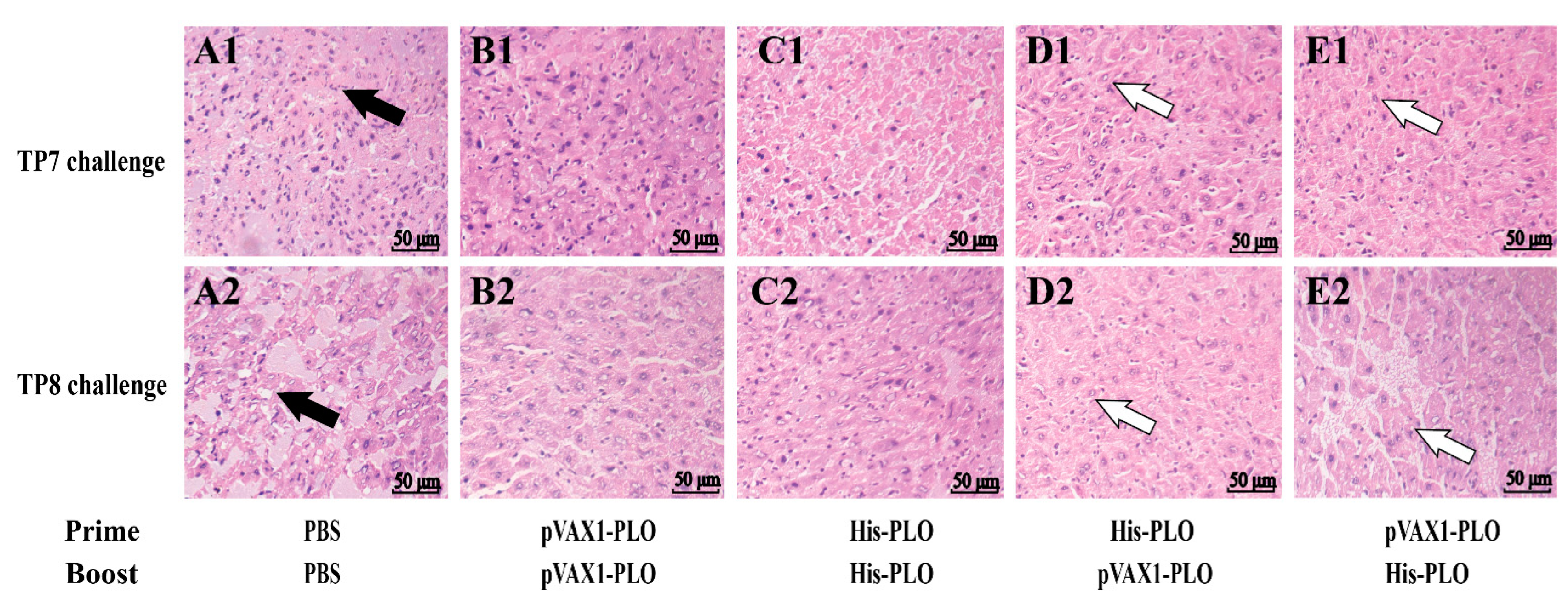
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
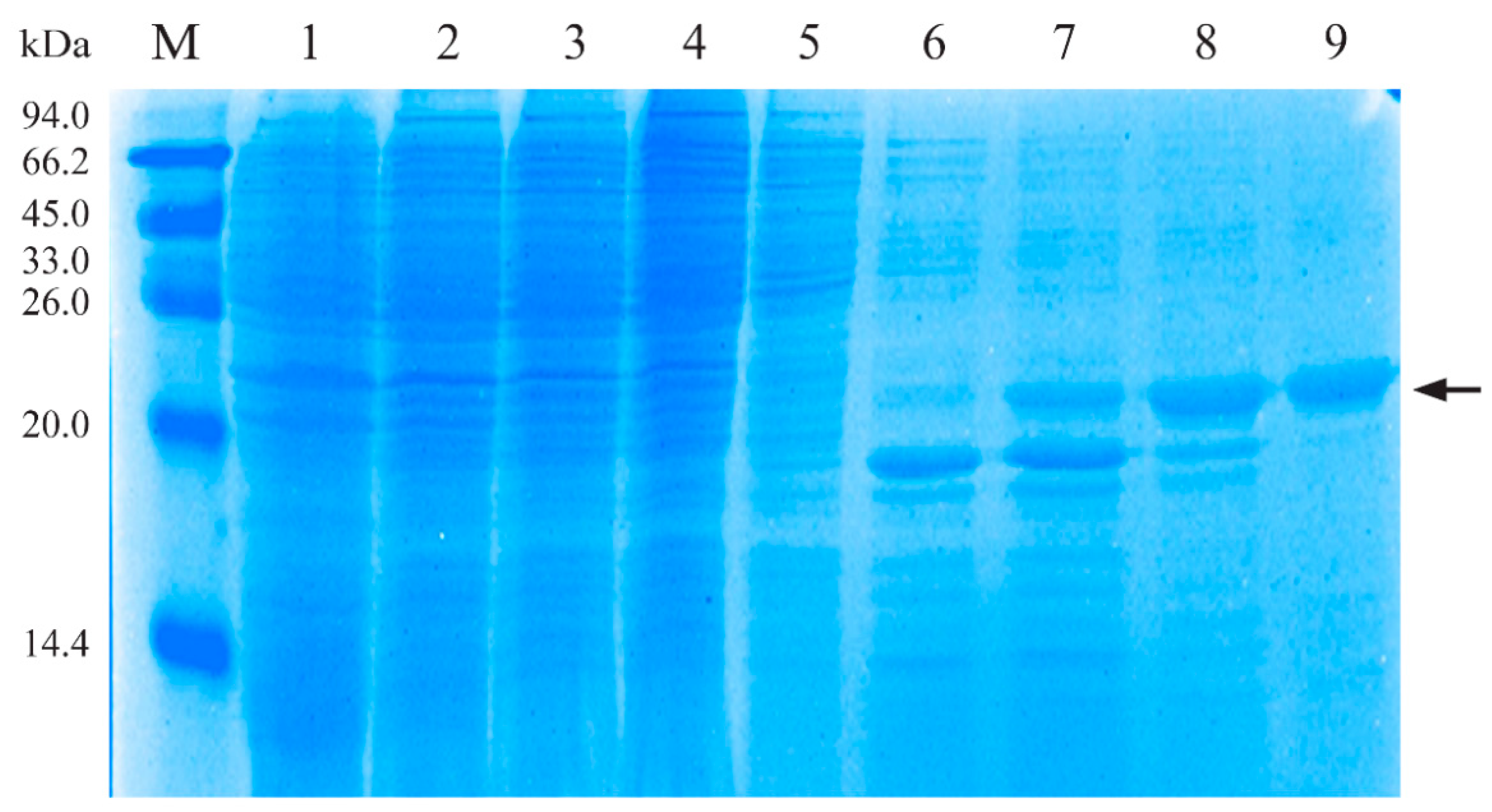
3.1. Characterization of Recombinant His-PLO Protein
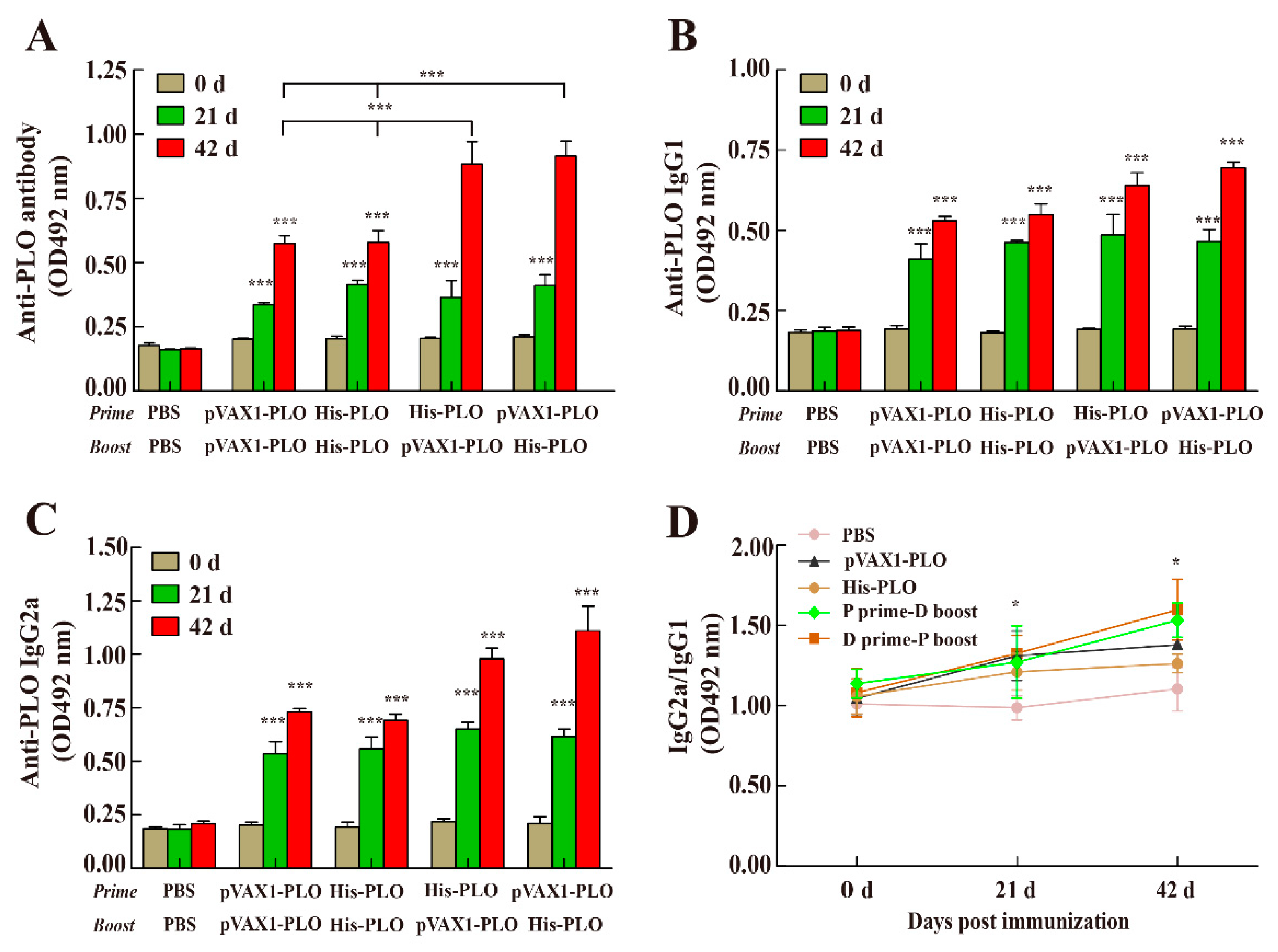
3.2. Prime-Boost Regimens Elicited Higher Antibody Production
3.3. Prime-Boost Regimens Induced Higher Cellular Immune Response
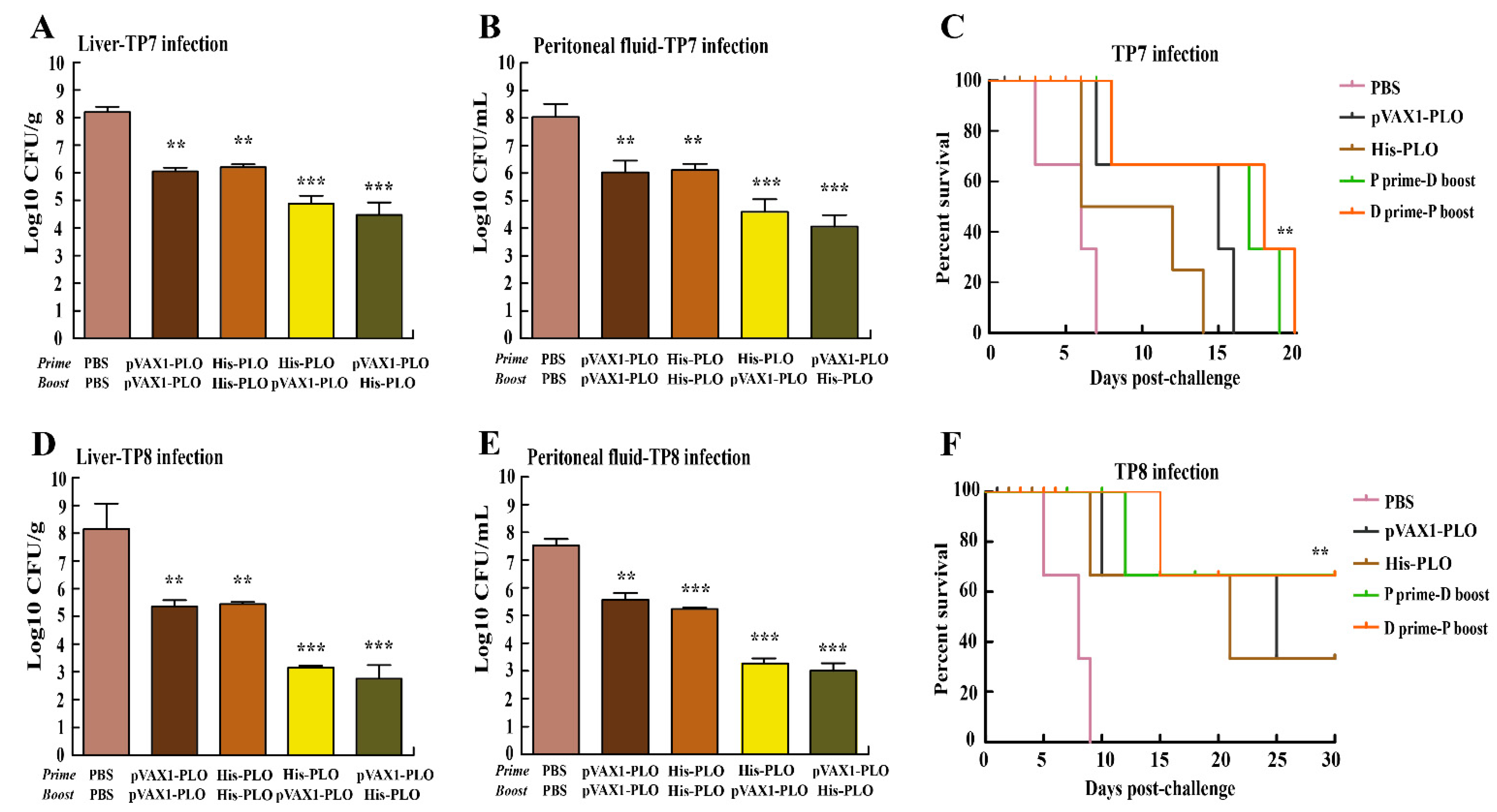
3.4. Prime-Boost Immunization Protected Mice against T. pyogenes Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rzewuska, M.; Kwiecień, E.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Stefańska, I.; Gieryńska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, M.G.; Risseti, R.M.; Bolaños, C.A.; Caffaro, K.A.; de Morais, A.C.; Lara, G.H.; Zamprogna, T.O.; Paes, A.C.; Listoni, F.J.; Franco, M.M. Trueperella pyogenes multispecies infections in domestic animals: A retrospective study of 144 cases (2002 to 2012). Vet. Q. 2015, 35, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Song, L.; Zhou, Z.; Liu, X.; Li, F.; Guo, Z.; Guan, Y.; Yang, L.; Feng, X.; Sun, C.; et al. vB-ApyS-JF1, the First Trueperella pyogenes Phage, Shows Potential as an Alternative Treatment Strategy for Trueperella pyogenes Infections. Front. Microbiol. 2021, 12, 736304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, W.; Huang, T.; Song, X.; Zhang, X.; Yue, B. Comparative transcriptome analysis of Trueperella pyogenes reveals a novel antimicrobial strategy. Arch. Microbiol. 2017, 199, 649–655. [Google Scholar] [CrossRef]
- Huang, T.; Cui, K.; Song, X.; Jing, J.; Lin, J.; Wang, X.; Zhang, X.; Chu, Y.; Yue, B. MTOR involved in bacterial elimination against Trueperella pyogenes infection based on mice model by transcriptome and biochemical analysis. Vet. Microbiol. 2019, 235, 199–208. [Google Scholar] [CrossRef]
- Meili, Z. Trueperella pyogenes pharyngitis in an immunocompetent 40-year-old man. BMJ Case Rep. 2020, 13, e236129. [Google Scholar] [CrossRef]
- Rzewuska, M.; Czopowicz, M.; Gawryś, M.; Markowska-Daniel, I.; Bielecki, W. Relationships between antimicrobial resistance, distribution of virulence factor genes and the origin of Trueperella pyogenes isolated from domestic animals and European bison (Bison bonasus). Microb. Pathog. 2016, 96, 35–41. [Google Scholar] [CrossRef]
- Zastempowska, E.; Lassa, H. Genotypic characterization and evaluation of an antibiotic resistance of Trueperella pyogenes (Arcanobacterium pyogenes) isolated from milk of dairy cows with clinical mastitis. Vet. Microbiol. 2012, 161, 153–158. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Wang, Q.; Liu, Y.; Tian, C.; Zhao, Y.; Yu, L.; Liu, M. Trueperella pyogenes isolated from dairy cows with endometritis in Inner Mongolia, China: Tetracycline susceptibility and tetracycline-resistance gene distribution. Microb. Pathog. 2017, 105, 51–56. [Google Scholar] [CrossRef]
- Yang, L.; Liang, H.; Wang, B.; Ma, B.; Wang, J.; Zhang, W. Evaluation of the Potency of Two Pyolysin-Derived Recombinant Proteins as Vaccine Candidates of Trueperella Pyogenes in a Mouse Model: Pyolysin Oligomerization and Structural Change Affect the Efficacy of Pyolysin-Based Vaccines. Vaccines 2020, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, P.; Wang, B.; Ma, B.; Wang, J. A combined Clostridium perfringens/Trueperella pyogenes inactivated vaccine induces complete immunoprotection in a mouse model. Biologicals 2017, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, K.; Zhang, Z.; Tang, C.; Zhang, X.; Yue, B. DNA vaccination based on pyolysin co-immunized with IL-1β enhances host antibacterial immunity against Trueperella pyogenes infection. Vaccine 2016, 34, 3469–3477. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hou, J.; Li, D.; Liu, Y.; Hu, N.; Hao, Y.; Fu, J.; Hu, Y.; Shao, Y. Enhancement of HIV-1 DNA vaccine immunogenicity by BCG-PSN, a novel adjuvant. Vaccine 2013, 31, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.P.; Apostolico, J.S.; Tomita, N.; Coirada, F.C.; Lunardelli, V.A.S.; Fernandes, E.R.; Souza, H.F.S.; Astray, R.M.; Boscardin, S.B.; Rosa, D.S. Homologous prime-boost with Zika virus envelope protein and poly (I:C) induces robust specific humoral and cellular immune responses. Vaccine 2020, 38, 3653–3664. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Zheng, M.; Xiong, F.; Liu, X.; Zhou, L.; Tan, W.; Chen, Z. Immunization with DNA prime-subunit protein boost strategy based on influenza H9N2 virus conserved matrix protein M1 and its epitope screening. Sci. Rep. 2020, 10, 4144. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Alberti, A.; Bivona, A.E.; Matos, M.N.; Cerny, N.; Schulze, K.; Weißmann, S.; Ebensen, T.; González, G.; Morales, C.; Cardoso, A.C.; et al. Mucosal Heterologous Prime/Boost Vaccination Induces Polyfunctional Systemic Immunity, Improving Protection Against Trypanosoma cruzi. Front. Immunol. 2020, 11, 128. [Google Scholar] [CrossRef] [Green Version]
- Sumithra, T.G.; Chaturvedi, V.K.; Gupta, P.K.; Bincy, J.; Siju, S.J.; Sunita, S.C.; Reshma, K.J.; Patel, C.L.; Rai, A.K. A novel bicistronic DNA vaccine with enhanced protective immune response against Bacillus anthracis through DNA prime-protein boost vaccination approach. Microb. Pathog. 2021, 158, 105104. [Google Scholar] [CrossRef]
- Dalmia, N.; Ramsay, A.J. Prime-boost approaches to tuberculosis vaccine development. Expert Rev. Vaccines 2012, 11, 1221–1233. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnol. 2018, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Tian, Y.; Yue, B.; Wang, H.; Zhang, X. Virulence determinants and biofilm production among Trueperella pyogenes recovered from abscesses of captive forest musk deer. Arch. Microbiol. 2013, 195, 203–209. [Google Scholar] [CrossRef]
- Zhao, K.; Li, W.; Li, J.; Ma, T.; Wang, K.; Yuan, Y.; Li, J.S.; Xie, R.; Huang, T.; Zhang, Y.; et al. TesG is a type I secretion effector of Pseudomonas aeruginosa that suppresses the host immune response during chronic infection. Nat. Microbiol. 2019, 4, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Karakavuk, M.; Can, H.; Gül, A.; Döşkaya, A.D.; Alak, S.E.; Ün, C.; Gürüz, A.Y.; Döşkaya, M. GRA8 DNA vaccine formulations protect against chronic toxoplasmosis. Microb. Pathog. 2021, 158, 105016. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Pu, Q.; Zhou, C.; Lin, P.; Gao, P.; Zhang, X.; Chu, Y.; Yue, B.; Wu, M. MicroRNA-302/367 Cluster Impacts Host Antimicrobial Defense via Regulation of Mitophagic Response Against Pseudomonas aeruginosa Infection. Front. Immunol. 2020, 11, 569173. [Google Scholar] [CrossRef] [PubMed]
- Amos, M.R.; Healey, G.D.; Goldstone, R.J.; Mahan, S.M.; Düvel, A.; Schuberth, H.J.; Sandra, O.; Zieger, P.; Dieuzy-Labaye, I.; Smith, D.G.; et al. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol. Reprod. 2014, 90, 54. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.; van der Lugt, J.J.; Gouws, J.J. Failure of an Actinomyces pyogenes vaccine to protect sheep against an intravenous challenge. Onderstepoort J. Vet. Res. 1990, 57, 239–241. [Google Scholar]
- Billington, S.J.; Songer, J.G.; Jost, B.H. Molecular characterization of the pore-forming toxin, pyolysin, a major virulence determinant of Arcanobacterium pyogenes. Vet. Microbiol. 2001, 82, 261–274. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Gu, Y.; Wang, Y.; Zhao, X.; Zhu, X. Protective immune response induced by co-immunization with the Trichinella spiralis recombinant Ts87 protein and a Ts87 DNA vaccine. Vet. Parasitol. 2013, 194, 207–210. [Google Scholar] [CrossRef]
- Soni, B.; Singh, S. Cytokine Milieu in Infectious Disease: A Sword or a Boon? J. Interferon Cytokine Res. 2020, 40, 24–32. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Jaramillo Ortiz, J.M.; Del Médico Zajac, M.P.; Zanetti, F.A.; Molinari, M.P.; Gravisaco, M.J.; Calamante, G.; Wilkowsky, S.E. Vaccine strategies against Babesia bovis based on prime-boost immunizations in mice with modified vaccinia Ankara vector and recombinant proteins. Vaccine 2014, 32, 4625–4632. [Google Scholar] [CrossRef]
- Jost, B.H.; Trinh, H.T.; Songer, J.G.; Billington, S.J. Immunization with genetic toxoids of the Arcanobacterium pyogenes cholesterol-dependent cytolysin, pyolysin, protects mice against infection. Infect. Immun. 2003, 71, 2966–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Zhao, K.; Song, X.; Song, T.; Wang, X.; Zhang, X.; Yue, B.; Chu, Y. Heterologous Prime-Boost Immunization with DNA Vaccine and Modified Recombinant Proteins Enhances Immune Response against Trueperella pyogenes in Mice. Vaccines 2022, 10, 839. https://doi.org/10.3390/vaccines10060839
Huang T, Zhao K, Song X, Song T, Wang X, Zhang X, Yue B, Chu Y. Heterologous Prime-Boost Immunization with DNA Vaccine and Modified Recombinant Proteins Enhances Immune Response against Trueperella pyogenes in Mice. Vaccines. 2022; 10(6):839. https://doi.org/10.3390/vaccines10060839
Chicago/Turabian StyleHuang, Ting, Kelei Zhao, Xuhao Song, Tao Song, Xinrong Wang, Xiuyue Zhang, Bisong Yue, and Yiwen Chu. 2022. "Heterologous Prime-Boost Immunization with DNA Vaccine and Modified Recombinant Proteins Enhances Immune Response against Trueperella pyogenes in Mice" Vaccines 10, no. 6: 839. https://doi.org/10.3390/vaccines10060839
APA StyleHuang, T., Zhao, K., Song, X., Song, T., Wang, X., Zhang, X., Yue, B., & Chu, Y. (2022). Heterologous Prime-Boost Immunization with DNA Vaccine and Modified Recombinant Proteins Enhances Immune Response against Trueperella pyogenes in Mice. Vaccines, 10(6), 839. https://doi.org/10.3390/vaccines10060839





