Real-World Effectiveness of the mRNA COVID-19 Vaccines in Japan: A Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Data Collection
2.3. Statistical Analysis
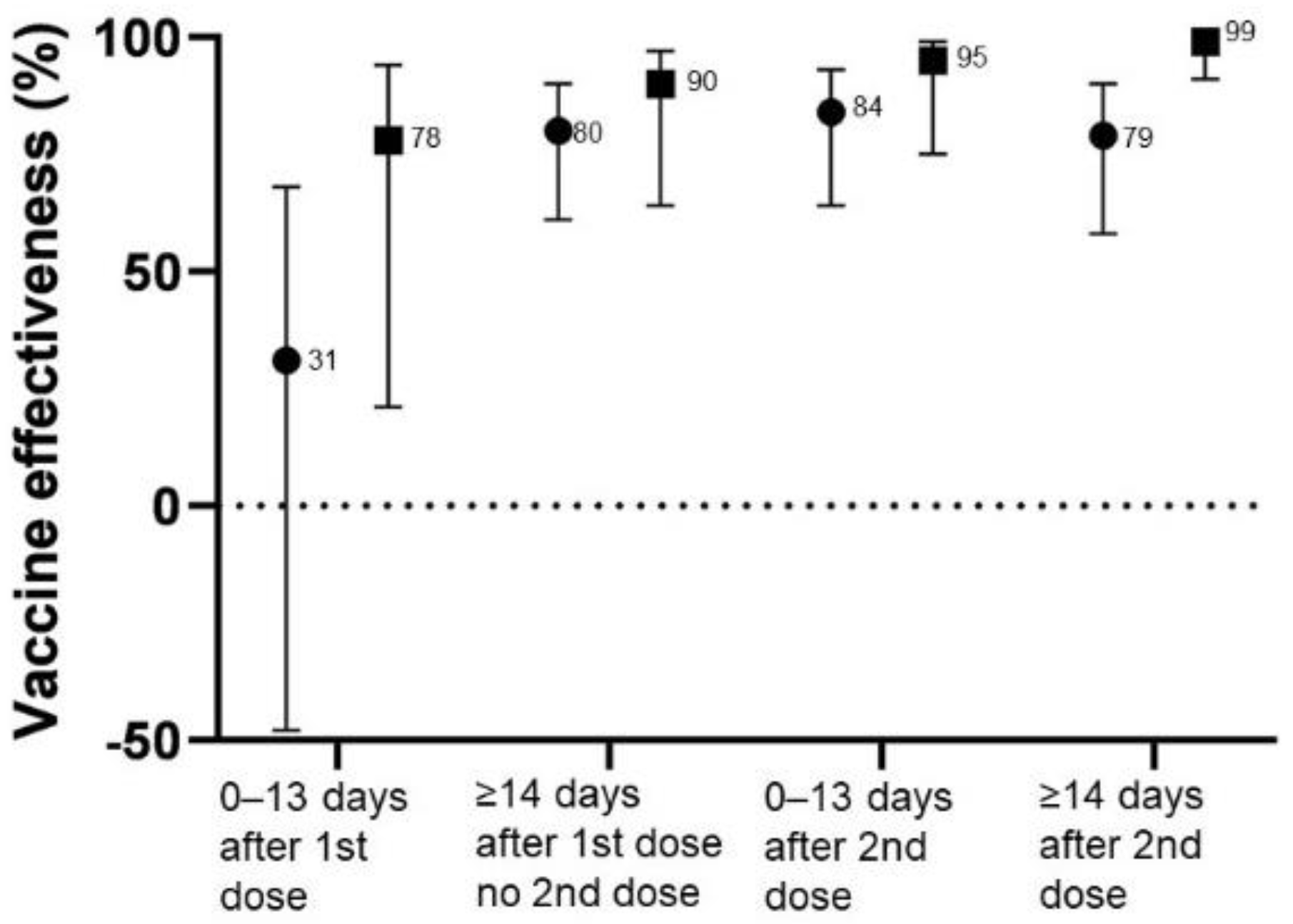
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Vitek, M.G.; Klavs, I.; Učakar, V.; Serdt, M.; Mrzel, M.; Vrh, M.; Fafangel, M. Vaccine effectiveness against severe acute respiratory infections (SARI) COVID-19 hospitalisations estimated from real-world surveillance data, Slovenia, October 2021. Eurosurveillance 2022, 27, 2101110. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Harder, T.; Külper-Schiek, W.; Reda, S.; Treskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Eurosurveillance 2021, 26, 2100920. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: A systematic review and meta-analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Teerawattananon, Y.; Anothaisintawee, T.; Pheerapanyawaranun, C.; Botwright, S.; Akksilp, K.; Sirichumroonwit, N.; Budtarad, N.; Isaranuwatchai, W. A systematic review of methodological approaches for evaluating real-world effectiveness of COVID-19 vaccines: Advising resource-constrained settings. PLoS ONE 2022, 17, e0261930. [Google Scholar] [CrossRef]
- Lio, C.F.; Cheong, H.H.; Lei, C.I.; Lo, I.L.; Yao, L.; Lam, C.; Leong, I.H. Effectiveness of personal protective health behaviour against COVID-19. BMC Public Health 2021, 21, 827. [Google Scholar] [CrossRef]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef]
- Iezadi, S.; Gholipour, K.; Azami-Aghdash, S.; Ghiasi, A.; Rezapour, A.; Pourasghari, H.; Pashazadeh, F. Effectiveness of non-pharmaceutical public health interventions against COVID-19: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0260371. [Google Scholar] [CrossRef]
- Hirt, J.; Janiaud, P.; Hemkens, L.G. Randomized trials on non-pharmaceutical interventions for COVID-19: A scoping review. BMJ Evid. Based Med. 2022. [Google Scholar] [CrossRef]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Our World Data. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 27 March 2022).
- Arashiro, Y.; Arima, Y.; Miyahara, R.; Suzuki, M.; Muraoka, H.; Ohba, K.; Uehara, Y.; Arioka, H.; Katou, H.; Oka, H.; et al. Provisional Report of a Case-Control Study on the COVID-19 Vaccine Effectiveness (First Report). Available online: https://www.niid.go.jp/niid/ja/2019-ncov/2484-idsc/10614-covid19-55.html (accessed on 16 February 2022).
- Arashiro, Y.; Arima, Y.; Suzuki, M.; Muraoka, H.; Ohba, K.; Uehara, Y.; Arioka, H.; Nouchi, H.; Katou, H.; Nagura, Y.; et al. Provisional Report of a Case-Control Study on the COVID-19 Vaccine Effectiveness (Second Report): Effectiveness During a Delta Strain Epidemic. Available online: https://www.niid.go.jp/niid/ja/2019-ncov/2484-idsc/10757-covid19-61.html (accessed on 16 February 2022).
- Maeda, H.; Morimoto, K. Vaccine Effectiveness Real-Time Surveillance for SARS-CoV-2 (VERSUS) Study: First report. 2021. Available online: https://covid-19-japan-epi.github.io/output/ (accessed on 16 February 2022).
- Maeda, H.; Morimoto, K. Vaccine Effectiveness Real-Time Surveillance for SARS-CoV-2 (VERSUS) Study: Second Report. Available online: https://covid-19-japan-epi.github.io/output/ve_nagasaki_v2.html (accessed on 16 February 2022).
- Franceschi, V.B.; Santos, A.S.; Glaeser, A.B.; Paiz, J.C.; Caldana, G.D.; Machado Lessa, C.L.; de Menezes Mayer, A.; Kuchle, J.G.; Gazzola Zen, P.R.; Vigo, A.; et al. Population-based prevalence surveys during the Covid-19 pandemic: A systematic review. Rev. Med. Virol. 2021, 31, e2200. [Google Scholar] [CrossRef]
- Ergonul, O.; Akyol, M.; Tanriover, C.; Tiemeier, H.; Petersen, E.; Petrosillo, N.; Gonen, M. National case fatality rates of the COVID-19 pandemic. Clin. Microbiol. Infect. 2021, 27, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Target Product Profiles for COVID-19 Vaccines. Available online: https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines (accessed on 16 February 2022).
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Neshin, S.A.S.; Khatami, A.; Turner, D.L.; Djalalinia, S.; Mousavi, S.A.; Mardani-Fard, H.A.; et al. Effectiveness of COVID-19 Vaccines against Delta (B.1.617.2) Variant: A Systematic Review and Meta-Analysis of Clinical Studies. Vaccines 2021, 10, 23. [Google Scholar] [CrossRef]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2021, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2021, 602, 654–656. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2021, 602, 676–681. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity and mRNA Vaccine Effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: A Prospective Observational Study. medRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Markoulatos, P.; Van de Peer, Y.; Oliver, S.G.; Amoutzias, G.D. The Neighborhood of the Spike Gene Is a Hotspot for Modular Intertypic Homologous and Nonhomologous Recombination in Coronavirus Genomes. Mol. Biol. Evol. 2022, 39, msab292. [Google Scholar] [CrossRef]
- Nikolaidis, M.; Papakyriakou, A.; Chlichlia, K.; Markoulatos, P.; Oliver, S.G.; Amoutzias, G.D. Comparative Analysis of SARS-CoV-2 Variants of Concern, Including Omicron, Highlights Their Common and Distinctive Amino Acid Substitution Patterns, Especially at the Spike ORF. Viruses 2022, 14, 707. [Google Scholar] [CrossRef] [PubMed]
| Cases (n = 398) | Controls (n = 179) | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | p-Value * | ||
| Sex | Female | 190 | 47.7 | 110 | 61.5 | 0.003 |
| Area | Fukuoka city | 381 | 95.7 | 158 | 88.3 | <0.001 |
| Age group | 16–19 | 22 | 5.5 | 25 | 14.0 | <0.001 |
| (years) | 20–29 | 68 | 17.1 | 18 | 10.1 | |
| 30–39 | 88 | 22.1 | 18 | 10.1 | ||
| 40–49 | 93 | 23.4 | 38 | 21.2 | ||
| 50–59 | 79 | 19.8 | 33 | 18.4 | ||
| 60–69 | 30 | 7.5 | 21 | 11.7 | ||
| ≥70 | 18 | 4.5 | 26 | 14.5 | ||
| PCR test | June | 3 | 0.8 | 4 | 2.2 | 0.47 |
| July | 61 | 15.3 | 30 | 16.8 | ||
| August | 318 | 79.9 | 138 | 77.1 | ||
| September | 16 | 4.0 | 7 | 3.9 | ||
| Any comorbidity | 96 | 24.1 | 46 | 25.7 | 0.68 | |
| Protective health behavior | ||||||
| Wear a mask during contact with anyone | 380 | 95.5 | 175 | 97.8 | 0.184 | |
| Wash hands for ≥20 s each time | 242 | 60.8 | 129 | 72.1 | 0.009 | |
| Use a hand sanitizer | 358 | 89.9 | 160 | 89.4 | 0.836 | |
| Keep >1.5 m distance during contact with anyone | 325 | 81.7 | 155 | 86.6 | 0.143 | |
| Regular ventilation and disinfection | 326 | 81.9 | 145 | 81.0 | 0.795 | |
| Dining with 5 or more people | 22 | 5.5 | 9 | 5.0 | 0.806 | |
| Obtain information on COVID-19 regularly | 327 | 82.2 | 148 | 82.7 | 0.879 | |
| Lifestyle | Current smoking | 102 | 25.6 | 41 | 22.9 | 0.297 |
| Current alcohol drinking | 219 | 55.0 | 78 | 43.6 | 0.034 | |
| Live in a single-family home | 137 | 34.4 | 78 | 43.6 | 0.037 | |
| Commute to work or school | 315 | 79.1 | 135 | 75.4 | 0.318 | |
| Family members commute to work or school | 285.0 | 71.6 | 158.0 | 88.3 | <0.001 | |
| Cases (n = 398) | Controls (n = 179) | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | p-Value * | ||
| Vaccination dose | 0 | 286 | 71.9 | 75 | 41.9 | <0.0001 |
| 1 | 66 | 16.6 | 41 | 22.9 | ||
| 2 | 46 | 11.6 | 63 | 35.2 | ||
| Vaccine type | BNT162b2 | 96 | 85.7 | 90 | 86.5 | 0.382 |
| mRNA-1273 | 16 | 14.3 | 14 | 13.5 | ||
| Days after the second dose; the mean (SD) | 42.9 | (39.4) | 27.4 | (21.5) | 0.02 | |
| Unvaccinated | 286 | 71.9 | 75 | 41.9 | <0.0001 | |
| Less than 13 days from 1st dose to PCR test | 39 | 9.8 | 14 | 7.8 | ||
| More than 14 days from 1st dose to PCR test | 27 | 6.8 | 27 | 15.1 | ||
| Less than 13 days from 2nd dose to PCR test | 16 | 4.0 | 23 | 12.8 | ||
| 14 to 29 days from 2nd dose to PCR test | 5 | 1.3 | 19 | 10.6 | ||
| 30 to 59 days from 2nd dose to PCR test | 12 | 3.0 | 13 | 7.3 | ||
| More than 60 days from 2nd dose to PCR test | 13 | 3.3 | 8 | 4.5 | ||
| Cases | Controls | Crude OR | 95% CI | ||
|---|---|---|---|---|---|
| Vaccination dose | 0 | 286 | 75 | 1 | (Reference) |
| 1 | 66 | 41 | 0.42 | (0.27–0.67) | |
| 2 | 46 | 63 | 0.19 | (0.12–0.30) | |
| Sex | Male | 208 | 69 | 1 | (Reference) |
| Female | 190 | 110 | 0.58 | (0.40–0.83) | |
| Area | Fukuoka city | 381 | 158 | 1 | (Reference) |
| Saga city | 17 | 21 | 0.34 | (0.17–0.65) | |
| Age group (years) | 16–19 | 22 | 25 | 1 | (Reference) |
| 20–29 | 68 | 18 | 4.29 | (1.98–9.30) | |
| 30–39 | 88 | 18 | 5.56 | (2.59–11.9) | |
| 40–49 | 93 | 38 | 2.78 | (1.40–5.52) | |
| 50–59 | 79 | 33 | 2.72 | (1.35–5.49) | |
| 60–69 | 30 | 21 | 1.62 | (0.73–3.61) | |
| ≥70 | 18 | 26 | 0.79 | (0.34–1.81) | |
| PCR test | June | 4 | 3 | 1 | (Reference) |
| July | 30 | 61 | 2.71 | (0.57–12.9) | |
| August | 138 | 318 | 3.07 | (0.68–13.9) | |
| September | 7 | 16 | 3.05 | (0.54–17.4) | |
| Any comorbidity | 96 | 46 | 0.92 | (0.61–1.38) | |
| Protective health behavior | |||||
| Wear a mask during contact with anyone | 380 | 175 | 0.48 | (0.16–1.45) | |
| Wash hands for over 20 s each time | 242 | 129 | 0.60 | (0.41–0.88) | |
| Use a hand sanitizer | 358 | 160 | 1.06 | (0.60–1.89) | |
| Keep >1.5 m distance during contact with anyone | 325 | 155 | 0.69 | (0.42–1.14) | |
| Regular ventilation and disinfection | 326 | 145 | 1.06 | (0.38–1.67) | |
| Dining with 5 or more people | 22 | 9 | 1.11 | (0.50–2.45) | |
| Obtain information on COVID-19 regularly | 327 | 148 | 0.97 | (0.61–1.54) | |
| Lifestyle | Current smoking | 102 | 41 | 1.16 | (0.77–1.76) |
| Current alcohol drinking | 219 | 78 | 1.58 | (1.11–2.25) | |
| Live in a single-family home | 137 | 78 | 0.68 | (0.48–0.98) | |
| Commute to work or school | 315 | 135 | 1.24 | (0.82–1.88) | |
| Family members commute to work or school | 285 | 158 | 0.34 | (0.21–0.57) | |
| Total | Diagnosis Period | Place | |||||
|---|---|---|---|---|---|---|---|
| June and July | August and September | Hospital | Hotel | Home | |||
| aOR * (95%CI) | aOR * (95%CI) | aOR * (95%CI) | aOR * (95%CI) | aOR * (95%CI) | aOR * (95%CI) | ||
| Vaccination dose | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| (Reference) | (Reference) | (Reference) | (Reference) | (Reference) | (Reference) | ||
| 1 | 0.35 | 0.38 | 0.32 | 0.15 | 0.29 | 0.36 | |
| (0.20–0.61) | (0.07–2.10) | (0.17–0.60) | (0.05–0.41) | (0.12–0.68) | (0.19–0.71) | ||
| 2 | 0.19 | 0.08 | 0.21 | 0.03 | 0.23 | 0.22 | |
| (0.11–0.35) | (0.01–0.65) | (0.11–0.40) | (0.01–0.13) | (0.08–0.60) | (0.11–0.45) | ||
| Unvaccinated | 1 | 1 | 1 | 1 | 1 | 1 | |
| (Reference) | (Reference) | (Reference) | (Reference) | (Reference) | |||
| Less than 13 days from 1st dose to PCR test | 0.69 | 1.21 | 0.68 | 0.22 | 0.76 | 0.79 | |
| (0.32–1.48) | (0.13–11.2) | (0.27–1.69) | (0.06–0.79) | (0.24–2.22) | (0.31–2.05) | ||
| More than 14 days from 1st dose to PCR test | 0.20 | 0.09 | 0.18 | 0.10 | 0.11 | 0.21 | |
| (0.10–0.39) | (0.01–0.94) | (0.08–0.39) | (0.03–0.36) | (0.03–0.38) | (0.09–0.48) | ||
| Less than 13 days from 2nd dose to PCR test | 0.16 | 0.69 | 0.12 | 0.05 | 0.14 | 0.16 | |
| (0.07–0.36) | (0.03–17.7) | (0.05–0.29) | (0.01–0.25) | (0.04–0.58) | (0.06–0.46) | ||
| More than 14 days from 2nd dose to PCR test | 0.21 | 0.01 | 0.30 | 0.01 | 0.31 | 0.25 | |
| (0.10–0.42) | (<0.001–0.15) | (0.14–0.65) | (<0.001–0.09) | (0.10–1.02) | (0.11–0.57) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, M.; Furue, T.; Fukuoka, M.; Iwanaga, K.; Matsuishi, E.; Miike, T.; Sakamoto, Y.; Mukai, N.; Kinugasa, Y.; Shigyo, M.; et al. Real-World Effectiveness of the mRNA COVID-19 Vaccines in Japan: A Case–Control Study. Vaccines 2022, 10, 779. https://doi.org/10.3390/vaccines10050779
Hara M, Furue T, Fukuoka M, Iwanaga K, Matsuishi E, Miike T, Sakamoto Y, Mukai N, Kinugasa Y, Shigyo M, et al. Real-World Effectiveness of the mRNA COVID-19 Vaccines in Japan: A Case–Control Study. Vaccines. 2022; 10(5):779. https://doi.org/10.3390/vaccines10050779
Chicago/Turabian StyleHara, Megumi, Takeki Furue, Mami Fukuoka, Kentaro Iwanaga, Eijo Matsuishi, Toru Miike, Yuichiro Sakamoto, Naoko Mukai, Yuki Kinugasa, Mutsumi Shigyo, and et al. 2022. "Real-World Effectiveness of the mRNA COVID-19 Vaccines in Japan: A Case–Control Study" Vaccines 10, no. 5: 779. https://doi.org/10.3390/vaccines10050779
APA StyleHara, M., Furue, T., Fukuoka, M., Iwanaga, K., Matsuishi, E., Miike, T., Sakamoto, Y., Mukai, N., Kinugasa, Y., Shigyo, M., Sonoda, N., Tanaka, M., Arase, Y., Tanaka, Y., Nakashima, H., Irie, S., & Hirota, Y. (2022). Real-World Effectiveness of the mRNA COVID-19 Vaccines in Japan: A Case–Control Study. Vaccines, 10(5), 779. https://doi.org/10.3390/vaccines10050779






