Global Implications for COVID-19 Vaccine Series Completion: Insights from Real-World Data from the United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Komodo Healthcare Map
2.2. TriNetX Dataworks USA
2.3. Cerner Real-World EHR Data
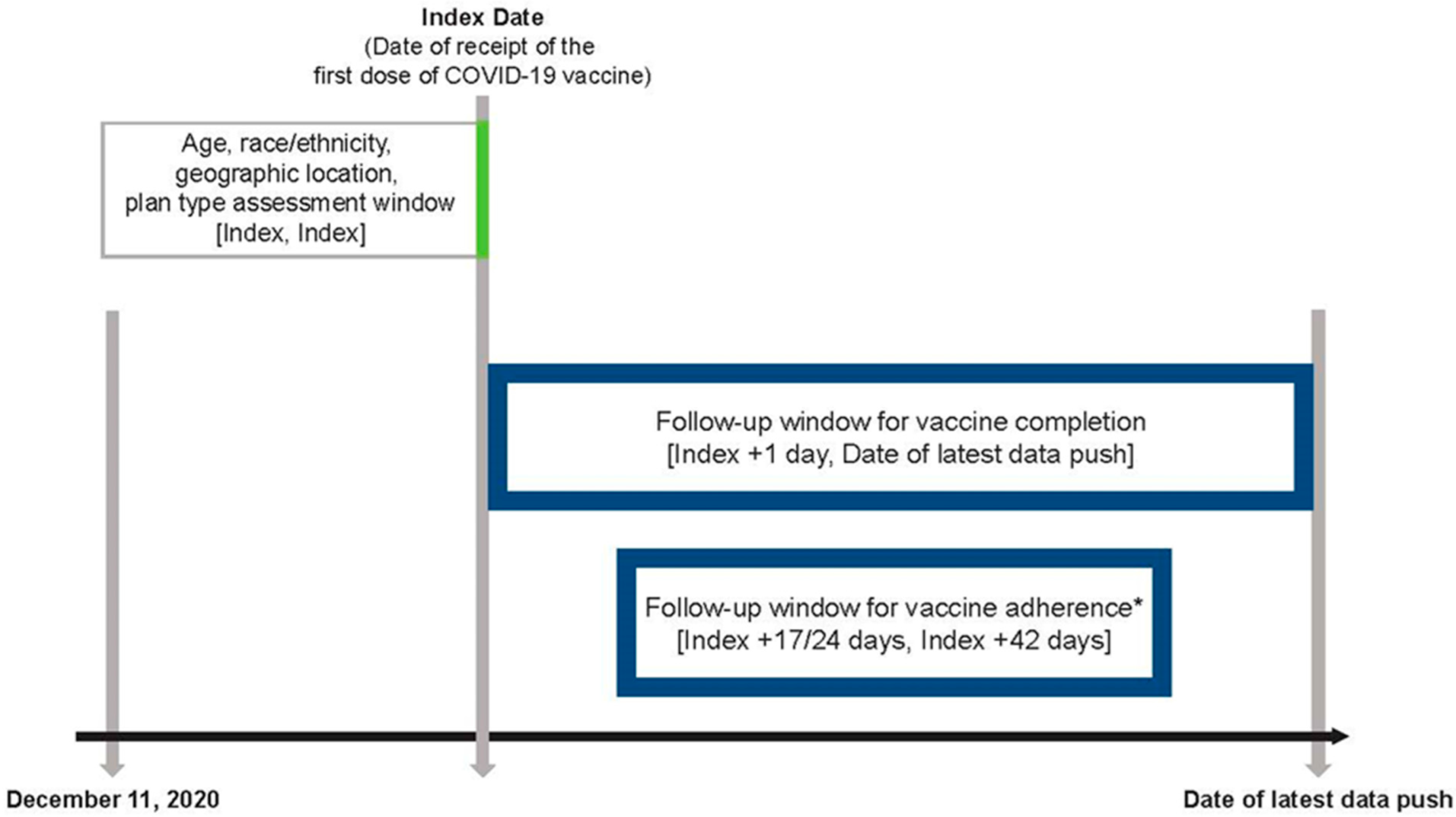
2.4. Study Design
2.5. Data Analysis
3. Results
3.1. Demographics of Vaccine Recipients
3.2. Overall Vaccine Adherence and Completion
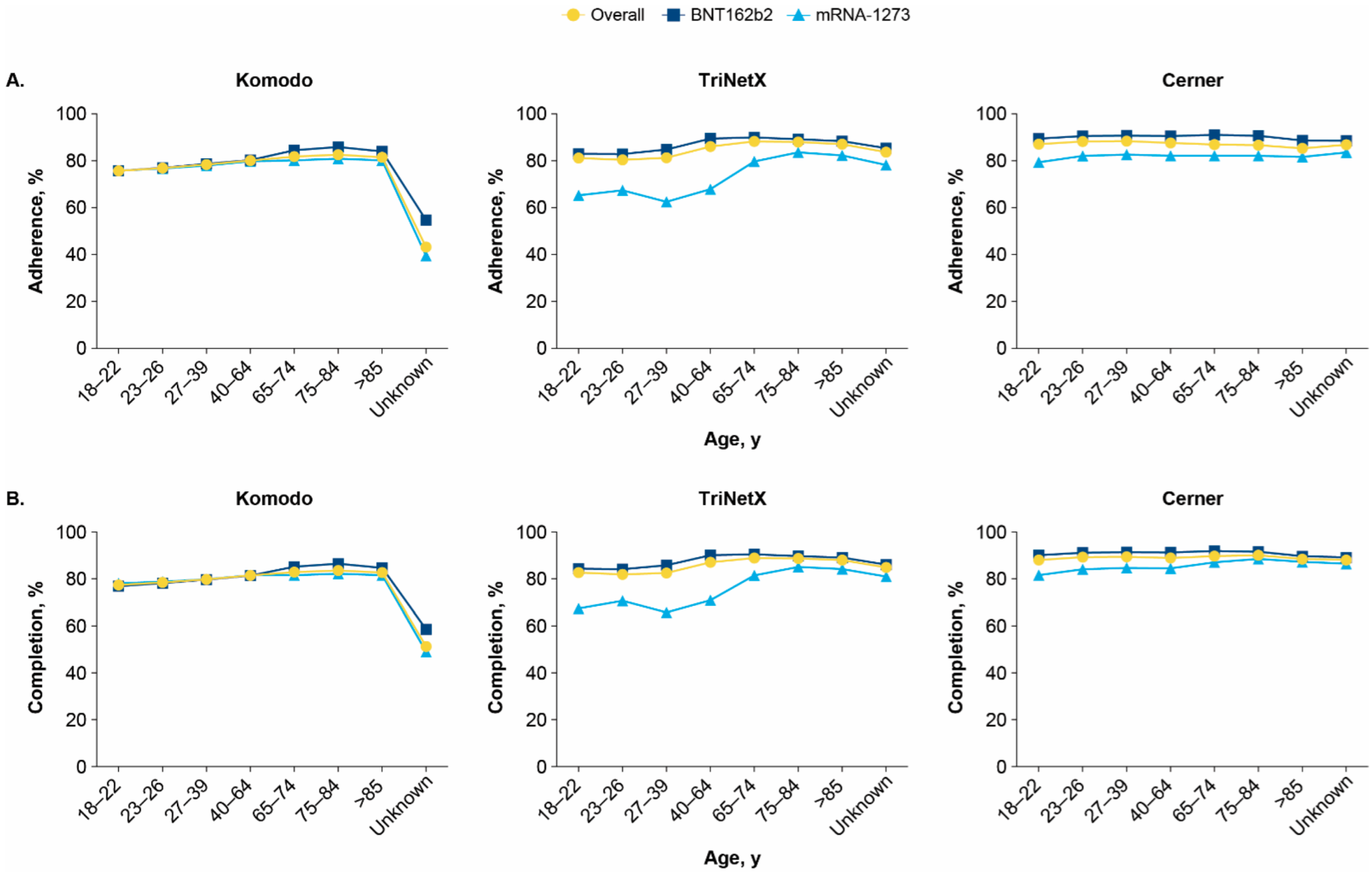
3.3. Vaccine Adherence and Completion by Age
3.4. Vaccine Adherence and Completion by Race, Ethnicity, Urban/Rural Status, and Geographic Region
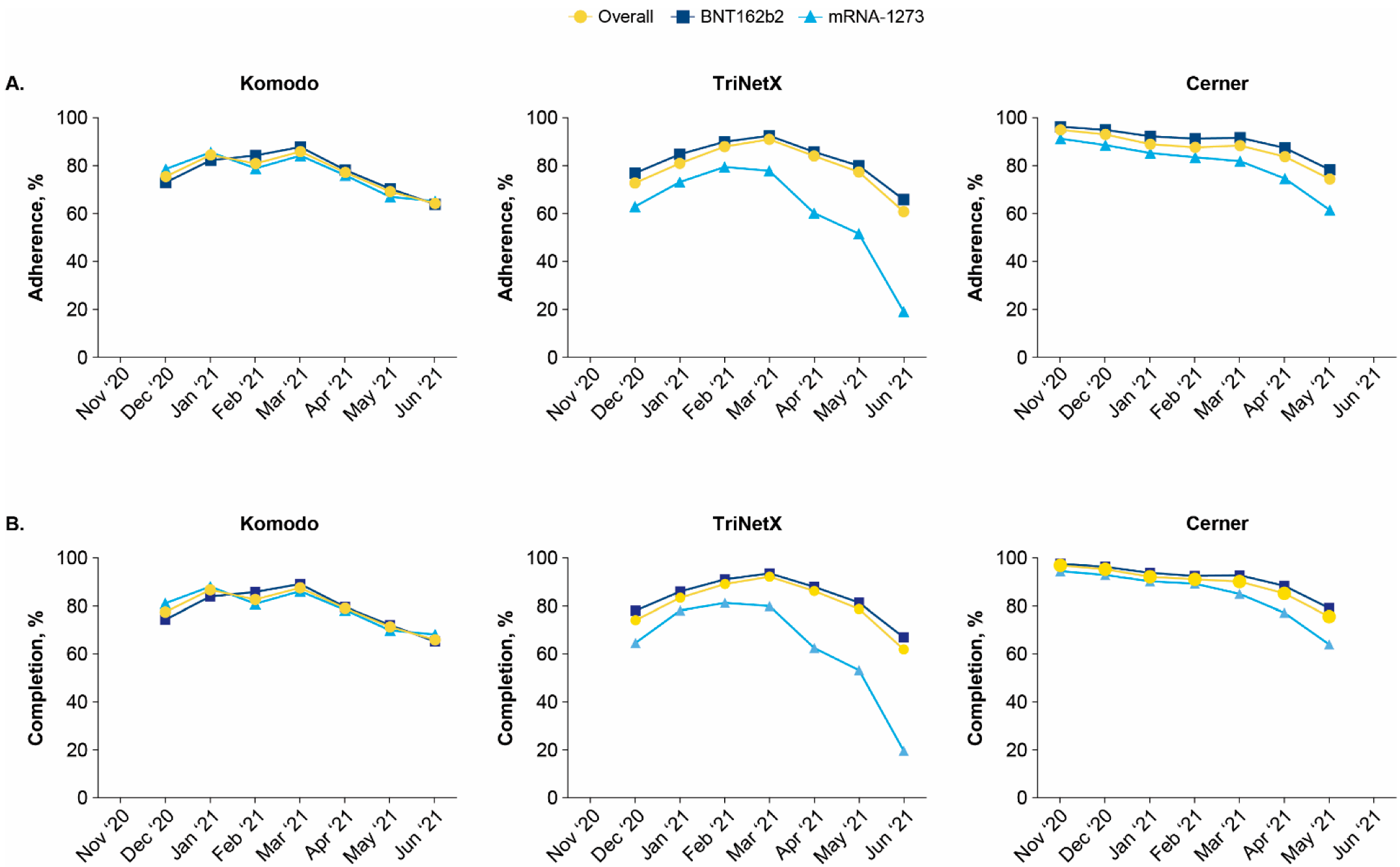
3.5. Vaccine Adherence and Completion by Index Month
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 June 2022).
- United States of America: WHO Coronavirus Disease COVID-19. Available online: https://covid19.who.int/region/amro/country/us (accessed on 1 July 2022).
- COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions (accessed on 1 July 2022).
- Stay Up to Date with Your Vaccines. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html (accessed on 1 July 2022).
- Fact Sheet for Recipients and Caregivers: Emergency Use Authorization (EUA) of the Janssen COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) in Individuals 18 Years of Age and Older. Available online: https://www.janssenlabels.com/emergency-use-authorization/Janssen+COVID-19+Vaccine-Recipient-fact-sheet.pdf (accessed on 1 July 2022).
- Natarajan, K.; Prasad, N.; Dascomb, K.; Irving, S.A.; Yang, D.H.; Gaglani, M.; Klein, N.P.; DeSilva, M.B.; Ong, T.C.; Grannis, S.J.; et al. Effectiveness of homologous and heterologous COVID-19 booster doses following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) vaccine dose against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults—VISION Network, 10 states, December 2021–March 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Hung, M.C.; Srivastav, A.; Grohskopf, L.A.; Kobayashi, M.; Harris, A.M.; Dooling, K.L.; Markowitz, L.E.; Rodriguez-Lainz, A.; Williams, W.W. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Packnett, E.; Irwin, D.E.; Novy, P.; Watson, P.S.; Whelan, J.; Moore-Schiltz, L.; Lucci, M.; Hogea, C. Meningococcal-group B (MenB) vaccine series completion and adherence to dosing schedule in the United States: A retrospective analysis by vaccine and payer type. Vaccine 2019, 37, 5899–5908. [Google Scholar] [CrossRef] [PubMed]
- Trantham, L.; Kurosky, S.K.; Zhang, D.; Johnson, K.D. Adherence with and completion of recommended hepatitis vaccination schedules among adults in the United States. Vaccine 2018, 36, 5333–5339. [Google Scholar] [CrossRef]
- Bruxvoort, K.; Slezak, J.; Huang, R.; Ackerson, B.; Sy, L.S.; Qian, L.; Reynolds, K.; Towner, W.; Solano, Z.; Mercado, C.; et al. Association of number of doses with hepatitis B vaccine series completion in US adults. JAMA Netw. Open 2020, 3, e2027577. [Google Scholar] [CrossRef]
- COVID Data Tracker. COVID-19 Vaccinations in the United States. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total (accessed on 1 July 2022).
- Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (accessed on 1 July 2022).
- Kriss, J.L.; Reynolds, L.E.; Wang, A.; Stokley, S.; Cole, M.M.; Harris, L.Q.; Shaw, L.K.; Black, C.L.; Singleton, J.A.; Fitter, D.L.; et al. COVID-19 vaccine second-dose completion and interval between first and second doses among vaccinated persons—United States, 14 December 2020–14 February 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 389–395. [Google Scholar] [CrossRef]
- Covid-19 Integrated County View. Available online: https://covid.cdc.gov/covid-data-tracker/#county-view?list_select_state=all_states&list_select_county=all_counties&data-type=Vaccinations&metric=Administered_Dose1_Pop_Pct&null=Vaccinations (accessed on 2 May 2022).
- Ioannou, G.N.; Green, P.; Locke, E.R.; Berry, K. Factors associated with early receipt of COVID-19 vaccination and adherence to second dose in the Veterans Affairs healthcare system. PLoS ONE 2021, 16, e0259696. [Google Scholar] [CrossRef]
- Galle, F.; Sabella, E.A.; Roma, P.; Ferracuti, S.; Da Molin, G.; Diella, G.; Montagna, M.T.; Orsi, G.B.; Liguori, G.; Napoli, C. Knowledge and lifestyle behaviors related to COVID-19 pandemic in people over 65 years old from Southern Italy. Int. J. Environ. Res. Public Health 2021, 18, 10872. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, R.; Chen, B.; Chen, H.; Li, Y.; Chen, Z.; Zhu, H.; Wang, H. Knowledge, perceived beliefs, and preventive behaviors related to COVID-19 among Chinese older adults: Cross-sectional web-based survey. J. Med. Internet Res. 2020, 22, e23729. [Google Scholar] [CrossRef]
- Clements, J.M. Knowledge and behaviors toward COVID-19 among US residents during the early days of the pandemic: Cross-sectional online questionnaire. JMIR Public Health Surveill. 2020, 6, e19161. [Google Scholar] [CrossRef]
- Murthy, B.P.; Sterrett, N.; Weller, D.; Zell, E.; Reynolds, L.; Toblin, R.L.; Murthy, N.; Kriss, J.; Rose, C.; Cadwell, B.; et al. Disparities in COVID-19 vaccination coverage between urban and rural counties—United States, December 14, 2020-April 10, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Saelee, R.; Zell, E.; Murthy, B.P.; Castro-Roman, P.; Fast, H.; Meng, L.; Shaw, L.; Gibbs-Scharf, L.; Chorba, T.; Harris, L.Q.; et al. Disparities in COVID-19 vaccination coverage between urban and rural counties—United States, December 14, 2020-January 31, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Diesel, J.; Sterrett, N.; Dasgupta, S.; Kriss, J.L.; Barry, V.; Vanden Esschert, K.; Whiteman, A.; Cadwell, B.L.; Weller, D.; Qualters, J.R.; et al. COVID-19 vaccination coverage among adults—United States, December 14, 2020-May 22, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 922–927. [Google Scholar] [CrossRef]
- Freeman, D.; Loe, B.S.; Chadwick, A.; Vaccari, C.; Waite, F.; Rosebrock, L.; Jenner, L.; Petit, A.; Lewandowsky, S.; Vanderslott, S.; et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2020, 1–15. [Google Scholar] [CrossRef]
- So, A.D.; Woo, J. Reserving coronavirus disease 2019 vaccines for global access: Cross sectional analysis. BMJ 2020, 371, m4750. [Google Scholar] [CrossRef]
- Usher, A.D. A beautiful idea: How COVAX has fallen short. Lancet 2021, 397, 2322–2325. [Google Scholar] [CrossRef]
- Teresa Aguado, M.; Barratt, J.; Beard, J.R.; Blomberg, B.B.; Chen, W.H.; Hickling, J.; Hyde, T.B.; Jit, M.; Jones, R.; Poland, G.A.; et al. Report on WHO meeting on immunization in older adults: Geneva, Switzerland, 22–23 March 2017. Vaccine 2018, 36, 921–931. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Boosters. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html (accessed on 1 July 2022).
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Whitaker, M.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Alden, N.B.; Yousey-Hindes, K.; Anderson, E.J.; et al. COVID-19–associated hospitalizations among adults during SARS-CoV-2 delta and omicron variant predominance, by race/ethnicity and vaccination status—COVID-NET, 14 states, July 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 466–473. [Google Scholar] [CrossRef]
- Korves, C.; Izurieta, H.S.; Smith, J.; Zwain, G.M.; Powell, E.I.; Balajee, A.; Ryder, K.; Young-Xu, Y. Relative effectiveness of booster vs. 2-dose mRNA Covid-19 vaccination in the Veterans Health Administration: Self-controlled risk interval analysis. Vaccine 2022, 40, 4742–4747. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- LaMori, J.; Feng, X.; Pericone, C.D.; Mesa-Frias, M.; Sogbetun, O.; Kulczycki, A. Hepatitis vaccination adherence and completion rates and factors associated with low compliance: A claims-based analysis of U.S. adults. PLoS ONE 2022, 17, e0264062. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, D.; Ou, W. Pneumococcal vaccination patterns among persons aged 65 years or older in the United States: A retrospective database analysis. Vaccine 2018, 36, 7574–7579. [Google Scholar] [CrossRef] [PubMed]
- Kamineni, A.; Blasi, P.R.; Gundersen, G.D.; Oliver, M.; Dunn, J.B.; Galloway, D.A.; Madeleine, M.M. Barriers to human papillomavirus vaccine series completion among insured individuals in an integrated healthcare setting. Infect. Dis. 2021, 14, 11786337211018712. [Google Scholar] [CrossRef]
- McLendon, L.; Puckett, J.; Green, C.; James, J.; Head, K.J.; Yun Lee, H.; Young Pierce, J.; Beasley, M.; Daniel, C.L. Factors associated with HPV vaccination initiation among United States college students. Hum. Vaccin. Immunother. 2021, 17, 1033–1043. [Google Scholar] [CrossRef]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; McNamara, L.A.; Stokley, S.; Singleton, J.A. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1183–1190. [Google Scholar] [CrossRef]
- Hirst, A.; Hyer, R.N.; Janssen, R.S. Comparative cost-effectiveness of a 2-dose versus 3-dose vaccine for hepatitis B prevention in selected adult populations. Vaccine 2021, 39, 4733–4741. [Google Scholar] [CrossRef]
- Prem, K.; Choi, Y.H.; Bénard, É.; Burger, E.A.; Mmath, L.H.; Laprise, J.F.; Regan, C.; Drolet, M.; Sy, S.; Abbas, K.; et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: A comparative modelling analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Barnabas, R.V.; Brown, E.R.; Onono, M.A.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Galloway, D.A.; Pinder, L.F.; Donnell, D.; Wakhungu, I.; et al. Efficacy of single-dose human papillomavirus vaccination among young African women. NEJM Evidence 2022, 1, EVIDoa2100056. [Google Scholar] [CrossRef]
- Oliver, S.E.; Wallace, M.; See, I.; Mbaeyi, S.; Godfrey, M.; Hadler, S.C.; Jatlaoui, T.C.; Twentyman, E.; Hughes, M.M.; Rao, A.K.; et al. Use of the Janssen (Johnson & Johnson) COVID-19 vaccine: Updated interim recommendations from the Advisory Committee on Immunization Practices—United States, December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, Z.; Zhang, J.; Sun, X.; Garrett, C.; Li, X. Social capital, urbanization level, and COVID-19 vaccination uptake in the United States: A national level analysis. Vaccines 2022, 10, 625. [Google Scholar] [CrossRef] [PubMed]

| Overall | BNT162b2 | mRNA-1273 | |
|---|---|---|---|
| Adherence, % a | |||
| Komodo | 79.4 | 79.5 | 79.4 |
| TriNetX | 85.6 | 88.1 | 72.9 |
| Cerner | 87.4 | 90.5 | 82.1 |
| Completion, % a | |||
| Komodo | 81.0 | 80.6 | 81.2 |
| TriNetX | 86.5 | 88.7 | 75.3 |
| Cerner | 89.2 | 91.2 | 86.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeMartino, J.K.; Wang, R.; Chen, C.Y.; Ahmad, N.; Bookhart, B.; Mascola, L. Global Implications for COVID-19 Vaccine Series Completion: Insights from Real-World Data from the United States. Vaccines 2022, 10, 1561. https://doi.org/10.3390/vaccines10091561
DeMartino JK, Wang R, Chen CY, Ahmad N, Bookhart B, Mascola L. Global Implications for COVID-19 Vaccine Series Completion: Insights from Real-World Data from the United States. Vaccines. 2022; 10(9):1561. https://doi.org/10.3390/vaccines10091561
Chicago/Turabian StyleDeMartino, Jessica K., Ruibin Wang, Cindy Y. Chen, Nina Ahmad, Brahim Bookhart, and Laurene Mascola. 2022. "Global Implications for COVID-19 Vaccine Series Completion: Insights from Real-World Data from the United States" Vaccines 10, no. 9: 1561. https://doi.org/10.3390/vaccines10091561
APA StyleDeMartino, J. K., Wang, R., Chen, C. Y., Ahmad, N., Bookhart, B., & Mascola, L. (2022). Global Implications for COVID-19 Vaccine Series Completion: Insights from Real-World Data from the United States. Vaccines, 10(9), 1561. https://doi.org/10.3390/vaccines10091561





