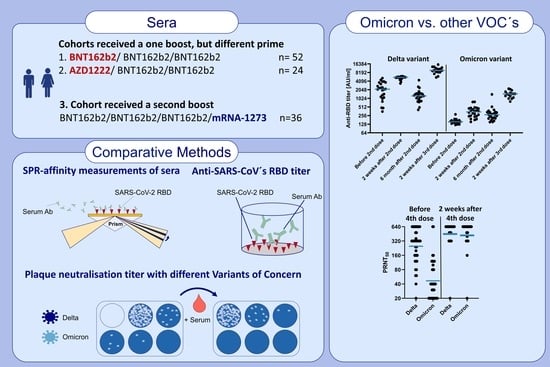
Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus Strains
2.2. RBD Protein Production
2.3. Plaque Reduction Neutralization Test (PRNT50)
2.4. ELISA
2.5. Surface Plasmon Resonance Spectroscopy (SPR)
2.6. Ethics and Collected Sera
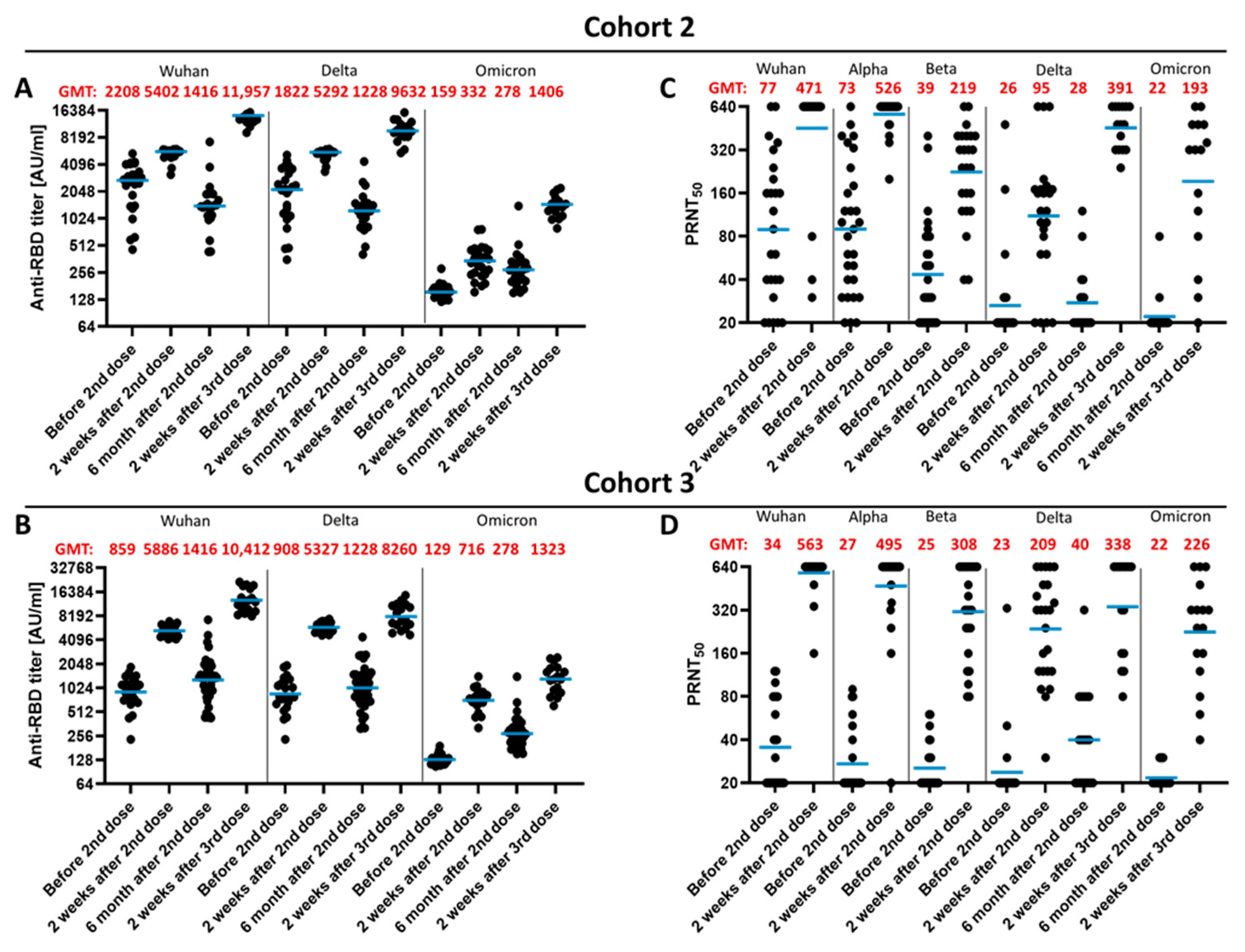
3. Results
3.1. Kinetic Parameters of Antibody Response within 14 D after Third Vaccination
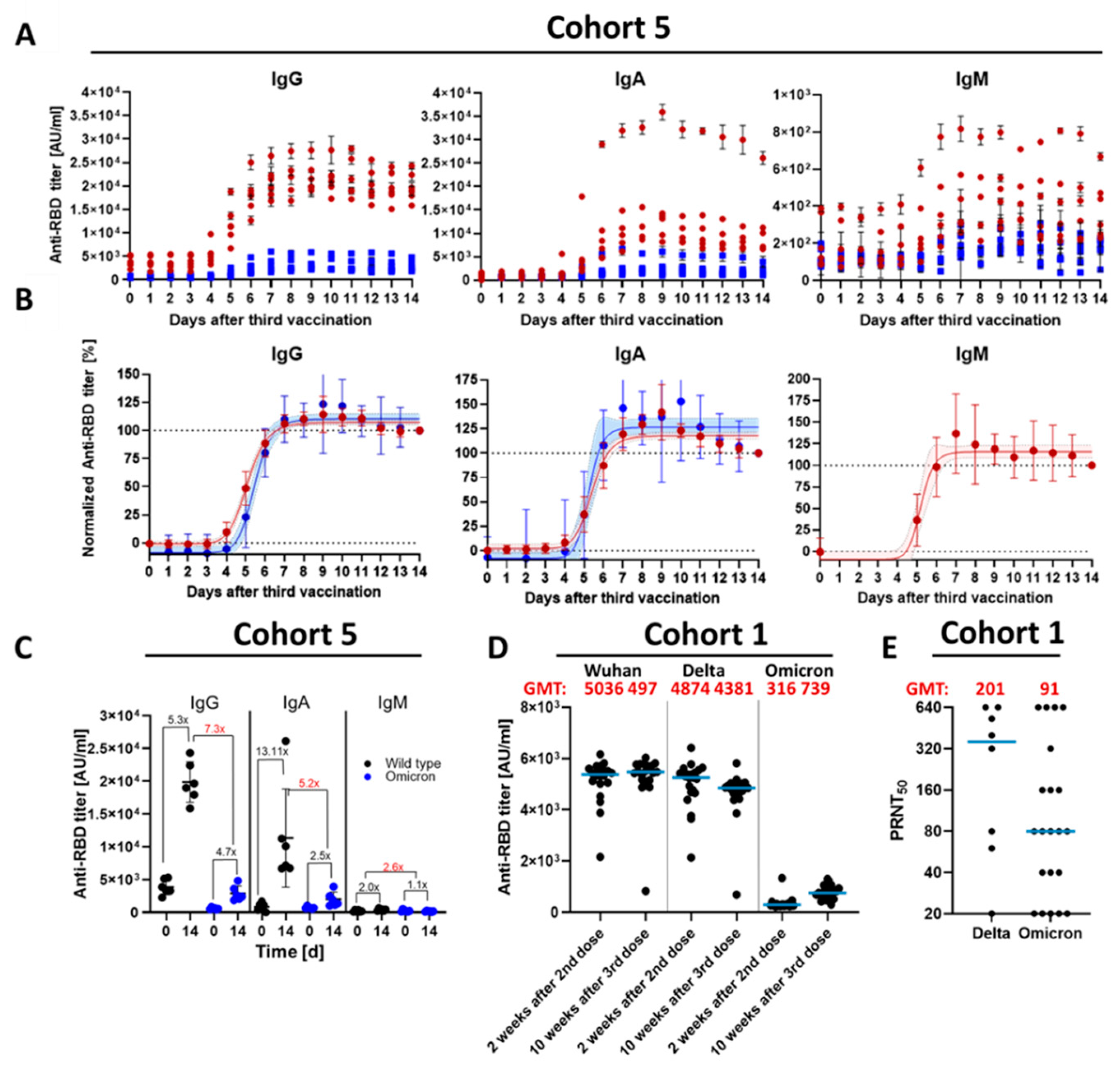
3.2. Qualitative Difference between Wuhan-Spike and Omicron-Spike Binding Antibodies in Sera of Three-Times Vaccinated Individuals
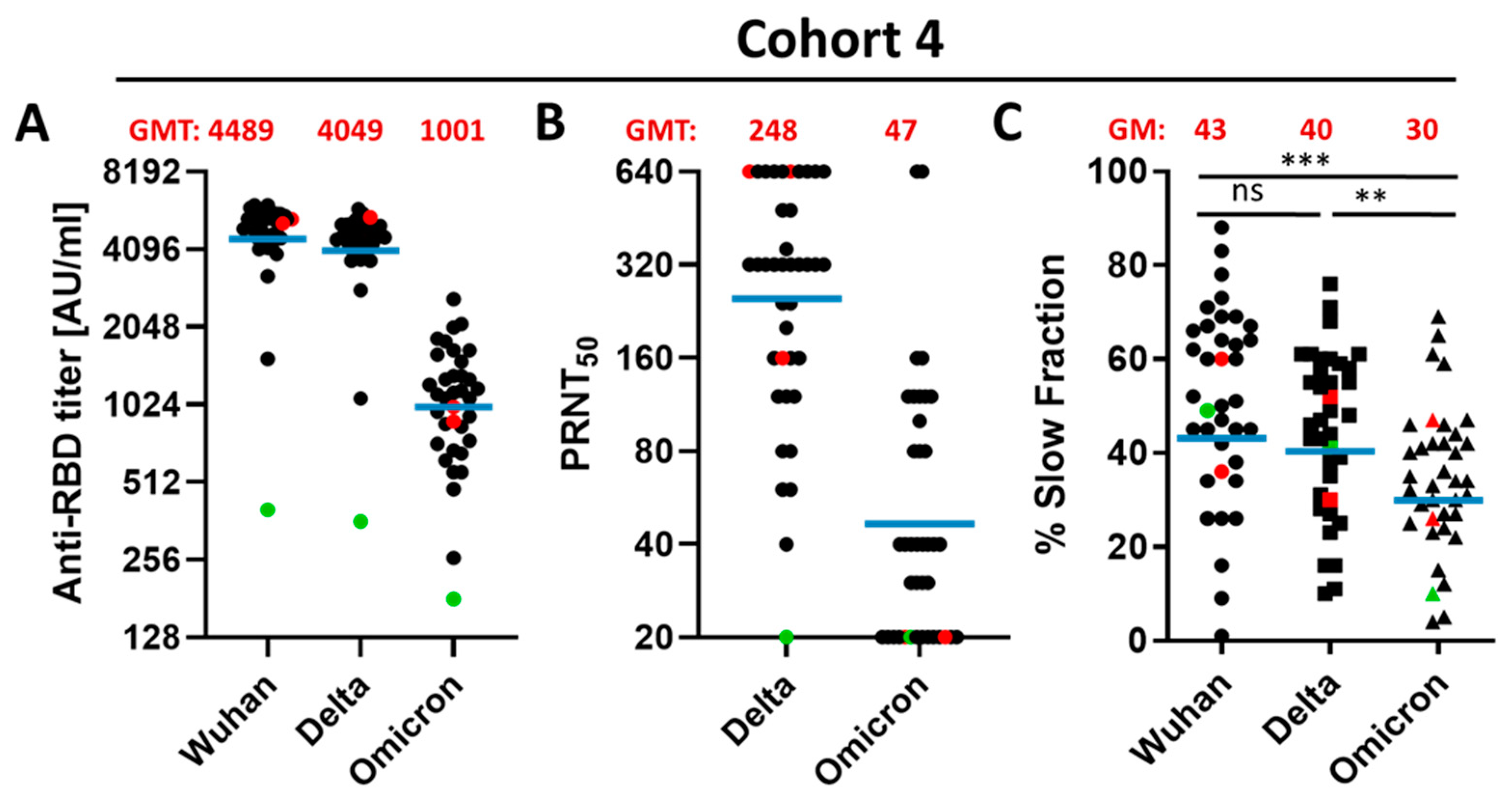
3.3. Impact of a Fourth Vaccination on Antibody Titer and Stability of the Antigen–Antibody Complexes
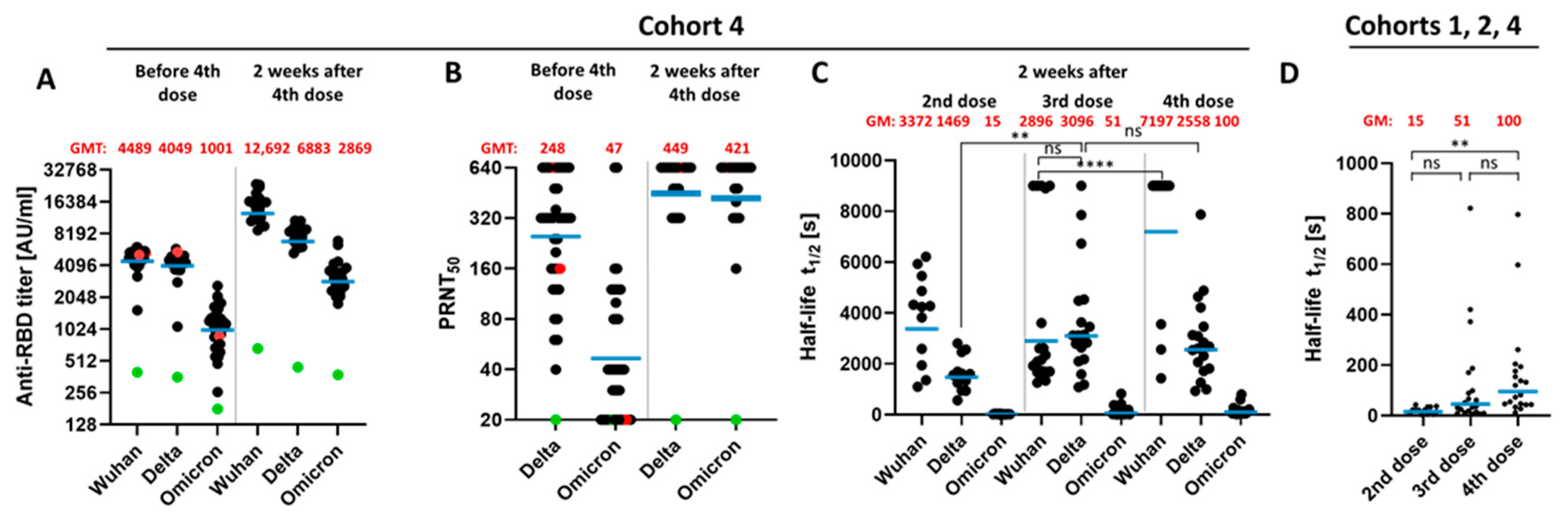
3.4. Impact of the Vaccination Regimen on Antibody Titer and Specificity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Holtgrave, D.R.; Dorabawila, V.; Conroy, M.; Greene, D.; Lutterloh, E.; Backenson, B.; Hoefer, D.; Morne, J.; Bauer, U.; et al. New COVID-19 Cases and Hospitalizations among Adults, by Vaccination Status—New York, May 3–July 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1306–1311. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; de Marco, A.; Di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Desingu, P.A.; Nagarajan, K. SARS-CoV-2 Omicron variant is spreading in different parts of the world in three different trends. J. Med. Virol. 2022, 94, 2354–2356. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J. Med. Virol. 2022, 94, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-Y.; Wang, W.-B.; Gao, R.-D.; Zhou, A.-M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Weisblum, Y.; Rutkowska, M.; Poston, D.; DaSilva, J.; Zhang, F.; Bednarski, E.; Cho, A.; Schaefer-Babajew, D.J.; Gaebler, C.; et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature 2021, 600, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Benz, N.I.; Eisert, J.; Herrlein, M.-L.; Oberle, D.; Dreher, M.; Stingl, J.C.; Hildt, C.; Hildt, E. Comirnaty-Elicited and Convalescent Sera Recognize Different Spike Epitopes. Vaccines 2021, 9, 1419. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Herrlein, M.-L.; Mhedhbi, I.; Bender, D.; Haberger, V.; Benz, N.; Eisert, J.; Stingl, J.; Dreher, M.; Oberle, D.; et al. Analysis of BNT162b2- and CVnCoV-elicited sera and of convalescent sera toward SARS-CoV-2 viruses. Allergy, 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC Recommends Additional Boosters for Certain Individuals. Available online: https://www.cdc.gov/media/releases/2022/s0328-covid-19-boosters.html (accessed on 11 April 2022).
- Ständige Impfkommission. Beschluss der STIKO zur 18. Aktualisierung der COVID-19-Impfempfehlung. Available online: https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/18_Aktualisierung_Covid.pdf?__blob=publicationFile (accessed on 11 April 2022).
- Kow, C.S.; Hasan, S.S. Real-world effectiveness of BNT162b2 mRNA vaccine: A meta-analysis of large observational studies. Inflammopharmacology 2021, 29, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Liu, H.; Wu, J.; Xiao, W.; Chen, S.; Qiu, S.; Duan, G.; Song, H.; Zhang, R. Effectiveness of BNT162b2 and mRNA-1273 Vaccines against COVID-19 Infection: A Meta-Analysis of Test-Negative Design Studies. Vaccines 2022, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Dorabawila, V.; Easton, D.; Bauer, U.E.; Kumar, J.; Hoen, R.; Hoefer, D.; Wu, M.; Lutterloh, E.; Conroy, M.B.; et al. COVID-19 Vaccine Effectiveness in New York State. N. Engl. J. Med. 2022, 386, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
| Cohort | Vaccination Schedule | Serum Collection | Samples | Age * | Sex * |
|---|---|---|---|---|---|
| 1 | D1 = 0 weeks; BNT162b2 D2 = 3 weeks; BNT162b2 D3 = 6 months; BNT162b2 | T1 = 2 weeks after second dose | n = 20 | M = 46; (20–64) | m = 12; f = 9 |
| T2 = 10 weeks after third dose | n = 20 | M = 46; (20–64) | m = 12; f = 9 | ||
| 2 | D1 = 0 weeks; BNT162b2 D2 = 3 weeks; BNT162b2 D3 = 6 months; BNT162b2 | T1 = 3 weeks after first dose (before second dose) | n = 26 | M = 43; (22–66) | m = 12; f = 14 |
| T2 = 2 weeks after second dose | n = 23 | M = 43; (22–66) | m = 11; f = 12 | ||
| T3 = 6 months after second dose (before third dose) | n = 18 | M = 43; (22–64) | m = 5; f = 13 | ||
| T4 = 2 weeks after third dose | n = 15 | M = 40; (22–60) | m = 3; f = 12 | ||
| 3 | D1 = 0 weeks; AZD1222 D3 = 8 weeks; BNT162b2 D4 = 6 months; BNT162b2 | T1 = 8 weeks after first dose (before second dose) | n = 24 | M = 50; (26–63) | m = 11; f = 13 |
| T2 = 2 weeks after second dose | n = 24 | M = 49; (26–63) | m = 10; f = 14 | ||
| T3 = 6 months after second dose (before third dose) | n = 20 | M = 50; (26–63) | m = 10; f = 10 | ||
| T4 = 2 weeks after third dose | n = 16 | M = 51; (26–63) | m = 8; f = 8 | ||
| 4 | D1 = 0 weeks; AZD1222 D2 = 3 weeks; BNT162b2 D3 = 6 months; BNT162b2 D4 = 12 months; mRNA-1273 | T1 = 6 months after third dose (before fourth dose) | n = 36 | M = 47; (25–65) | m = 12; f = 24 |
| T2 = 2 weeks after fourth dose | n = 19 | M = 46; (25–63) | m = 7; f = 12 | ||
| 5 | D1 = 0 weeks; BNT162b2 D2 = 3 weeks; BNT162b2 D3 = 6 months; BNT162b2 | T1 = One day before third vaccination | n = 6 | M = 28; (25–33) | m = 1; f = 5 |
| T2-15 = Daily | n = 6 | M = 28; (25–33) | m = 1; f = 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hein, S.; Mhedhbi, I.; Zahn, T.; Sabino, C.; Benz, N.I.; Husria, Y.; Renelt, P.M.; Braun, F.; Oberle, D.; Maier, T.J.; et al. Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination. Vaccines 2022, 10, 796. https://doi.org/10.3390/vaccines10050796
Hein S, Mhedhbi I, Zahn T, Sabino C, Benz NI, Husria Y, Renelt PM, Braun F, Oberle D, Maier TJ, et al. Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination. Vaccines. 2022; 10(5):796. https://doi.org/10.3390/vaccines10050796
Chicago/Turabian StyleHein, Sascha, Ines Mhedhbi, Tobias Zahn, Catarina Sabino, Nuka Ivalu Benz, Younes Husria, Patricia Maria Renelt, Floriane Braun, Doris Oberle, Thorsten J. Maier, and et al. 2022. "Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination" Vaccines 10, no. 5: 796. https://doi.org/10.3390/vaccines10050796
APA StyleHein, S., Mhedhbi, I., Zahn, T., Sabino, C., Benz, N. I., Husria, Y., Renelt, P. M., Braun, F., Oberle, D., Maier, T. J., Hildt, C., & Hildt, E. (2022). Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination. Vaccines, 10(5), 796. https://doi.org/10.3390/vaccines10050796