Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design, Expression, and Purification of Catcher-cVLP
2.2. Design, Expression, and Purification of Recombinant IL-1β Proteins
2.3. Vaccine Formulation
2.4. Quality Assessment of cVLP:IL-1β Vaccines
2.5. Biological Activity of IL-1β Antigens Using HEK-Blue Cells
2.6. Mouse Immunization Studies
2.7. Analysis of Vaccine-Induced Antibody Responses
2.8. IL-1 Subfamily Cross-Reactivity Western Blot Analysis
2.9. Quantification of Interleukins in Vaccinated Mice
2.10. Statistical Analysis
3. Results
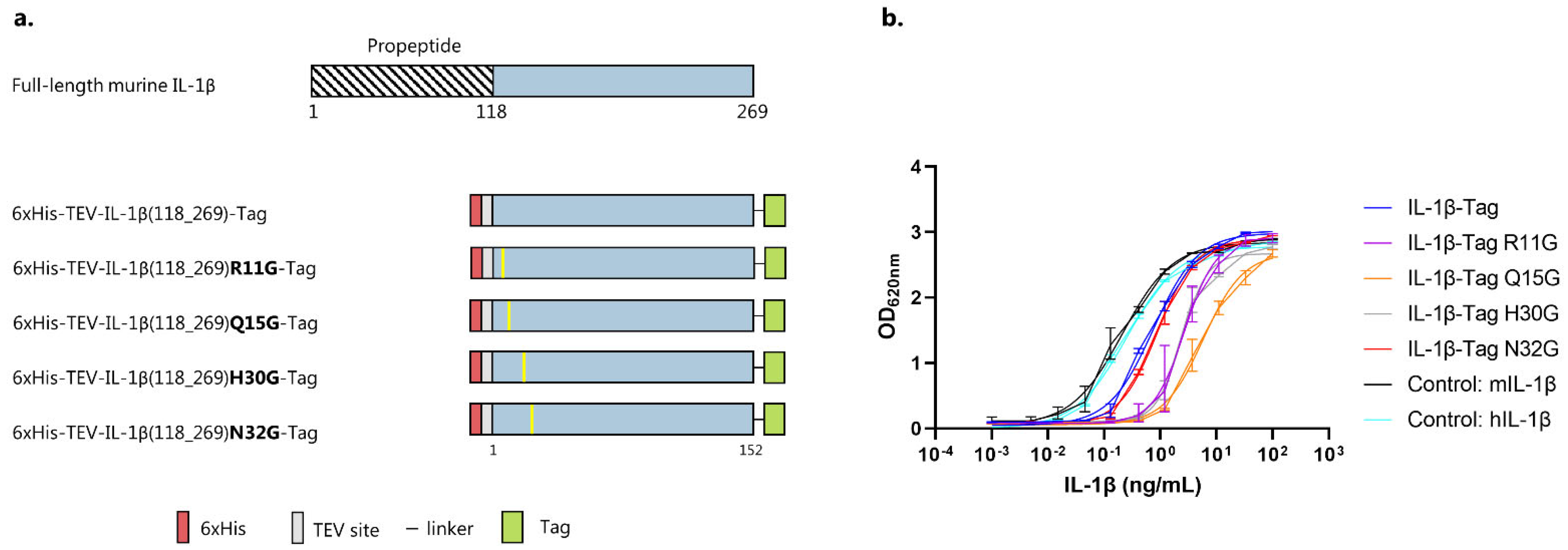
3.1. IL-1β Antigen Design
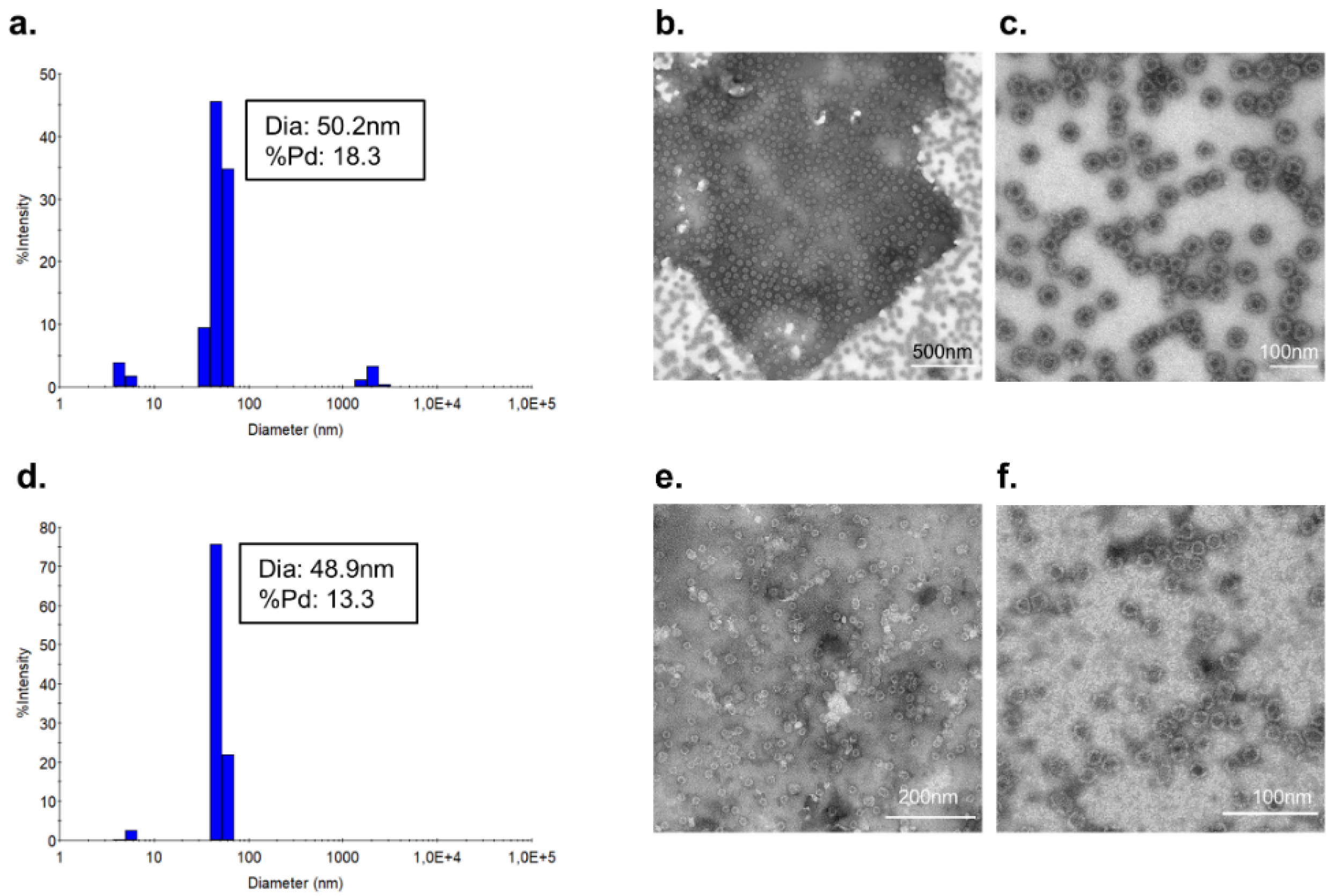
3.2. Vaccine Characterization and Quality Assessment
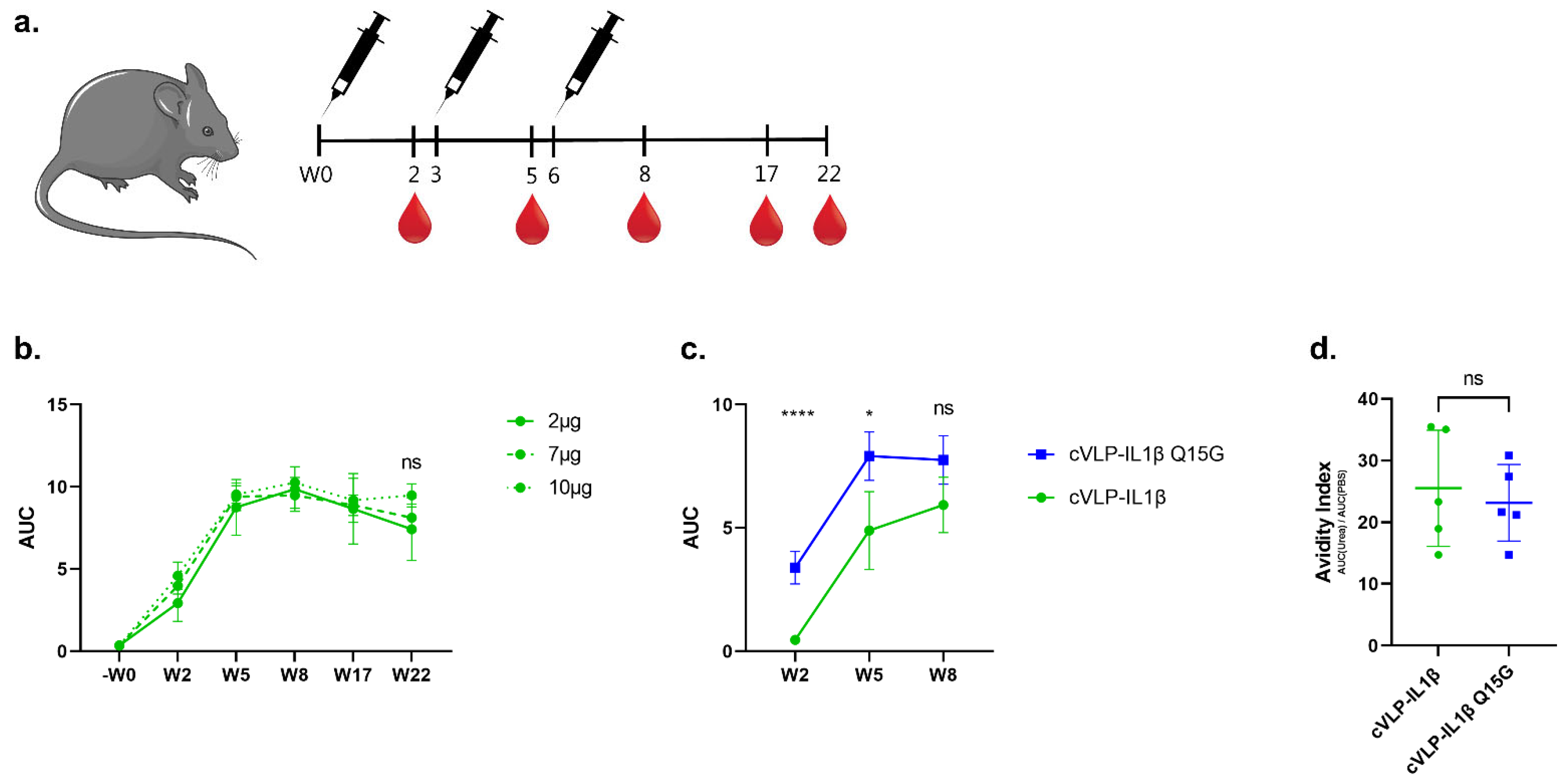
3.3. Immunogenicity of cVLP:IL-1β Vaccines
3.4. IL-1 Family Member Cross-Reactivity of Vaccine-Induced Anti-IL-1β Antibodies
3.5. Vaccination against IL-1β Inhibits the Challenge Response to DNFB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alinaghi, F.; Bennike, N.H.; Egeberg, A.; Thyssen, J.P.; Johansen, J.D. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermatitis 2019, 80, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Werfel, T.; White, I.R.; Johansen, J.D. Contact allergy: A review of current problems from a clinical perspective. Int. J. Environ. Res. Public Health 2018, 15, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thyssen, J.P.; Linneberg, A.; Menné, T.; Johansen, J.D. The epidemiology of contact allergy in the general population--prevalence and main findings. Contact Dermatitis 2007, 57, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Karlberg, A.T.; Bergström, M.A.; Börje, A.; Luthman, K.; Nilsson, J.L.G. Allergic contact dermatitis—Formation, structural requirements, and reactivity of skin sensitizers. Chem. Res. Toxicol. 2008, 21, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, P.S. The relationships between exposure dose and response in induction and elicitation of contact hypersensitivity in humans. Br. J. Dermatol. 2007, 157, 1093–1102. [Google Scholar] [CrossRef]
- Martin, S.F.; Esser, P.R.; Weber, F.C.; Jakob, T.; Freudenberg, M.A.; Schmidt, M.; Goebeler, M. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 1152–1163. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis 2019, 81, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.L.; Yiannias, J.A. Contact Dermatitis to Medications and Skin Products. Clin. Rev. Allergy Immunol. 2019, 56, 41–59. [Google Scholar] [CrossRef]
- Watanabe, H.; Gaide, O.; Pétrilli, V.; Martinon, F.; Contassot, E.; Roques, S.; Kummer, J.A.; Tschopp, J.; French, L.E. Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J. Investig. Dermatol. 2007, 127, 1956–1963. [Google Scholar] [CrossRef] [Green Version]
- Vennegaard, M.T.; Dyring-Andersen, B.; Skov, L.; Nielsen, M.M.; Schmidt, J.D.; Bzorek, M.; Poulsen, S.S.; Thomsen, A.R.; Woetmann, A.; Thyssen, J.P.; et al. Epicutaneous exposure to nickel induces nickel allergy in mice via a MyD88-dependent and interleukin-1-dependent pathway. Contact Dermatitis 2014, 71, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.; Mraz, V.; Geisler, C.; Skov, L.; Bonefeld, C.M. The role of interleukin-1β in the immune response to contact allergens. Contact Dermatitis 2021, 85, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Enk, A.H.; Katz, S.I. Early molecular events in the induction phase of contact sensitivity. Proc. Natl. Acad. Sci. USA 1992, 89, 1398–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsini, E.; Galli, C.L. Epidermal cytokines in experimental contact dermatitis. Toxicology 2000, 142, 203–211. [Google Scholar] [CrossRef]
- Enk, A.H.; Angeloni, V.L.; Udey, M.C.; Katz, S.I. An essential role for Langerhans cell-derived IL-1 beta in the initiation of primary immune responses in skin. J. Immunol. 1993, 150, 3698–3704. [Google Scholar]
- Kaiser, C.; Knight, A.; Nordström, D.; Pettersson, T.; Fransson, J.; Florin-Robertsson, E.; Pilström, B. Injection-site reactions upon Kineret (anakinra) administration: Experiences and explanations. Rheumatol. Int. 2012, 32, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Bartelds, G.M.; Krieckaert, C.L.M.; Nurmohamed, M.T.; Van Schouwenburg, P.A.; Lems, W.F.; Twisk, J.W.R.; Dijkmans, B.A.C.; Aarden, L.; Wolbink, G.J. Development of Antidrug Antibodies Against Adalimumab and Association With Disease Activity and Treatment Failure During Long-term Follow-up. JAMA 2011, 305, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Chackerian, B.; Kathryn, M. Frietze Moving Towards a New Class of Vaccines for Non- infectious Chronic Diseases. Expert Rev. Vaccines 2016, 15, 561–563. [Google Scholar] [CrossRef] [Green Version]
- Rondeau, J.M.; Ramage, P.; Zurini, M.; Gram, H. The molecular mode of action and species specificity of canakinumab, a human monoclonal antibody neutralizing IL-1β. MAbs 2015, 7, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Kuemmerle-Deschner, J.B.; Ramos, E.; Blank, N.; Roesler, J.; Felix, S.D.; Jung, T.; Stricker, K.; Chakraborty, A.; Tannenbaum, S.; Wright, A.M.; et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res. Ther. 2011, 13, R34. [Google Scholar] [CrossRef] [Green Version]
- Lachmann, H.J.; Kone-Paut, I.; Kuemmerle-Deschner, J.B.; Leslie, K.S.; Hachulla, E.; Quartier, P.; Gitton, X.; Widmer, A.; Patel, N.; Hawkins, P.N. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 2009, 360, 2416–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, M.F.; Dyer, M.R. Therapeutic vaccination for chronic diseases: A new class of drugs in sight. Nat. Rev. Drug Discov. 2004, 3, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G.P.; Singh, O.M.; Pal, R.; Chatterjee, N.; Sahai, P.; Dhall, K.; Kaur, J.; Das, S.K.; Suri, S.; Bucrshee, K.; et al. A vaccine that prevents pregnancy in women. Proc. Natl. Acad. Sci. USA 1994, 91, 8532–8536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, M.F.; Hoffmann Rohrer, U.; Kündig, T.M.; Bürki, K.; Hengartner, H.; Zinkernagel, R.M. The Influence of Antigen Organization on B Cell Responsiveness. Science 1993, 262, 1448–1451. [Google Scholar] [CrossRef]
- Leneghan, D.B.; Miura, K.; Taylor, I.J.; Li, Y.; Jin, J.; Brune, K.D.; Bachmann, M.F.; Howarth, M.; Long, C.A.; Biswas, S. Nanoassembly routes stimulate conflicting antibody quantity and quality for transmission-blocking malaria vaccines. Nat. Sci. Rep. 2017, 7, 3811. [Google Scholar] [CrossRef]
- Jegerlehner, A.; Storni, T.; Lipowsky, G.; Schmid, M.; Pumpens, P.; Bachmann, M.F. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur. J. Immunol. 2002, 32, 3305–3314. [Google Scholar] [CrossRef]
- Bertin-Maghit, S.M.; Capini, C.J.; Bessis, N.; Chomilier, J.; Muller, S.; Abbas, A.; Autin, L.; Spadoni, J.L.; Rappaport, J.; Therwath, A.; et al. Improvement of collagen-induced arthritis by active immunization against murine IL-1β peptides designed by molecular modelling. Vaccine 2005, 23, 4228–4235. [Google Scholar] [CrossRef]
- Spohn, G.; Keller, I.; Beck, M.; Grest, P.; Jennings, G.T.; Bachmann, M.F. Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis. Eur. J. Immunol. 2008, 38, 877–887. [Google Scholar] [CrossRef]
- Spohn, G.; Schori, C.; Keller, I.; Sladko, K.; Sina, C.; Guler, R.; Schwarz, K.; Johansen, P.; Jennings, G.T.; Bachmann, M.F. Preclinical efficacy and safety of an anti-IL-1β vaccine for the treatment of type 2 diabetes. Mol. Ther. Methods Clin. Dev. 2014, 1, 14048. [Google Scholar] [CrossRef]
- Cavelti-Weder, C.; Timper, K.; Seelig, E.; Keller, C.; Osranek, M.; Lssing, U.; Spohn, G.; Maurer, P.; Müller, P.; Jennings, G.T.; et al. Development of an interleukin-1β vaccine in patients with type 2 diabetes. Mol. Ther. 2016, 24, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Thrane, S.; Janitzek, C.M.; Matondo, S.; Resende, M.; Gustavsson, T.; de Jongh, W.A.; Clemmensen, S.; Roeffen, W.; van de Vegte-Bolmer, M.; van Gemert, G.J.; et al. Bacterial superglue enables easy development of efficient virus-like particle based vaccines. J. Nanobiotechnology 2016, 14, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brune, K.D.; Buldun, C.M.; Li, Y.; Taylor, I.J.; Brod, F.; Biswas, S.; Howarth, M. Dual Plug-and-Display Synthetic Assembly Using Orthogonal Reactive Proteins for Twin Antigen Immunization. Bioconjug. Chem. 2017, 28, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- Funch, A.B.; Mraz, V.; Gadsbøll, A.S.Ø.; Jee, M.H.; Weber, J.F.; Ødum, N.; Woetmann, A.; Johansen, J.D.; Geisler, C.; Bonefeld, C.M. CD8 + tissue-resident memory T cells recruit neutrophils that are essential for flare-ups in contact dermatitis. Allergy 2022, 77, 513–524. [Google Scholar] [CrossRef]
- Auron, P.E.; Quigley, G.J.; Rosenwasser, L.J.; Gehrke, L. Multiple Amino Acid Substitutions Suggest a Structural Basis for the Separation of Biological Activity and Receptor Binding in a Mutant Interleukin-1/8 Protein1. Biochemistry 1992, 31, 6632–6638. [Google Scholar] [CrossRef] [PubMed]
- Vigers, G.P.A.; Anderson, L.J.; Caffes, P.; Brandhuber, B.J. Crystal structure of the type-I interleukin-1 receptor complexed with interleukin-1β. Nature 1997, 386, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.J.; Bray, J.; Childs, J.D.; Vigers, G.P.A.; Brandhuber, B.J.; Skalicky, J.J.; Thompson, R.C.; Eisenberg, S.P. Mapping receptor binding sites in interleukin (IL)-1 receptor antagonist and IL-1β by site-directed mutagenesis. Identification of a single site in IL-1ra and two sites in IL-1β. J. Biol. Chem. 1995, 270, 11477–11483. [Google Scholar] [CrossRef] [Green Version]
- Vigers, G.P.K.; Caffes, P.; Evans, R.J.; Thompson, R.C.; Eisenberg, S.P.; Brandhubers, B.J. X-ray Structure of Interleukin-1 Receptor Antagonist at 2.0-A Resolution. J. Biol. Chem. 1994, 269, 12874–12879. [Google Scholar] [CrossRef]
- Gehrke, L.; Jobling, S.A.; Paik, L.S.; McDonald, B.; Rosenwasser, L.J.; Auron, P.E. A point mutation uncouples human interleukin-1? biological activity and receptor binding. J. Biol. Chem. 1990, 265, 5922–5925. [Google Scholar] [CrossRef]
- Palladini, A.; Thrane, S.; Janitzek, C.M.; Pihl, J.; Clemmensen, S.B.; Adriaan De Jongh, W.; Clausen, T.M.; Nicoletti, G.; Landuzzi, L.; Penichet, M.L.; et al. Virus-like particle display of HER2 induces potent anti-cancer responses. Oncoimmunology 2018, 7, e1408749. [Google Scholar] [CrossRef] [Green Version]
- Tissot, A.C.; Maurer, P.; Nussberger, J.; Sabat, R.; Pfister, T.; Ignatenko, S.; Volk, H.D.; Stocker, H.; Müller, P.; Jennings, G.T.; et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: A double-blind, randomised, placebo-controlled phase IIa study. Lancet 2008, 371, 821–827. [Google Scholar] [CrossRef]
- Farlow, M.R.; Andreasen, N.; Riviere, M.-E.; Vostiar, I.; Vitaliti, A.; Sovago, J.; Caputo, A.; Winblad, B.; Graf, A. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers. Res. Ther. 2015, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, M.F.; Whitehead, P. Active immunotherapy for chronic diseases. Vaccine 2013, 31, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.-L.; Horsted, E.W.; et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Mohsen, M.O.; Kramer, M.F.; Heath, M.D. Vaccination against Allergy: A Paradigm Shift? Trends Mol. Med. 2020, 26, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Dietmeier, K.; Bauer, M.; Maudrich, M.; Utzinger, S.; Muntwiler, S.; Saudan, P.; Bachmann, M.F. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: A novel therapy for cat allergy. J. Exp. Med. 2009, 206, 1941–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engeroff, P.; Caviezel, F.; Storni, F.; Thoms, F.; Vogel, M.; Bachmann, M.F.; Martin Bachmann, C.F. Allergens displayed on virus-like particles are highly immunogenic but fail to activate human mast cells. Allergy Eur. J. Allergy Clin. Immunol. 2017, 73, 341–349. [Google Scholar] [CrossRef]
- Tissot, A.C.; Spohn, G.; Jennings, G.T.; Shamshiev, A.; Kurrer, M.O.; Windak, R.; Meier, M.; Viesti, M.; Hersberger, M.; Kündig, T.M.; et al. A VLP-based vaccine against interleukin-1α protects mice from atherosclerosis. Eur. J. Immunol. 2013, 43, 716–722. [Google Scholar] [CrossRef]
- Zeilhofer, U.; Bachmann, M.F.; Jennings, G.T.; Hernandez, M.; Witschi, R.; Grest, P.; Till, H.; Röhn, A.; Ralvenius, W.T.; Paul, J.; et al. A Virus-Like Particle-Based Anti-Nerve Growth Factor Vaccine Reduces Inflammatory Hyperalgesia: Potential Long-Term Therapy for Chronic Pain. J. Immunol. 2011, 186, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.D.; Ahlström, M.G.; Johansen, J.D.; Dyring-Andersen, B.; Agerbeck, C.; Nielsen, M.M.; Poulsen, S.S.; Woetmann, A.; Ødum, N.; Thomsen, A.R.; et al. Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8 + T cells. Contact Dermatitis 2017, 76, 218–227. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Schechtman, J.; Bennett, R.; Handel, M.L.; Burmester, G.R.; Tesser, J.; Modafferi, D.; Poulakos, J.; Sun, G. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: A large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003, 48, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Tesser, J.; Schiff, M.H.; Schechtman, J.; Burmester, G.R.; Bennett, R.; Modafferi, D.; Zhou, L.; Bell, D.; Appleton, B. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Ghadirian, E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect. Immun. 1994, 62, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, E.; Irikura, V.M.; Paul, S.M.; Hirsh, D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc. Natl. Acad. Sci. USA 1996, 93, 11008–11013. [Google Scholar] [CrossRef] [Green Version]
- Labow, M.; Shuster, D.; Zetterstrom, M.; Nunes, P.; Terry, R.; Cullinan, E.B.; Bartfai, T.; Solorzano, C.; Moldawer, L.L.; Chizzonite, R.; et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J. Immunol. 1997, 159, 2452–2461. [Google Scholar] [PubMed]
- Irikura, V.M.; Hirsch, E.; Hirsh, D. Effects of Interleukin-1 Receptor Antagonist Overexpression on Infection by Listeria monocytogenes. Infect. Immun. 1999, 67, 1901–1909. [Google Scholar] [CrossRef]
- Irikura, V.M.; Lagraoui, M.; Hirsh, D. The Epistatic Interrelationships of IL-1, IL-1 Receptor Antagonist, and the Type I IL-1 Receptor. J. Immunol. 2002, 169, 393–398. [Google Scholar] [CrossRef]
- Guler, R.; Parihar, S.P.; Spohn, G.; Johansen, P.; Brombacher, F.; Bachmann, M.F. Blocking IL-1α but not IL-1β increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine 2011, 29, 1339–1346. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Li, L.; Liu, X.; Mei, K.; Wang, X. Structural insights into the assembly and activation of IL-1b with its receptors. Nat. Immunol. 2010, 11, 905–912. [Google Scholar] [CrossRef]
- Krumm, B.; Xiang, Y.; Deng, J. Structural biology of the IL-1 superfamily: Key cytokines in the regulation of immune and inflammatory responses. Protein Sci. 2014, 23, 526–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliviero, F.; Cavalli, G.; Boissier, M.-C.; Assier, E.; Bessis, N.; Zagury, J.-F. IL-1 Vaccination Is Suitable for Treating Inflammatory Diseases. Front. Pharmacol. 2017, 8, 6. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goksøyr, L.; Funch, A.B.; Okholm, A.K.; Theander, T.G.; de Jongh, W.A.; Bonefeld, C.M.; Sander, A.F. Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis. Vaccines 2022, 10, 828. https://doi.org/10.3390/vaccines10050828
Goksøyr L, Funch AB, Okholm AK, Theander TG, de Jongh WA, Bonefeld CM, Sander AF. Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis. Vaccines. 2022; 10(5):828. https://doi.org/10.3390/vaccines10050828
Chicago/Turabian StyleGoksøyr, Louise, Anders B. Funch, Anna K. Okholm, Thor G. Theander, Willem Adriaan de Jongh, Charlotte M. Bonefeld, and Adam F. Sander. 2022. "Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis" Vaccines 10, no. 5: 828. https://doi.org/10.3390/vaccines10050828
APA StyleGoksøyr, L., Funch, A. B., Okholm, A. K., Theander, T. G., de Jongh, W. A., Bonefeld, C. M., & Sander, A. F. (2022). Preclinical Efficacy of a Capsid Virus-like Particle-Based Vaccine Targeting IL-1β for Treatment of Allergic Contact Dermatitis. Vaccines, 10(5), 828. https://doi.org/10.3390/vaccines10050828





