Evaluation of Meningococcal Serogroup C Bactericidal Antibodies after Primary Vaccination: A Multicentre Study, Italy
Abstract
:1. Introduction
2. Methods
2.1. Ethical Committee Approval
2.2. Serological Assay
2.3. Statistical Analysis
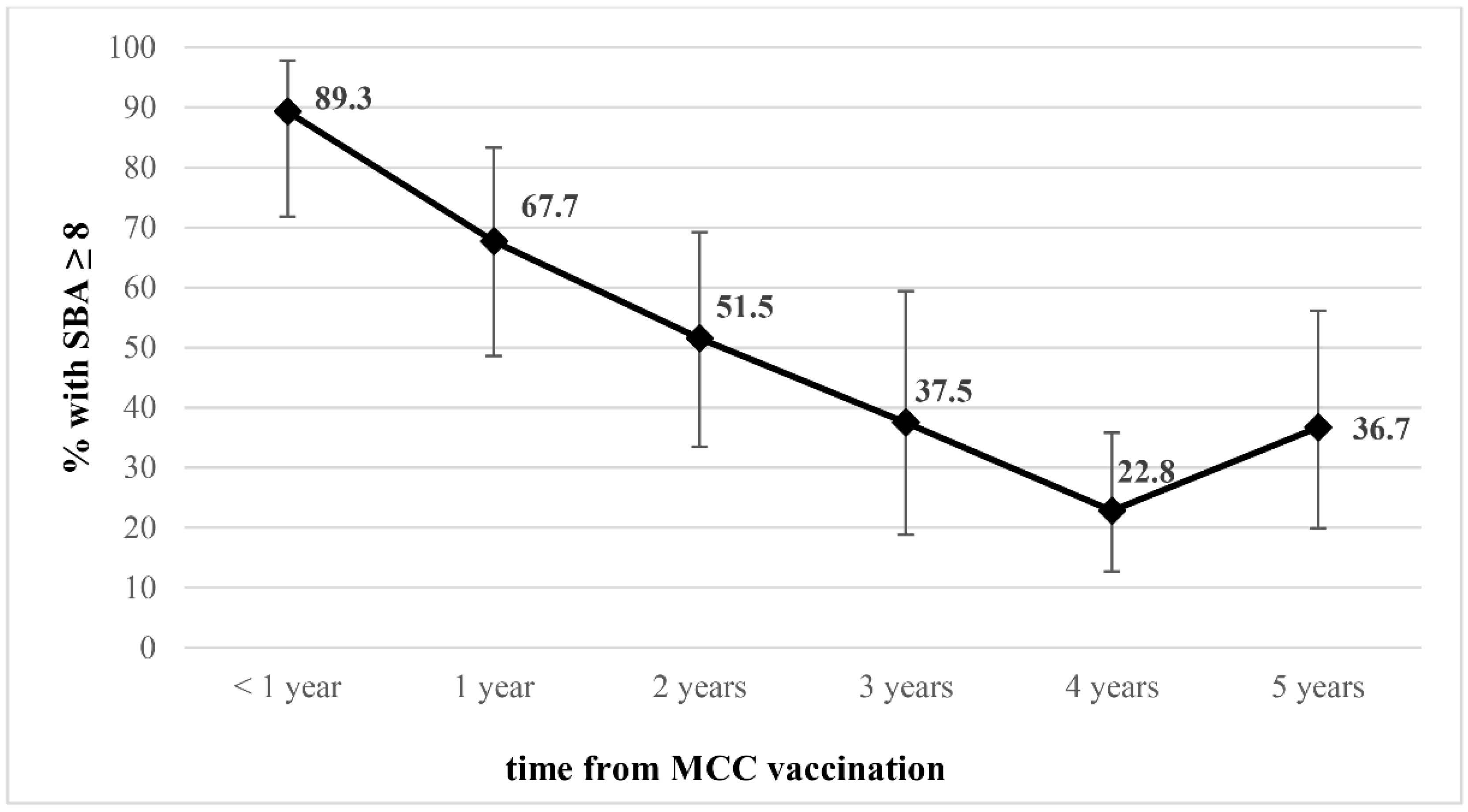
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stefanelli, P.; Fazio, C.; Sofia, T.; Neri, A.; Mastrantonio, P. Serogroup C meningococci in Italy in the era of conjugate menC vaccination. BMC Infect. Dis. 2009, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore Di Sanità. ISS Surveillance Data on Invasive Bacterial Diseases, Consolidated Report 2019. Available online: https://www.iss.it/-/rapporto-consolidato-mib-2019 (accessed on 20 April 2022).
- PNPV 2005–2007. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_543_allegato.pdf (accessed on 20 April 2022).
- PNPV 2012–2014. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf (accessed on 20 April 2022).
- PNPV 2017-2019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 20 April 2022).
- Ministry of Health. Vaccine Coverage. Available online: https://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20 (accessed on 20 April 2022).
- Sabbatucci, M.; Odone, A.; Signorelli, C.; Siddu, A.; Maraglino, F.; Rezza, G. Improved Temporal Trends of Vaccination Coverage Rates in Childhood after the Mandatory Vaccination Act, Italy 2014–2019. Vaccines 2022, 10, 2540. [Google Scholar] [CrossRef] [PubMed]
- Maiden, M.C.J.; Ibarz-Pavo´n, A.B.; Urwin, R.; Gray, S.J.; Andrews, N.J.; Clarke, S.C.; Walker, A.M.; Evans, M.R.; Kroll, J.S.; Neal, K.R.; et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 2008, 197, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Trotter, C.L.; Chandra, M.; Cano, R.; Larrauri, A.; Ramsay, M.E.; Brehony, C.; Jolley, K.A.; Maiden, M.C.; Heuberger, S.; Frosch, M. A surveillance network for meningococcal disease in Europe. FEMS Microbiol. Rev. 2007, 31, 27–36. [Google Scholar] [CrossRef]
- Bettinger, J.A.; Scheifele, D.W.; Saux, N.L.; Halperin, S.A.; Vaudry, W.; Tsang, R. The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada, Canadian Immunization Monitoring Program, Active (IMPACT). Pediatr. Infect. Dis. J. 2009, 28, 220–224. [Google Scholar] [CrossRef]
- Booy, R.; Jelfs, J.; El Bashir, H.; Nissen, M.D. Impact of meningococcal C conjugate vaccine use in Australia (Editorial). MJA 2007, 186, 108–109. [Google Scholar]
- Borrow, R.; Abad, R.; Trotter, C.; van der Klis, F.R.M.; Vazquez, J.A. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013, 31, 4477–4486. [Google Scholar] [CrossRef]
- Goldblatt, D. Conjugate vaccines. Clin. Exp. Immunol. 2000, 119, 1–3. [Google Scholar] [CrossRef]
- Richmond, P.; Borrow, R.; Goldblatt, D.; Findlow, J.; Martin, S.; Morris, R.; Cartwright, K.; Miller, E. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 2001, 183, 160–163. [Google Scholar] [CrossRef]
- Southern, J.; Crowley-Luke, A.; Borrow, R.; Andrews, N.; Miller, E. Immunogenicity of one, two or three doses of a meningococcal C conjugate vaccine conjugated to tetanus toxoid, given as a three-dose primary vaccination course in UK infants at 2, 3 and 4 months of age with acellular pertussis-containing DTP/Hib vaccine. Vaccine 2006, 24, 215–219. [Google Scholar] [CrossRef]
- Snape, M.D.; Kelly, D.F.; Green, B.; Moxon, E.R.; Borrow, R.; Pollard, A.J. Lack of serum bactericidal activity in preschool children two years after a single dose of serogroup C meningococcal polysaccharide-protein conjugate vaccine. Pediatr. Infect. Dis. J. 2005, 24, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Cohn, A.C.; MacNeil, J.R.; Harrison, L.H.; Lynfield, R.; Reingold, A.; Schaffner, W.; Zell, E.R.; Plikaytis, B.; Wang, X.; Messonnier, N.E. Active Bacterial Core Surveillance (ABCs) Team and MeningNet Surveillance Partners. Effectiveness and Duration of Protection of One Dose of a Meningococcal Conjugate Vaccine. Pediatrics 2017, 139, e20162193. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Keshavan, P.; Welsch J., A.; Han, L.; Smolenov, I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum. Vaccin. Immunother. 2016, 12, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Borrow, R.; Forsten, A.; Findlow, I.; Dhingra, M.S.; Jordanov, E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: A Phase II randomized study. Hum. Vaccin. Immunother. 2020, 16, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, S.E.; Gheesling, D.E.; LiButti, K.; Donaldson, B.J.; Harakeh, H.S.; Dykes, J.K.; Arhin, F.F.; Devi, S.J.N.; Frasch, C.E.; Huang, J.C.; et al. Standardization and a multi-laboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 1997, 4, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Balmer, P.; Miller, E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine 2005, 23, 2222–2227. [Google Scholar] [CrossRef]
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global epidemiology of invasive meningococcal disease. Popul. Health Metr. 2013, 11, 17. [Google Scholar] [CrossRef]
- Trotter, C.L.; Maiden, M.C. Meningococcal vaccines and herd immunity: Lessons learned from serogroup C conjugate vaccination programs. Expert Rev. Vaccines 2009, 8, 851–861. [Google Scholar] [CrossRef]
- Frasch, C.E.; Borrow, R.; Donnelly, J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine 2009, 27 (Suppl. 2), B112–B116. [Google Scholar] [CrossRef]
- Spinosa, M.R.; Progida, C.; Talà, A.; Cogli, L.; Alifano, P.; Bucci, C. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect. Immun. 2007, 75, 3594–3603. [Google Scholar] [CrossRef]
- Lewis, L.A.; Ram, S. Meningococcal disease and the complement system. Virulence 2014, 5, 98–126. [Google Scholar] [CrossRef] [PubMed]
- Emonts, M.; Hazelzet, J.A.; de Groot, R.; Hermans, P.W. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 2003, 3, 565–577. [Google Scholar] [CrossRef]
- Presa, J.; Findlow, J.; Vojicic, J.; Williams, S.; Serra, L. Epidemiologic Trends, Global Shifts in Meningococcal Vaccination Guidelines, and Data Supporting the Use of MenACWY-TT Vaccine: A Review. Infect. Dis. Ther. 2019, 8, 307–333. [Google Scholar] [CrossRef] [PubMed]
- Sakou, I.I.; Tzanakaki, G.; Tsolia, M.N.; Sioumala, M.; Barbouni, A.; Kyprianou, M.; Papaevangelou, V.; Tsitsika, A.; Blackwell, C.C.; Kafetzis, D.; et al. Investigation of serum bactericidal activity in childhood and adolescence 3-6 years after vaccination with a single dose of serogroup C meningococcal conjugate vaccine. Vaccine 2009, 27, 4408–4411. [Google Scholar] [CrossRef] [PubMed]
- Borrow, R.; Goldblatt, D.; Andrews, N.; Southern, J.; Ashton, L.; Deane, S.; Morris, R.; Cartwright, K.; Miller, E. Antibody persistence and immunological memory at age 4 years after meningococcal group C conjugate vaccination in children in the United Kingdom. J. Infect. Dis. 2002, 186, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Reisinger, K.; Block, S.L.; Percell, S.; Odrljin, T.; Dull, P.M.; Smolenov, I. Antibody persistence after primary and booster doses of a quadrivalent meningococcal conjugate vaccine in adolescents. Pediatr. Infect. Dis. J. 2014, 33, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Block, S.L.; Christensen, S.; Verma, B.; Xie, F.; Keshavan, P.; Dull, P.M.; Smolenov, I. Antibody persistence 5 years after vaccination at 2 to 10 years of age with Quadrivalent MenACWY-CRM conjugate vaccine, and responses to a booster vaccination. Vaccine 2015, 33, 2175–2182. [Google Scholar] [CrossRef]
- Vesikari, T.; Forsten, A.; Bianco, V.; Van der Wielen, M.; Miller, J.M. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum. Vaccin. Immunother. 2016, 12, 132–139. [Google Scholar] [CrossRef]
- Knuf, M.; Romain, O.; Kindler, K.; Walther, U.; Tran, P.M.; Pankow-Culot, H.; Fischbach, T.; Kieninger-Baum, D.; Bianco, V.; Baine, Y.; et al. Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2–10-year-old children: Results of an open, randomised, controlled study. Eur. J. Pediatr. 2013, 172, 601–612. [Google Scholar] [CrossRef]
- Borrow, R.; Andrews, N.; Findlow, H.; Waight, P.; Southern, J.; Crowley-Luke, A.; Stapley, L.; England, A.; Findlow, J.; Miller, E. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin. Vaccine Immunol. 2010, 17, 154–159. [Google Scholar] [CrossRef]

| Age at Vaccination | MCC | ACYW | Booster Vaccination a | Total |
|---|---|---|---|---|
| No. Vaccinated (%) | ||||
| <2 years (7–23 months) | 189 (93.1) | 45 (84.9) | 0 (0.0) | 234 (85.1) |
| 2 years (24–35 months) | 7 (3.5) | 1 (1.9) | 0 (0.0) | 8 (2.9) |
| ≥3 years (36–133 months) | 7 (3.5) | 7 (13.2) | 16 (100.0) | 30 (12.0) |
| Total | 203 (100.0) | 53 (100.0) | 16 (100.0) | 272 (100.0) |
| MCC Vaccine | |||
|---|---|---|---|
| Adjusted PPR | 95% CI | p-Value | |
| Time since vaccination | 0.77 | 0.71–0.84 | <0.001 |
| Age at vaccination | 1.10 | 1.01–1.20 | 0.024 |
| ACWY Vaccine | |||
| Time since vaccination | 0.87 | 0.71–1.06 | 0.171 |
| Age at vaccination | 1.03 | 0.99–1.06 | 0.145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, A.; Fabiani, M.; Barbui, A.M.; Vocale, C.; Miglietta, A.; Fazio, C.; Carannante, A.; Palmieri, A.; Vacca, P.; Ambrosio, L.; et al. Evaluation of Meningococcal Serogroup C Bactericidal Antibodies after Primary Vaccination: A Multicentre Study, Italy. Vaccines 2022, 10, 778. https://doi.org/10.3390/vaccines10050778
Neri A, Fabiani M, Barbui AM, Vocale C, Miglietta A, Fazio C, Carannante A, Palmieri A, Vacca P, Ambrosio L, et al. Evaluation of Meningococcal Serogroup C Bactericidal Antibodies after Primary Vaccination: A Multicentre Study, Italy. Vaccines. 2022; 10(5):778. https://doi.org/10.3390/vaccines10050778
Chicago/Turabian StyleNeri, Arianna, Massimo Fabiani, Anna Maria Barbui, Caterina Vocale, Alessandro Miglietta, Cecilia Fazio, Anna Carannante, Annapina Palmieri, Paola Vacca, Luigina Ambrosio, and et al. 2022. "Evaluation of Meningococcal Serogroup C Bactericidal Antibodies after Primary Vaccination: A Multicentre Study, Italy" Vaccines 10, no. 5: 778. https://doi.org/10.3390/vaccines10050778
APA StyleNeri, A., Fabiani, M., Barbui, A. M., Vocale, C., Miglietta, A., Fazio, C., Carannante, A., Palmieri, A., Vacca, P., Ambrosio, L., & Stefanelli, P. (2022). Evaluation of Meningococcal Serogroup C Bactericidal Antibodies after Primary Vaccination: A Multicentre Study, Italy. Vaccines, 10(5), 778. https://doi.org/10.3390/vaccines10050778






