A Review of Different Vaccines and Strategies to Combat COVID-19
Abstract
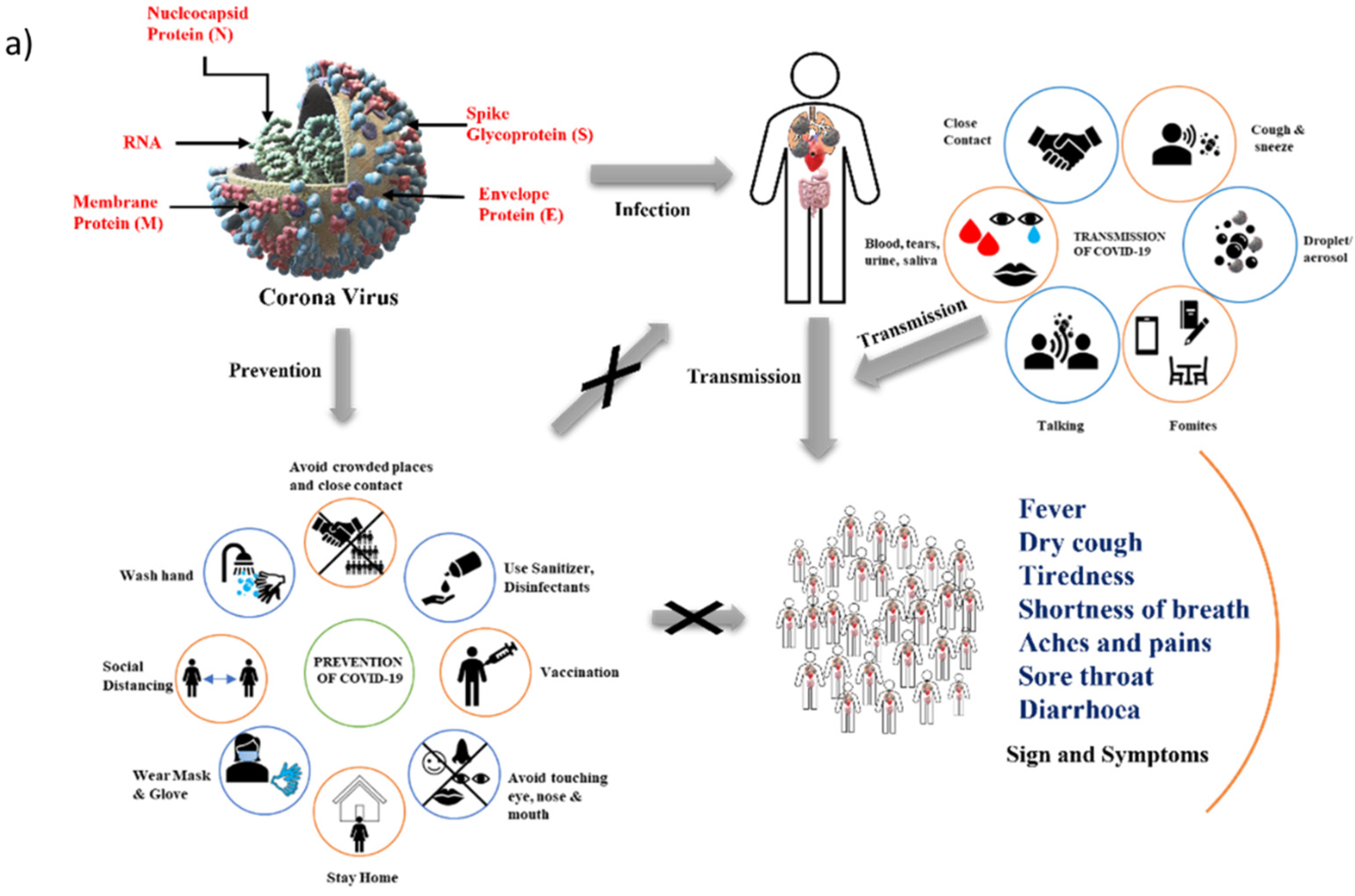
:1. Introduction
2. Background (Origin and Spread of COVID-19)
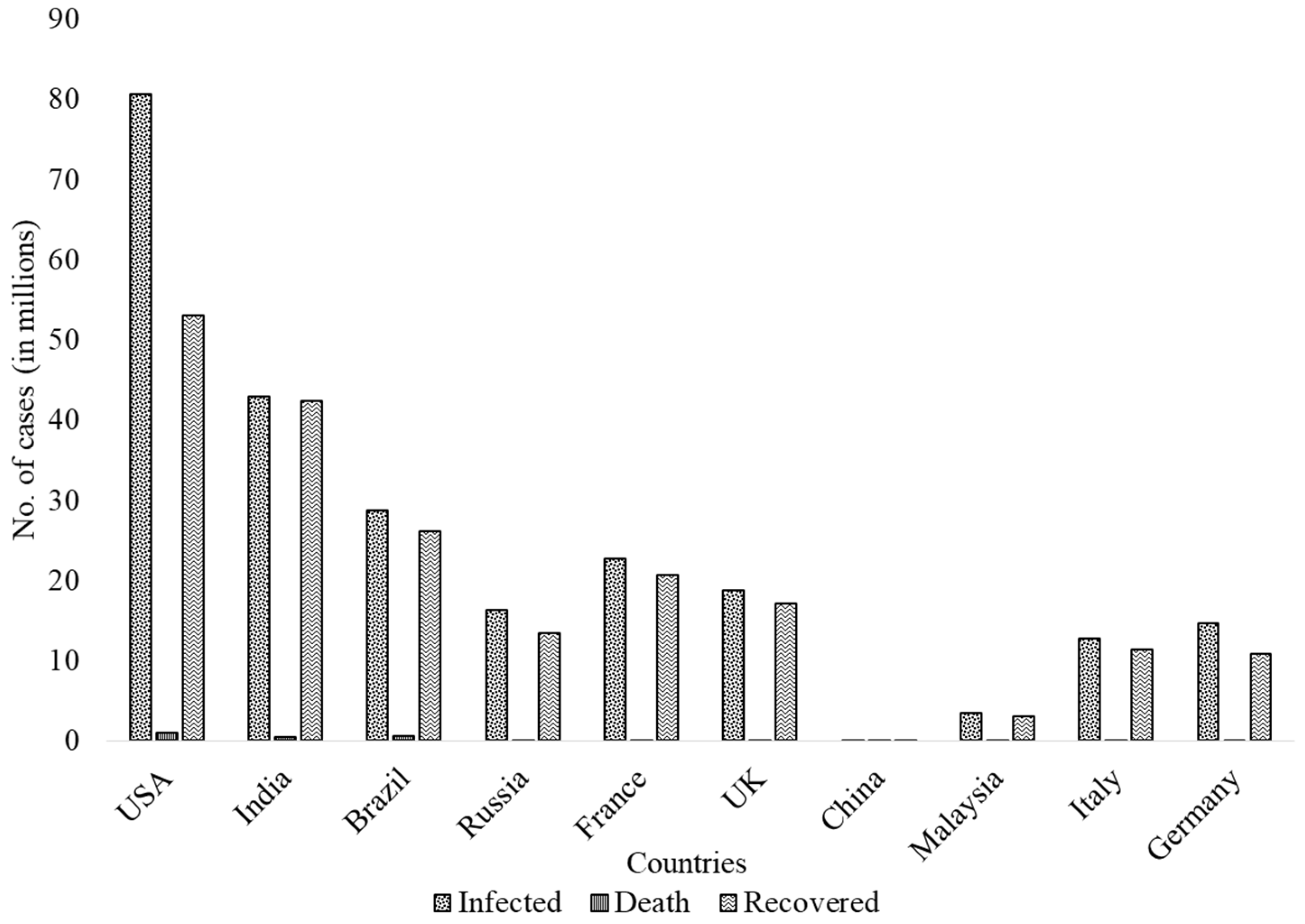
3. COVID-19 Cases across the World
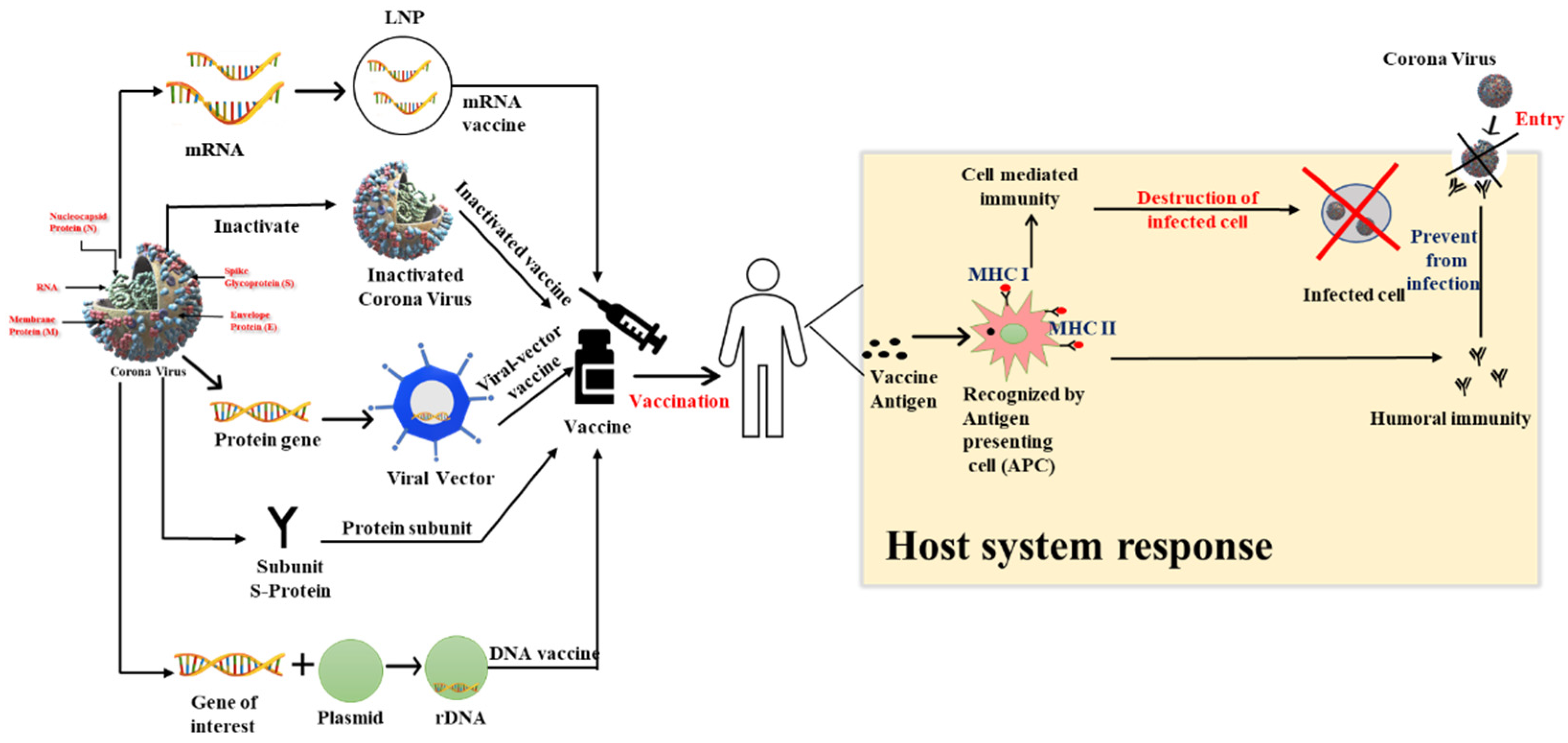
4. Vaccine Strategies
4.1. mRNA-Based Vaccines
4.1.1. Comirnaty® Vaccine-Pfizer-BioNTech
4.1.2. mRNA-1273 Vaccine-Moderna
4.2. Inactivated Virus Vaccines
4.2.1. CoronaVac Vaccine-Sinovac Biotech
4.2.2. BBIBP-CorV Vaccine-Sinopharm
4.2.3. Covaxin (BBV152) Vaccine-Bharat Biotech
4.3. Viral Vector-Based Vaccines
4.3.1. CoviShield (ChAdOx1 nCoV) Vaccine-AstraZeneca
4.3.2. Sputnik V (Gam-COVID-Vac) Vaccine-Gamaleya
4.3.3. JNJ-78436735 (Ad26.COV2.S) Vaccine-Janssen
4.4. Protein Subunit
NVX-CoV2373-Novavax
4.5. DNA-Based Vaccines
ZyCov-D Vaccine-Cadila Healthcare
5. Delta and Omicron Variants and the Current Scenario
6. Role of Antioxidants in COVID-19 Prevention
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almaghaslah, D.; Kandasamy, G.; Almanasef, M.; Vasudevan, R.; Chandramohan, S. Review on the coronavirus disease (COVID-19) pandemic: Its outbreak and current status. Int. J. Clin. Pract. 2020, 74, e13637. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Ye, D.; Liu, Q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents 2020, 56, 106137. [Google Scholar] [CrossRef]
- Harapan, H.; Itoh, N.; Yufika, A.; Winardi, W.; Keam, S.; Te, H.; Megawati, D.; Hayati, Z.; Wagner, A.L.; Mudatsir, M. Coronavirus disease 2019 (COVID-19): A literature review. J. Infect. Public Health 2020, 13, 667–6673. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.C.; Mishra, R.R.; Thatoi, H. Recent Biotechnological Tools for Diagnosis of COVID-19 Disease: A review. Biotechnol. Prog. 2021, 37, e3078. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.M.; Yu, X.H.; Tang, S.L.; Tang, C.K. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int. J. Antimicrob. Agents. 2020, 55, 105951. [Google Scholar] [CrossRef]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019-COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Ullah, H.; Ullah, A.; Gul, A.; Mousavi, T.; Khan, M.W. Novel coronavirus 2019 (COVID-19) pandemic outbreak: A comprehensive review of the current literature. Vacunas 2021, 22, 106–113. [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What we know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, M.; Norouzi, Z.; Maleki, A. COVID-19 Infection: A Novel Fatal Pandemic of the World in 2020. In Practical Cardiology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 731–735. [Google Scholar]
- Zayet, S.; Kadiane-Oussou, N.J.; Royer, P.-Y.; Toko, L.; Gendrin, V.; Klopfenstein, T. Coronavirus disease 2019: New things to know! J. Med. Virol. 2020, 92, 1767–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes Infect. 2020, 9, 469–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, P.; Lu, X.; Xu, C.; Sun, W.; Pan, B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020, 92, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Kuo, R.L.; Shih, S.R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A Multidisciplinary Review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Dhananjaya, K.V. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, SARS and MERS: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Gómez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Salje, H.; Kiem, C.T.; Lefrancq, N.; Courtejoie, N.; Bosetti, P.; Paireau, J.; Andronico, A.; Hozé, N.; Richet, J.; Dubost, C.-L.; et al. Estimating the burden of SARS-CoV-2 in France. Science 2020, 369, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, T.; Platt, D.; Parida, L. Variant analysis of SARS-CoV-2 genomes. Bull. World Health Organ. 2020, 98, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Abukhalaf, A.A.; Alomar, A.A.; AlMutairi, F.J.; Usmani, A.M.; Klonoff, D.C. Impact of lockdown on COVID-19 prevalence and mortality during 2020 pandemic: Observational analysis of 27 countries. Eur. J. Med. Res. 2020, 25, 56. [Google Scholar] [CrossRef]
- Brodeur, A.; Gray, D.; Islam, A.; Bhuiyan, S. A literature review of the economics of COVID-19. J. Econ. Surv. 2021, 35, 1007–1044. [Google Scholar] [CrossRef]
- Kharroubi, S.; Saleh, F. Are Lockdown Measures Effective Against COVID-19? Front. Public Health 2020, 8, 549692. [Google Scholar] [CrossRef]
- Thakar, A.; Panara, K.; Goyal, M.; Kumari, R.; Sungchol, K. Impact of AYUSH interventions on COVID-19: A protocol for a living systematic review and meta-analysis. F1000Research 2020, 10, 674. [Google Scholar] [CrossRef]
- Basak, A.; Rahaman, S.; Guha, A.; Sanyal, T. Dynamics of the Third wave, modelling COVID-19 pandemic with an outlook towards India. medRxiv-Epidemiol. 2021. [Google Scholar] [CrossRef]
- Rawat, D.; Dixit, V.; Gulati, S.; Gulati, S.; Gulati, A. Impact of COVID-19 outbreak on lifestyle behaviour: A review of studies published in India. Diabetes Metab. Syndr. 2021, 15, 331–336. [Google Scholar] [CrossRef]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef]
- Wibawa, T. COVID-19 vaccine research and development: Ethical issues. Trop. Med. Int. Health 2021, 26, 14–19. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Lahariya, C. Vaccine epidemiology: A review. J. Fam. Med. Prim. Care 2016, 5, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, S.M.; O’Shea, K.J.; Ferguson, M.C.; Bottazzi, M.E.; Wedlock, P.T.; Strych, U.; McKinnell, J.A.; Siegmund, S.S.; Cox, S.N.; Hotez, P.J.; et al. Vaccine Efficacy Needed for a COVID-19 Coronavirus Vaccine to Prevent or Stop an Epidemic as the Sole Intervention. Am. J. Prev. Med. 2020, 59, 493–503. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.; Rao, Y.; Rao, X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Soleimanpour, S.; Yaghoubi, A. COVID-19 vaccine: Where are we now and where should we go? Expert Rev. Vaccines 2021, 20, 23–44. [Google Scholar] [CrossRef]
- Forni, G.; Mantovani, A. On behalf of the COVID-19 Commission of Accademia Nazionale deiLincei, Rome. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Altawalah, H. Antibody Responses to Natural SARS-CoV-2 Infection or after COVID-19 Vaccination. Vaccines 2021, 9, 910. [Google Scholar] [CrossRef]
- Lundstrom, K. The Current Status of COVID-19 Vaccines. Front. Genome Ed. 2020, 2, 579297. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alturki, S.O.; Alturki, S.O.; Connors, J.; Cusimano, G.; Kutzler, M.A.; Izmirly, A.M.; Haddad, E.K. The 2020 Pandemic: Current SARS-CoV-2 Vaccine Development. Front. Immunol. 2020, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Srivastava, N.; Mishra, G.; Dhama, K.; Kumar, S.; Puri, B.; Saxena, S.K. Recombinant vaccines for COVID-19. Hum. Vaccines Immunother. 2020, 16, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Coppeta, L.; Balbi, O.; Grattagliano, Z.; Mina, G.G.; Pietroiusti, A.; Magrini, A.; Bolcato, M.; Trabucco Aurilio, M. First Dose of the BNT162b2 mRNA COVID-19 Vaccine Reduces Symptom Duration and Viral Clearance in Healthcare Workers. Vaccines 2021, 9, 659. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Badiani, A.A.; Patel, J.A.; Ziolkowski, K.; Nielsen, F. Pfizer: The miracle vaccine for COVID-19? Public Health Pract. 2020, 1, 100061. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis after Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stahel, V.P. The safety of Covid-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Pfizer COVID vaccine protects against worrying coronavirus variants. Nature 2021, 593, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine-United States. MMWR Morb. Mortal. Wkly Rep. 2020, 69, 1653–1656. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-COV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Fernandes, A.; Chaudhari, S.; Jamil, N.; Gopalakrishnan, G. COVID-19 Vaccine. Endocr. Pract. 2021, 27, 170–172. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Hasan, M.R.; Malek, J.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat. Med. 2021, 27, 1614–1621. [Google Scholar] [CrossRef]
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2020, 50, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Ophinni, Y.; Hasibuan, A.S.; Widhani, A.; Maria, S.; Koesnoe, S.; Yunihastuti, E.; Karjadi, T.H.; Rengganis, I.; Djauzi, S. COVID-19 Vaccines: Current Status and Implication for Use in Indonesia. Acta Med. Indones. 2020, 52, 388–412. [Google Scholar]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side Effects and Perceptions Following Sinopharm COVID-19 Vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef]
- Thiagarajan, K. What do we know about India’s Covaxin vaccine? BMJ 2021, 373, n997. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Kaur, U.; Ojha, B.; Pathak, B.; Singh, A.; Giri, K.; Singh, A.; Das, A.; Misra, A.; Yadav, A.; Chakrabarti, S.S.; et al. A prospective observational safety study on ChAdOx1 nCoV-19 corona virus vaccine (recombinant) use in healthcare workers-first results from India. EClinicalMedicine 2021, 38, 101038. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Rab, S.; Afjal; Javaid, M.; Haleem, A.; Vaishya, R. An update on the global vaccine development for coronavirus. Diabetes Metab. Syndr. 2020, 14, 2053–2055. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Kostoff, R.N.; Brigs, M.B.; Porter, A.L.; Spandidos, D.A.; Tsatsakis, A. COVID-19 vaccine safety. Int. J. Mol. Med. 2020, 46, 1599–1602. [Google Scholar] [PubMed]
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643. [Google Scholar] [CrossRef]
- Nogrady, B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature 2021, 595, 339–340. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef]
- Shay, D.K.; Gee, J.; Su, J.R.; Myers, T.R.; Marquez, P.; Liu, R.; Zhang, B.; Licata, C.; Clark, T.A.; Shimabukuro, T.T. Safety Monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine—United States, March–April 2021. MMWR 2021, 70, 680–684. [Google Scholar]
- Edwards, K.; Orenstein, W. COVID-19: Vaccines to Prevent SARS-CoV-2 Infection. U: UpToDate [Internet]. 2022. Available online: https://www.uptodate.com/contents/covid-19-vaccines (accessed on 2 February 2022).
- Zhang, N.; Tang, J.; Lu, L.; Jiang, S.; Du, L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2015, 202, 151–159. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [Green Version]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Park, K.S.; Sun, X.; Aikins, M.E.; Moon, J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021, 169, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development. Arch. Med. Res. 2021, 52, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and efficacy of NVX-CoV2373 covid-19 vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, S.; Imran, I.; Al Mughairbi, F.; Sheikh, F.S.; Hussain, J.; Khan, A.; Al-Harrasi, A. Vaccine Development against COVID-19: Study from Pre-Clinical Phases to Clinical Trials and Global Use. Vaccines 2021, 9, 836. [Google Scholar] [CrossRef]
- Lee, L.Y.Y.; Izzard, L.; Hurt, A.C. A Review of DNA Vaccines against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef] [Green Version]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Prompetchara, E.; Ketloy, C.; Tharakhet, K.; Kaewpang, P.; Buranapraditkun, S.; Techawiwattanaboon, T.; Sathean-Anan-Kun, S.; Pitakpolrat, P.; Watcharaplueksadee, S.; Phumiamorn, S.; et al. DNA vaccine candidate encoding SARS-CoV-2 spike proteins elicited potent humoral and Th1 cell-mediated immune responses in mice. PLoS ONE 2021, 16, e0248007. [Google Scholar] [CrossRef]
- Silveira, M.M.; Moreira, G.M.S.G.; Mendonça, M. DNA vaccines against COVID-19: Perspectives and challenges. Life Sci. 2021, 267, 118919. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Malinczak, C.A. Harnessing Cellular Immunity for Vaccination against Respiratory Viruses. Vaccines 2020, 8, 783. [Google Scholar] [CrossRef]
- Debock, I.; Flamand, V. Unbalanced Neonatal CD4(+) T-Cell Immunity. Front. Immunol. 2014, 5, 393. [Google Scholar] [CrossRef] [PubMed]
- Lukens, J.R.; Anand, P.K. Adapt(ed) to repair-TH2 immune responses in the bladder promote recurrent infections. Nat. Immunol. 2020, 21, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shang, J.; Jiang, S.; Du, L. Subunit Vaccines against Emerging Pathogenic Human Coronaviruses. Front. Microbiol. 2020, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Rajanathan, C.T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef]
- Jain, S.; Batra, H.; Yadav, P.; Chand, S. COVID-19 Vaccines Currently under Preclinical and Clinical Studies, and Associated Antiviral Immune Response. Vaccines 2020, 8, 649. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Pung, R.; Mak, T.M.; CMMID COVID-19 Working Group; Kucharski, A.J.; Lee, V.J. Serial intervals in SARS-CoV-2 B.1.617.2 variant cases. Lancet 2021, 398, 837–838. [Google Scholar] [CrossRef]
- Nasreen, S.; He, S.; Chung, H.; Brown, K.A.; Gubbay, J.B.; Buchan, S.A.; Fell, D.B.; Austin, P.C.; Schwartz, K.L.; Sundaram, M.E.; et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. medRxiv 2021, 21259420. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Adams, L.; Hobson, P.; Hatipoglu, E.; et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet 2021, 398, 207–209. [Google Scholar] [CrossRef]
- Kirola, L. Genetic emergence of B.1.617.2 in COVID-19. New Microbes New Infect. 2021, 43, 100929. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Moona, A.A.; Daria, S.; Asaduzzaman, M.; Islam, M.R. Bangladesh reported delta variant of coronavirus among its citizen: Actionable items to tackle the potential massive third wave. Infect. Prev. Pract. 2021, 3, 100159. [Google Scholar] [CrossRef]
- Farinholt, T.; Doddapaneni, H.; Qin, X.; Menon, V.; Meng, Q.; Metcalf, G.; Chao, H.; Gingras, M.; Avadhanula, V.; Farinholt, P.; et al. Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021, 19, 255. [Google Scholar] [CrossRef]
- Edara, V.V.; Pinsky, B.A.; Suthar, M.S.; Lai, L.; Davis-Gardner, M.E.; Floyd, K.; Flowers, M.W.; Wrammert, J.; Hussaini, L.; Ciric, C.R.; et al. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N. Engl. J. Med. 2021, 385, 664–666. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 and Novel Therapeutics against Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Shi, Y.; Xiong, Q.; Liu, Z.; Gao, Q.; Zou, L.; et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Nat. Commun. 2021, 13, 460. [Google Scholar] [CrossRef]
- Shawn, R. The COVID-19 Delta Variant: Here’s Everything You Need to Know. Healthline. 2021. Available online: https://www.healthline.com/health-news/the-covid-19-delta-variant-heres-everything-you-need-to-know (accessed on 8 February 2022).
- Bernal, L.J.; Andrews, N.; Gower, C.; Gallagher, E.; Utsi, L.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; et al. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Landau, N.R. Review 1: “Neutralization against B.1.351 and B.1.617.2 with sera of COVID-19 recovered cases and vaccinees of BBV152”. Rapid Rev. COVID-19 2021. [Google Scholar] [CrossRef]
- Baraniuk, C. Covid-19: How effective are vaccines against the delta variant? BMJ 2021, 374, n1960. [Google Scholar] [CrossRef] [PubMed]
- Ettaboina, S.K.; Nakkala, K.; Laddha, K.S. A Mini Review on SARS-COVID-19-2 Omicron Variant (B.1.1.529). SciMed. J. 2021, 3, 399–406. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, S.; Wu, B.; Yang, Q.; Chen, A.; Li, Y.; Zhang, Y.; Pan, T.; Zhang, H.; He, X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Sig. Transduct. Target. Ther. 2021, 6, 430. [Google Scholar] [CrossRef]
- Ranjan, R. Omicron Impact in India: Analysis of the Ongoing COVID-19 Third Wave Based on Global Data. medRxiv 2022. [Google Scholar] [CrossRef]
- He, X.; Hong, W.; Pan, X.; Lu, G.; Wei, X. SARS-CoV-2 Omicron variant: Characteristics and prevention: Characteristics and prevention. MedComm 2021, 2, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Kumar, S.; Ansari, S.; Paweska, J.T.; Maurya, V.K.; Tripathi, A.K.; Abdel-Moneim, A.S. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J. Med. Virol. 2022, 94, 1738–1744. [Google Scholar] [CrossRef]
- Corum, J.; Zimmer, C. Tracking Omicron and Other Coronavirus Variants. The Newyork Times. Available online: https://www.nytimes.com/interactive/2021/health/coronavirus-variant-tracker.html (accessed on 8 February 2022).
- Elflein, J. Number of SARS-CoV-2 Omicron Variant Cases Worldwide as of February 7, 2022, by Country or Territory. Available online: https://www.statista.com/statistics/1279100/number-omicron-variant-worldwide-by-country/ (accessed on 8 February 2022).
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Vihta, K.-D.; Pouwels, K.B.; Peto, T.E.; Pritchard, E.; House, T.; Studley, R.; Rourke, E.; Cook, D.; Diamond, I.; Crook, D.; et al. Omicron-Associated Changes in Sars-Cov-2 Symptoms in the United Kingdom. medRxiv 2022. [Google Scholar] [CrossRef]
- Christie, B. Covid-19: Early studies give hope omicron is milder than other variants. BMJ 2021, 375, n3144. [Google Scholar] [CrossRef]
- Sheikh, A.; Kerr, S.; Woolhouse, M.; McMenamin, J.; Robertson, C. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: National cohort with nested test negative design study in Scotland (EAVE II): A national cohort study with nested test-negative design. Lancet Infect Dis. 2022. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Kaçmaz, R. The Role of Antioxidants in the Covid-19 Pandemic. Int. J. Biochem. Physiol. 2020, 5, 000190. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. How to characterize an antioxidant—An update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [PubMed]
- De Flora, S.; Balansky, R.; La Maestra, S. Antioxidants and COVID-19. J. Prev. Med. Hyg. 2021, 62 (Suppl. S3), E34–E45. [Google Scholar]
- Lammi, C.; Arnoldi, A. Food-derived antioxidants and COVID-19. J. Food Biochem. 2021, 45, e13557. [Google Scholar] [CrossRef]
- Abian, O.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Ceballos-Laita, L.; Vega, S.; Reyburn, H.T.; Rizzuti, B.; Velazquez-Campoy, A. Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening. Int. J. Biol. Macromol. 2020, 164, 1693–1703. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Botwina, P.; Menichetti, E.; Bagnoli, L.; Rosati, O.; Marini, F.; Fonseca, S.F.; Abenante, L.; Alves, D.; Dabrowska, A.; et al. Seleno-Functionalization of Quercetin Improves the Non-Covalent Inhibition of Mpro and Its Antiviral Activity in Cells against SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 7048. [Google Scholar] [CrossRef]
- Haritha, C.V.; Sharun, K.; Jose, B. Ebselen a new candidate therapeutic against SARS-CoV-2. Int. J. Surg. 2020, 84, 53–56. [Google Scholar] [CrossRef]
- Sies, H.; Parnham, M.J. Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections. Free Radic. Biol. Med. 2020, 156, 107–112. [Google Scholar] [CrossRef]
- Amporndanai, K.; Meng, X.; Shang, W.; Jin, Z.; Rogers, M.; Zhao, Y.; Rao, Z.; Liu, Z.J.; Yang, H.; Zhang, L.; et al. Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. Nat. Commun. 2021, 12, 3061. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol. Ther. 2018, 189, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-NRF2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Abulmeaty, M.M.A.; Aljuraiban, G.S.; Shaikh, S.M.; ALEid, N.E.; Mazrou, L.R.A.; Turjoman, A.A.; Aldosari, M.S.; Razak, S.; El-Sayed, M.M.; Areabi, T.M.; et al. The Efficacy of Antioxidant Oral Supplements on the Progression of COVID-19 in Non-Critically Ill Patients: A Randomized Controlled Trial. Antioxidants 2021, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA Virus Interactions: Potential Implications for SARS-CoV-2 Infection (COVID-19). Front. Nutr. 2020, 7, 164. [Google Scholar] [CrossRef]
- Elanthendral, G.; Shobana, N.; Meena, R.; Prakash, P.; Samrot, A.V. Utilizing pharmacological properties of polyphenolic curcumin in nanotechnology. Biocatal. Agric. Biotechnol. 2021, 38, 102212. [Google Scholar] [CrossRef]
- Prakash, P.; Meena, R.; Abraham, L.S.; Sunkar, S.; Govindaraju, K.; Pully, D.; Samrot, A.V. Evidence-based traditional Siddha formulations for prophylaxis and management of respiratory symptoms in COVID-19 pandemic-A review. Biocatal. Agric. Biotechnol. 2021, 35, 102056. [Google Scholar] [CrossRef]


| TYPE | mRNA Vaccine | Inactivated Virus Vaccine | Viral Vector Vaccine | Protein Subunit | DNA Based Vaccine | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VACCINE NAME | Comirmaty® | mRNA-1273 | CoronaVac | BBIBP-CorV | Covaxin | Covishield | Sputnik V | Janssen Vaccines | Nuvaxovid | ZyCoV-D |
| MANUFACTURER | Pfizer Inc & BioNTech | Moderna | Sinovac | Sinopharm | Bharat Biotech | AstraZeneca/ University of Oxford | Gamaleya | Janssen | Novavax | Cadila Healthcare |
| PREPARATION | S-Protein (SARS-CoV2) + P2 S → LNP | S-Protein → LNP | Inactivated virus created from African green monkey kidney cells (Vero cells) | β-propiolactone (inactivate COV) + adjuvant (Al(OH)3) + Vero cell | Whole-virion inactivated SARSCoV-2 antigen | S-protein (SARS-CoV-2) + chimpanzee adenovirus vector | rAd26 & rAd5-both carry the gene for S-protein (SARS-CoV-2) | Non-replicating adenovirus serotype 26 +S-protein (SARS-CoV-2) | Long S-Protein (SARS-CoV-2) + M adjuvant | S-protein (SARS-CoV-2) + pVAX1 |
| DOSAGE | 2 doses, 0.3 mL each 21 days apart | 2 doses, 0.5 mL each 28 days apart | 2 doses, 0.5 mL each 28 days apart | 2 doses, 0.5 mL each 21 days apart | 2 doses, 0.5 mL each 28 days apart | 2 doses, 0.5 mL each 3 months apart | 2 doses, 0.5 mL each 21 days apart | 1 dose 0.5 mL | 2 doses, 0.5 mL each 21 days apart | 3 doses, 0.5 mL each 28 days apart |
| AGE GROUP | 12 and older | 18 and older | 18 and above | 18–59 | 18 and above | 18 and older | 18 and above | 18 and older | 18 and above | 12 and above |
| % EFFICACY | 95% | 94.1% | 83.5% | 79% | 77.8% | 90% | 91.6% | 66.9% | 89.7% | 66.6% |
| SIDE EFFECTS | Headache, Fatigue, chills, Muscle and joint pain | Fatigue, Myalgia, Arthralgia, Headache | Headache, Fatigue, Muscle pain, Vomiting | Pain at the injection site, Fatigue, Lethargy, Headache, Tenderness | Injection site pain, Itching Headache, Fever, body ache, Nausea, Vomiting | Headache, Fever, Dizziness Nausea Vomiting, Myalgia. | Body pain, Injection site pain, Headache and Fatigue | Fatigue, Headache, Myalgia, Fever, Chills, Nausea, Diarrhoea | Injection-site tenderness, Fatigue, Headache, Muscle pain | Body ache Nausea Vomiting, Fever, Chills |
| EFFICACY AGAINST DELTA VARIANT | 88% | 86.7% | 59% | Not much information available | 65.2% | 65.2% | 83% | 71% | Not much information available | 66.6% |
| REFERENCES | [50,51,53] | [61,62] | [66,67] | [69,71] | [74] | [79] | [82,84,85] | [89] | [97,98] | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabitha, S.; Shobana, N.; Prakash, P.; Padmanaban, S.; Sathiyashree, M.; Saigeetha, S.; Chakravarthi, S.; Uthaman, S.; Park, I.-K.; Samrot, A.V. A Review of Different Vaccines and Strategies to Combat COVID-19. Vaccines 2022, 10, 737. https://doi.org/10.3390/vaccines10050737
Sabitha S, Shobana N, Prakash P, Padmanaban S, Sathiyashree M, Saigeetha S, Chakravarthi S, Uthaman S, Park I-K, Samrot AV. A Review of Different Vaccines and Strategies to Combat COVID-19. Vaccines. 2022; 10(5):737. https://doi.org/10.3390/vaccines10050737
Chicago/Turabian StyleSabitha, Srinivasan, Nagarajan Shobana, Pandurangan Prakash, Sathiyamoorthy Padmanaban, Mahendran Sathiyashree, Subramanian Saigeetha, Srikumar Chakravarthi, Saji Uthaman, In-Kyu Park, and Antony V. Samrot. 2022. "A Review of Different Vaccines and Strategies to Combat COVID-19" Vaccines 10, no. 5: 737. https://doi.org/10.3390/vaccines10050737
APA StyleSabitha, S., Shobana, N., Prakash, P., Padmanaban, S., Sathiyashree, M., Saigeetha, S., Chakravarthi, S., Uthaman, S., Park, I.-K., & Samrot, A. V. (2022). A Review of Different Vaccines and Strategies to Combat COVID-19. Vaccines, 10(5), 737. https://doi.org/10.3390/vaccines10050737






