COVID-19 Vaccine Effectiveness at a Referral Hospital in Northern Peru: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
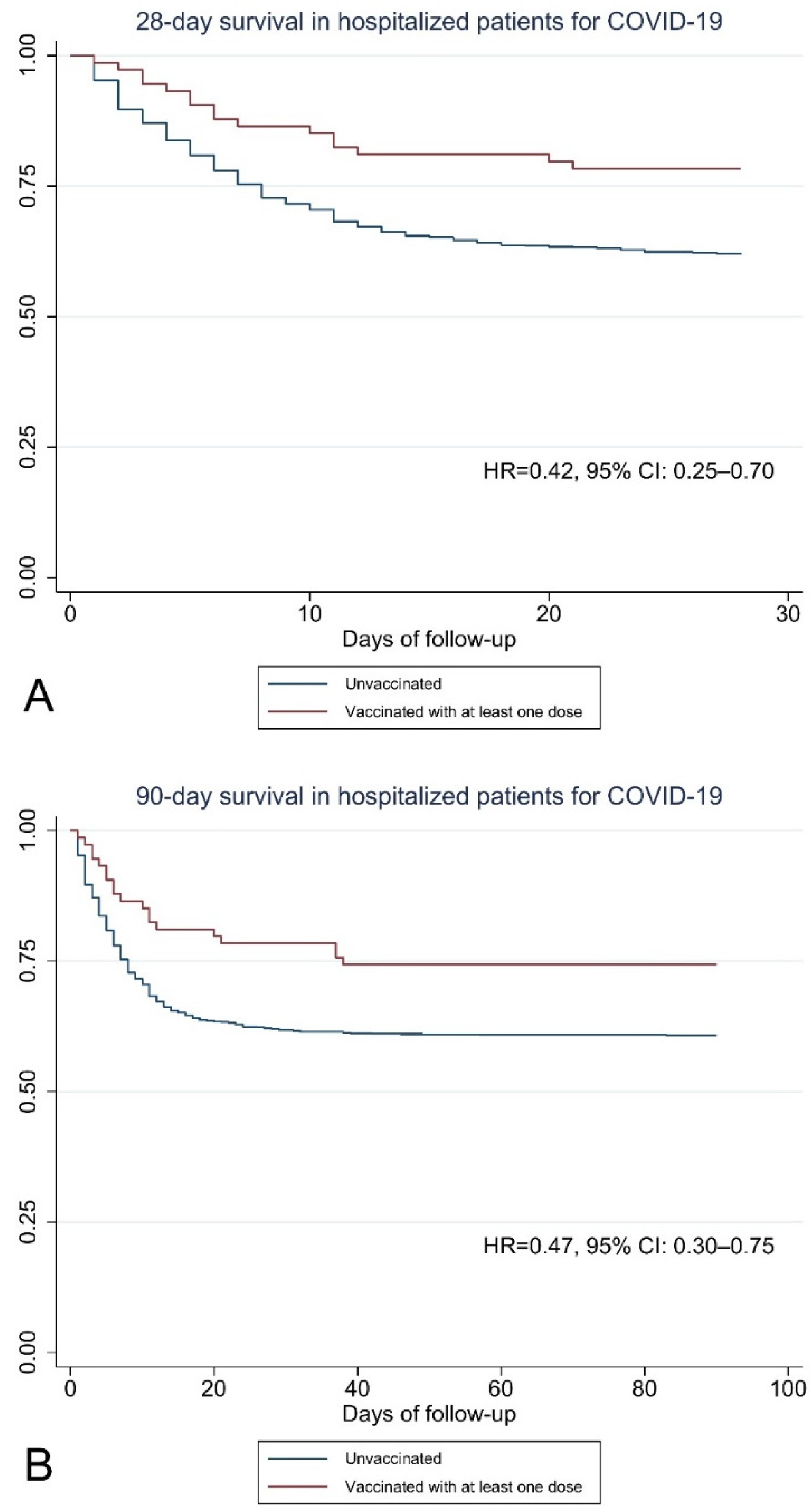
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orús, A. Número de Personas Fallecidas a Consecuencia Del Coronavirus a Nivel Mundial a Fecha de 23 de Septiembre de 2021, Por Continente. Available online: https://es.statista.com/estadisticas/1107719/covid19-numero-de-muertes-a-nivel-mundial-por-region/ (accessed on 26 September 2020).
- OPS/OMS. Respuesta de la OPS/OMS. 27 de Agosto Del 2021; Pan American Health Organization: Washington, DC, USA, 2021; pp. 1–13. [Google Scholar]
- MINSA. Sala Situacional COVID-19 Perú. Available online: https://covid19.minsa.gob.pe/sala_situacional.asp (accessed on 26 September 2020).
- Lam-Cabanillas, E.; León-Risco, A.; León-Risco, K.; Llamo-Hoyos, G.; López-Zavaleta, R.; Luzuriaga-Tirado, E.; Mendoza-Blas, A.; Huamán-Saavedra, J. Bases moleculares de la patogénesis de Covid-19 y estudios in silico de posibles tratamientos farmacológicos. Rev. De La Fac. De Med. Hum. 2021, 21, 417–432. [Google Scholar]
- Aguilar-Gamboa, F.R.; Suclupe-Campos, D.O.; Vega-Fernández, J.A.; Silva-Diaz, H. Diversidad genómica en SARS-CoV-2: Mutaciones y variantes. Rev. Cuerpo Med. 2021, 14, 572–578. [Google Scholar] [CrossRef]
- Pírez, C.; Peluffo, G.; Barrios, P.; Pujadas, M. Inmunizaciones como estrategia de salud pública. Arch. Pediatría Urug. 2021, 92, S1–S5. [Google Scholar]
- Giglio, N.; Bakir, J.; Gentile, A. Eficacia, efectividad e impacto en vacunas: ¿es lo mismo. Rev. Hosp. Niños 2018, 60, 34–41. [Google Scholar]
- IETSI-EsSalud. Lineamientos Clínicos sobre Vacunación contra la COVID-19 en el Seguro Social de Perú; Instituto de Evaluación de Tecnologías en Salud e Investigación EsSalud: Lima, Perú, 2021; pp. 1–28. [Google Scholar]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Hung, I.F.; Poland, G.A. Single-dose Oxford–AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet 2021, 397, 854–855. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N. Effectiveness of Covid-19 vaccines in ambulatory and inpatient care settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Desai, R.J.; Franklin, J.M. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ 2019, 367, l5657. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-J.; Lu, C.-Y.; Chang, Y.-H.; Sun, Y.; Chu, H.-J.; Lee, C.-Y.; Liu, C.-H.; Lin, C.-H.; Lu, C.-J.; Li, C.-Y. Effectiveness of the WHO-Authorized COVID-19 Vaccines: A Rapid Review of Global Reports till 30 June 2021. Vaccines 2021, 9, 1489. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef]
- Vaishya, R.; Sibal, A.; Sharma, H.; Singh, S.K. Lack of vaccination and associated comorbidities predispose to the need for intensive care in individuals infected with the delta variant—A case cohort study from a tertiary care hospital in New Delhi, India. Diabetes Metab. Syndr. 2021, 15, 102203. [Google Scholar] [CrossRef] [PubMed]
- McGovern, A.P.; Thomas, N.J.; Vollmer, S.J.; Hattersley, A.T.; Mateen, B.A.; Dennis, J.M. The disproportionate excess mortality risk of COVID-19 in younger people with diabetes warrants vaccination prioritisation. Diabetologia 2021, 64, 1184–1186. [Google Scholar] [CrossRef]
- Ali, H.; Alterki, A.; Sindhu, S.; Alahmad, B.; Hammad, M.; Al-Sabah, S.; Alghounaim, M.; Jamal, M.H.; Aldei, A.; Mairza, M.J.; et al. Robust Antibody Levels in Both Diabetic and Non-Diabetic Individuals After BNT162b2 mRNA COVID-19 Vaccination. Front. Immunol. 2021, 12, 752233. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Thindwa, D.; Garcia Quesada, M.; Liu, Y.; Bennett, J.; Cohen, C.; Knoll, M.D.; von Gottberg, A.; Hayford, K.; Flasche, S. Use of seasonal influenza and pneumococcal polysaccharide vaccines in older adults to reduce COVID-19 mortality. Vaccine 2020, 38, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Chen, C.; Li, Y.; Yan, D.; Zhang, X.; Jiang, D.; Yang, S.; Li, L. Impact of Coinfection With SARS-CoV-2 and Influenza on Disease Severity: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 773130. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) | ||
|---|---|---|---|
| Vaccinated | |||
| Unvaccinated | 1480 (95.2) | ||
| Only the first dose | 43 (2.8) | ||
| First and second dose | 31 (2.0) | ||
| Vaccine type | |||
| Pfizer | 64 (86.5) | ||
| Sinopharm | 8 (10.8) | ||
| AstraZeneca | 2 (2.7) | ||
| Age (years) * | 55.2 ± 16.8 | ||
| Sex | |||
| Female | 647 (41.6) | ||
| Male | 907 (58.4) | ||
| Medical insurance | |||
| SIS | 899 (57.8) | ||
| EsSalud | 152 (9.8) | ||
| Others | 26 (1.7) | ||
| No medical insurance | 477 (30.7) | ||
| Symptoms at the onset of the disease | |||
| Dyspnea | |||
| No | 706 (45.5) | ||
| Yes | 847 (54.5) | ||
| Fever | |||
| No | 989 (63.7) | ||
| Yes | 564 (36.3) | ||
| Cough | |||
| No | 411 (26.5) | ||
| Yes | 1142 (73.5) | ||
| Comorbidity | |||
| Hypertension ** | |||
| No | 1454 (93.7) | ||
| Yes | 97 (6.3) | ||
| Type 2 diabetes ** | |||
| No | 1521 (98.1) | ||
| Yes | 30 (1.9) | ||
| Chronic kidney disease ** | |||
| No | 1533 (98.8) | ||
| Yes | 18 (1.2) | ||
| Obesity ** | |||
| No | 1516 (97.7) | ||
| Yes | 35 (2.3) | ||
| Cancer ** | |||
| No | 1516 (97.7) | ||
| Breast | 10 (0.65) | ||
| Cervical | 3 (0.19) | ||
| Prostate | 3 (0.19) | ||
| Stomach | 2 (0.13) | ||
| Brain | 2 (0.13) | ||
| Non-Hodgkin’s lymphoma | 2 (0.13) | ||
| Unspecified or uncommon site | 13 (0.83) | ||
| Neurological * | |||
| No | 1526 (98.4) | ||
| Yes | 25 (1.6) | ||
| Death after 28 days | 592 (38.1) | ||
| Death after 90 days | 614 (39.5) | ||
| Variables | Mortality after 28 Days | Mortality after 90 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| No (n = 961) | Yes (n = 592) | p | No (n = 939) | Yes (n = 614) | p | |||
| n (%) | n (%) | n (%) | n (%) | |||||
| Vaccinated * | 0.003 | 0.012 | ||||||
| Unvaccinated | 903 (61.1) | 576 (38.9) | 884 (59.8) | 595 (40.2) | ||||
| At least one dose | 58 (78.4) | 16 (21.6) | 55 (74.3) | 19 (25.7) | ||||
| Age (years) ** | 50.4 ± 16.2 | 63.1 ± 14.7 | 0.001 | 50.1 ± 16.2 | 63.1 ± 14.6 | 0.001 | ||
| Sex * | 0.001 | 0.001 | ||||||
| Female | 447 (69.1) | 200 (30.9) | 437 (67.5) | 210 (32.5) | ||||
| Male | 514 (56.7) | 392 (43.3) | 502 (55.4) | 404 (44.6) | ||||
| Symptoms at the onset of the disease | ||||||||
| Dyspnea * | 0.001 | 0.001 | ||||||
| No | 564 (78.9) | 142 (20.1) | 556 (78.7) | 150 (21.3) | ||||
| Yes | 397 (46.9) | 450 (53.1) | 383 (45.2) | 464 (54.8) | ||||
| Fever * | 0.009 | 0.013 | ||||||
| No | 636 (64.3) | 353 (35.7) | 621 (62.8) | 368 (37.2) | ||||
| Yes | 325 (57.6) | 239 (42.4) | 318 (56.4) | 246 (43.6) | ||||
| Cough * | 0.001 | 0.001 | ||||||
| No | 198 (48.1) | 213 (51.8) | 190 (46.2) | 221 (53.8) | ||||
| Yes | 763 (66.8) | 379 (33.2) | 749 (65.6) | 393 (34.4) | ||||
| Comorbidity | ||||||||
| Hypertension * | 0.001 | 0.001 | ||||||
| No | 945 (65.0) | 509 (35.0) | 923 (63.5) | 531 (36.5) | ||||
| Yes | 14 (14.4) | 83 (85.6) | 14 (14.4) | 83 (85.6) | ||||
| Type 2 diabetes * | 0.001 | 0.001 | ||||||
| No | 953 (62.7) | 568 (37.3) | 931 (61.2) | 590 (38.8) | ||||
| Yes | 6 (20.0) | 24 (80.0) | 6 (20.0) | 24 (80.0) | ||||
| Chronic kidney disease * | 0.001 | <0.001 | ||||||
| No | 955 (62.3) | 578 (37.7) | 934 (60.9) | 599 (39.1) | ||||
| Yes | 4 (22.2) | 14 (77.8) | 3 (16.7) | 15 (83.3) | ||||
| Obesity * | <0.001 | <0.001 | ||||||
| No | 954 (62.9) | 562 (37.1) | 932 (61.5) | 584 (38.5) | ||||
| Yes | 5 (14.3) | 30 (85.7) | 5 (14.3) | 30 (85.7) | ||||
| Cancer * | 0.822 | 0.453 | ||||||
| No | 938 (61.9) | 578 (38.1) | 918 (60.5) | 598 (39.5) | ||||
| Yes | 21 (60.0) | 14 (40.0) | 19 (54.3) | 16 (45.7) | ||||
| Neurological * | <0.001 | <0.001 | ||||||
| No | 956 (62.6) | 570 (37.4) | 934 (61.2) | 592 (39.8) | ||||
| Yes | 3 (12.0) | 22 (88.0) | 3 (12.0) | 22 (88.0) | ||||
| After 28 Days | After 90 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | HR | 95% CI | aHR * | 95% CI | HR | 95% CI | aHR * | 95% CI | |
| Vaccinated | |||||||||
| Unvaccinated | Ref. | Ref. | Ref. | Ref. | |||||
| At least one dose | 0.51 | 0.31–0.84 | 0.42 | 0.25–0.70 | 0.58 | 0.37–0.92 | 0.47 | 0.30–0.75 | |
| Age (years) | 1.03 | 1.03–1.04 | 1.03 | 1.03–1.04 | 1.04 | 1.03–1.04 | 1.03 | 1.03–1.04 | |
| Sex | |||||||||
| Female | Ref. | Ref. | |||||||
| Male | 1.49 | 1.25–1.78 | 1.47 | 1.24–1.74 | |||||
| Symptoms at the onset of the disease | |||||||||
| Dyspnea (yes) | 3.38 | 2.79–4.10 | 3.33 | 2.76–4.03 | |||||
| Fever (yes) | 1.26 | 1.07–1.49 | 1.24 | 1.06–1.47 | |||||
| Cough (yes) | 0.56 | 0.47–0.66 | 0.56 | 0.47–0.66 | |||||
| Comorbidity | |||||||||
| Hypertension (yes) | 3.74 | 2.95–4.75 | 3.68 | 2.90–4.67 | |||||
| Type 2 diabetes (yes) | 3.03 | 1.98–4.64 | 2.97 | 1.94–4.55 | |||||
| Chronic kidney disease (yes) | 2.51 | 1.47–4.26 | 2.65 | 1.59–4.42 | |||||
| Obesity (yes) | 3.80 | 2.61–5.53 | 3.74 | 2.57–5.44 | |||||
| Cancer (yes) | 1.06 | 0.62–1.80 | 1.17 | 0.71–1.92 | |||||
| Neurological (yes) | 3.71 | 2.42–5.69 | 3.66 | 2.39–5.61 | |||||
| Characteristics | After 28 Days | After 90 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR * | 95% CI | p | Effectiveness | HR * | 95% CI | p | Effectiveness | ||
| Vaccinated | |||||||||
| Unweighted | 0.42 | 0.25–0.70 | <0.001 | 58.0% | 0.47 | 0.30–0.75 | 0.002 | 53.0% | |
| Weighted | 0.49 | 0.26–0.89 | 0.019 | 51.0% | 0.50 | 0.28–0.89 | 0.019 | 50.0% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valladares-Garrido, M.J.; Zeña-Ñañez, S.; Peralta, C.I.; Puicón-Suárez, J.B.; Díaz-Vélez, C.; Failoc-Rojas, V.E. COVID-19 Vaccine Effectiveness at a Referral Hospital in Northern Peru: A Retrospective Cohort Study. Vaccines 2022, 10, 812. https://doi.org/10.3390/vaccines10050812
Valladares-Garrido MJ, Zeña-Ñañez S, Peralta CI, Puicón-Suárez JB, Díaz-Vélez C, Failoc-Rojas VE. COVID-19 Vaccine Effectiveness at a Referral Hospital in Northern Peru: A Retrospective Cohort Study. Vaccines. 2022; 10(5):812. https://doi.org/10.3390/vaccines10050812
Chicago/Turabian StyleValladares-Garrido, Mario J., Sandra Zeña-Ñañez, C. Ichiro Peralta, Jacqueline B. Puicón-Suárez, Cristian Díaz-Vélez, and Virgilio E. Failoc-Rojas. 2022. "COVID-19 Vaccine Effectiveness at a Referral Hospital in Northern Peru: A Retrospective Cohort Study" Vaccines 10, no. 5: 812. https://doi.org/10.3390/vaccines10050812
APA StyleValladares-Garrido, M. J., Zeña-Ñañez, S., Peralta, C. I., Puicón-Suárez, J. B., Díaz-Vélez, C., & Failoc-Rojas, V. E. (2022). COVID-19 Vaccine Effectiveness at a Referral Hospital in Northern Peru: A Retrospective Cohort Study. Vaccines, 10(5), 812. https://doi.org/10.3390/vaccines10050812








