Correlation of Fc Receptor Polymorphisms with Pneumococcal Antibodies in Vaccinated Kidney Transplant Recipients
Abstract
:1. Introduction
| Fc Receptor | SNP (Substitution) | Amino Acid | Function | Reference |
|---|---|---|---|---|
| FcγRIIa | FCGR2A | H131R | R131 homozygous: | |
| rs1801274 (A vs. G) |
| [2] | ||
| [10] | |||
| [11] | |||
| FcγRIIIa | FCGR3A | V158F | Affects the affinity of IgG1 to IgG4 | [17,18,19,20,21] |
| rs396991 | and influences immune cell activation | |||
| (G vs. A) | V158 carriers: | |||
| [15] | |||
| ||||
| FcγRIIIb | FCGR3B | (Intron) | Neutrophil antigen resulting in the | [17,19] |
| rs35139848 | isoform neutrophil antigen 1 (NA1) vs. | |||
| (A vs. G) | NA2, affecting N-linked glycosylation | |||
| of the FcγR | ||||
| Influences neutrophil recovery, severe | [16] | |||
| infections, and transplant-related | ||||
| mortality after hematopoietic stem | ||||
| cell transplantation |
2. Materials and Methods
2.1. Patients
2.2. Vaccine
2.3. Genotyping of FCAR and FCGR Polymorphisms
2.4. Determination of Antibodies against Pneumococci
2.5. Determination of HLA and MICA Antibodies
2.6. Statistical Analysis
3. Results
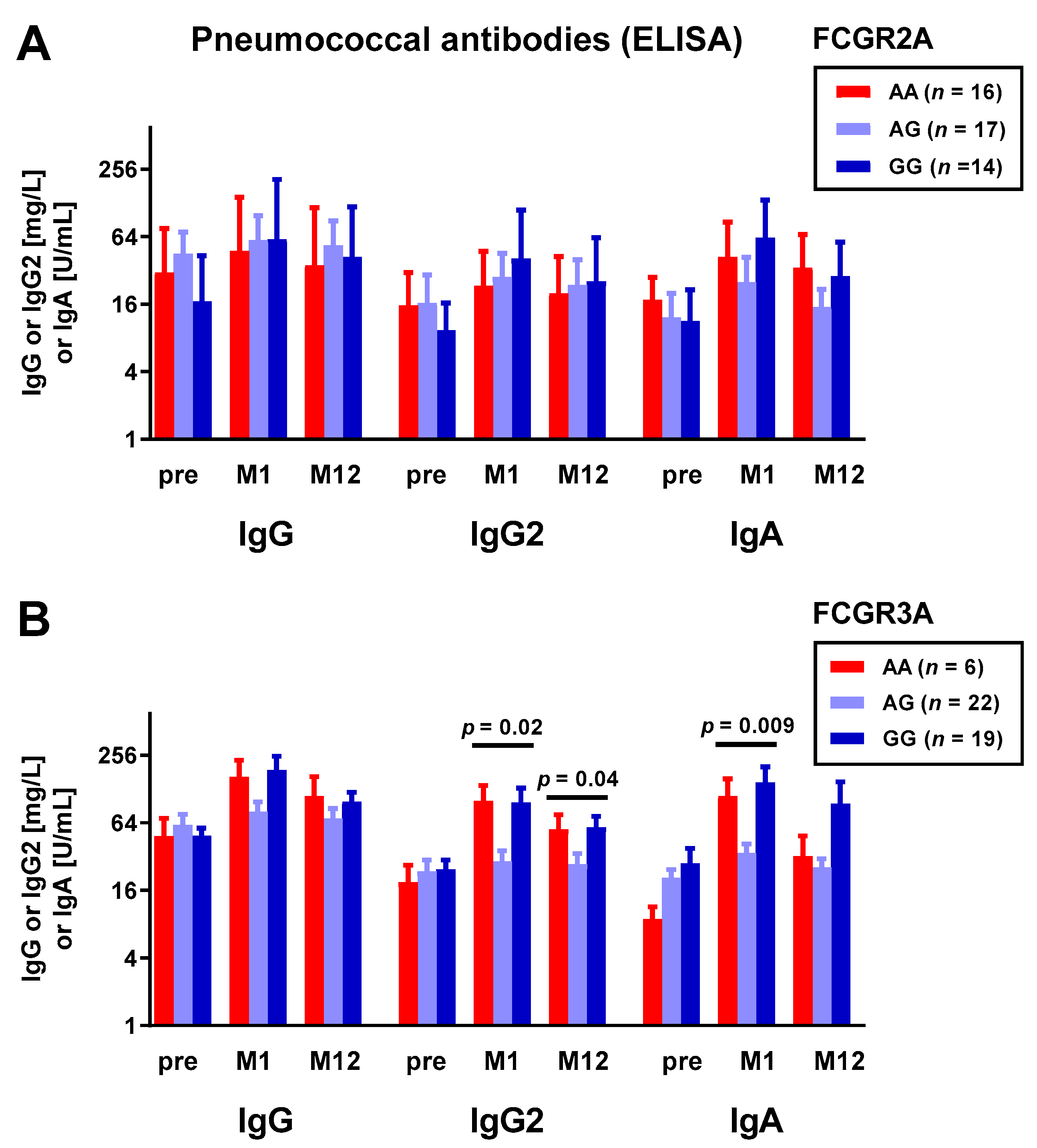
3.1. Correlation between Fc Receptor Polymorphisms and Pneumococcal Antibodies
3.2. Correlation between Fc Receptor Polymorphisms and HLA and MICA Antibodies
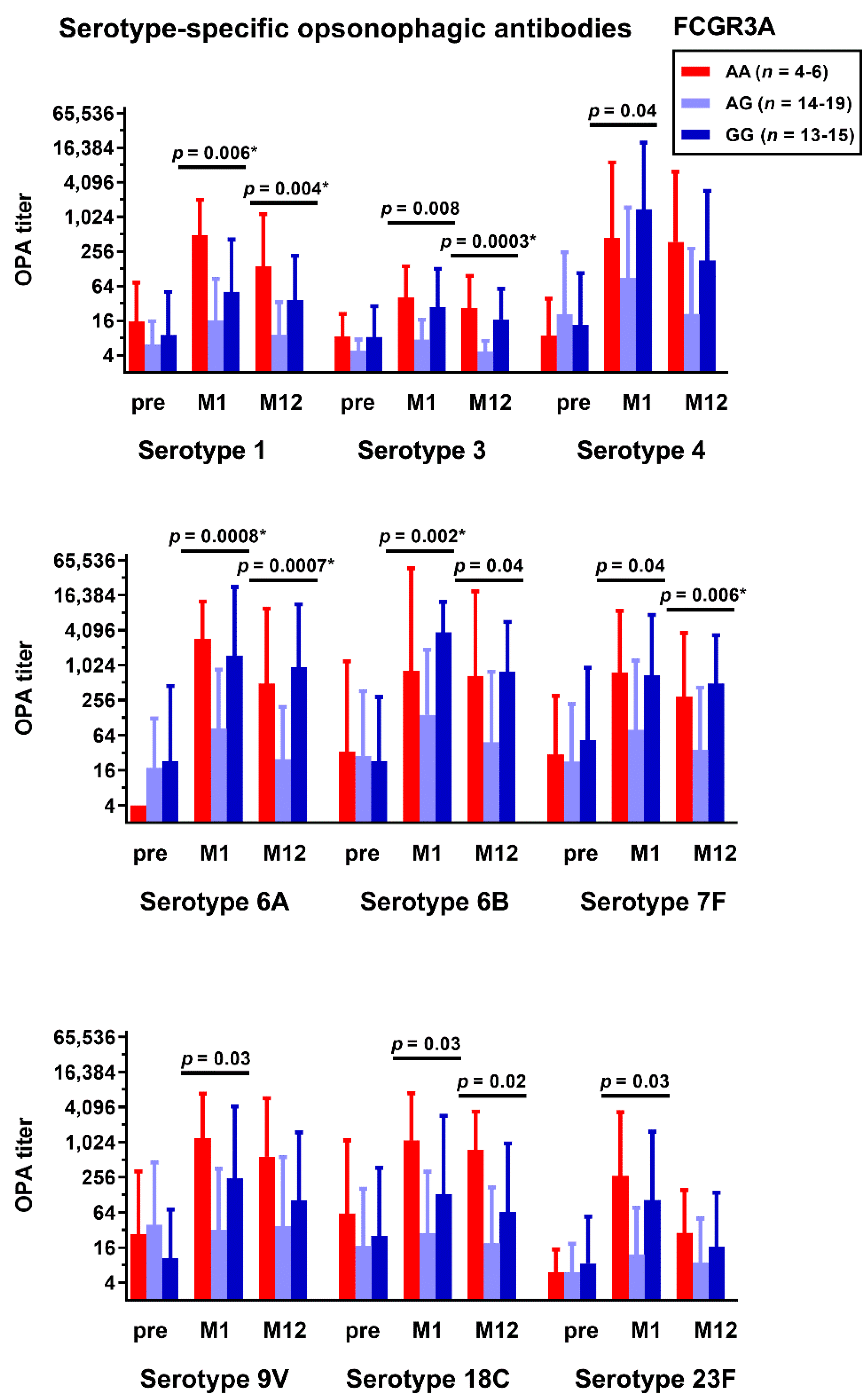
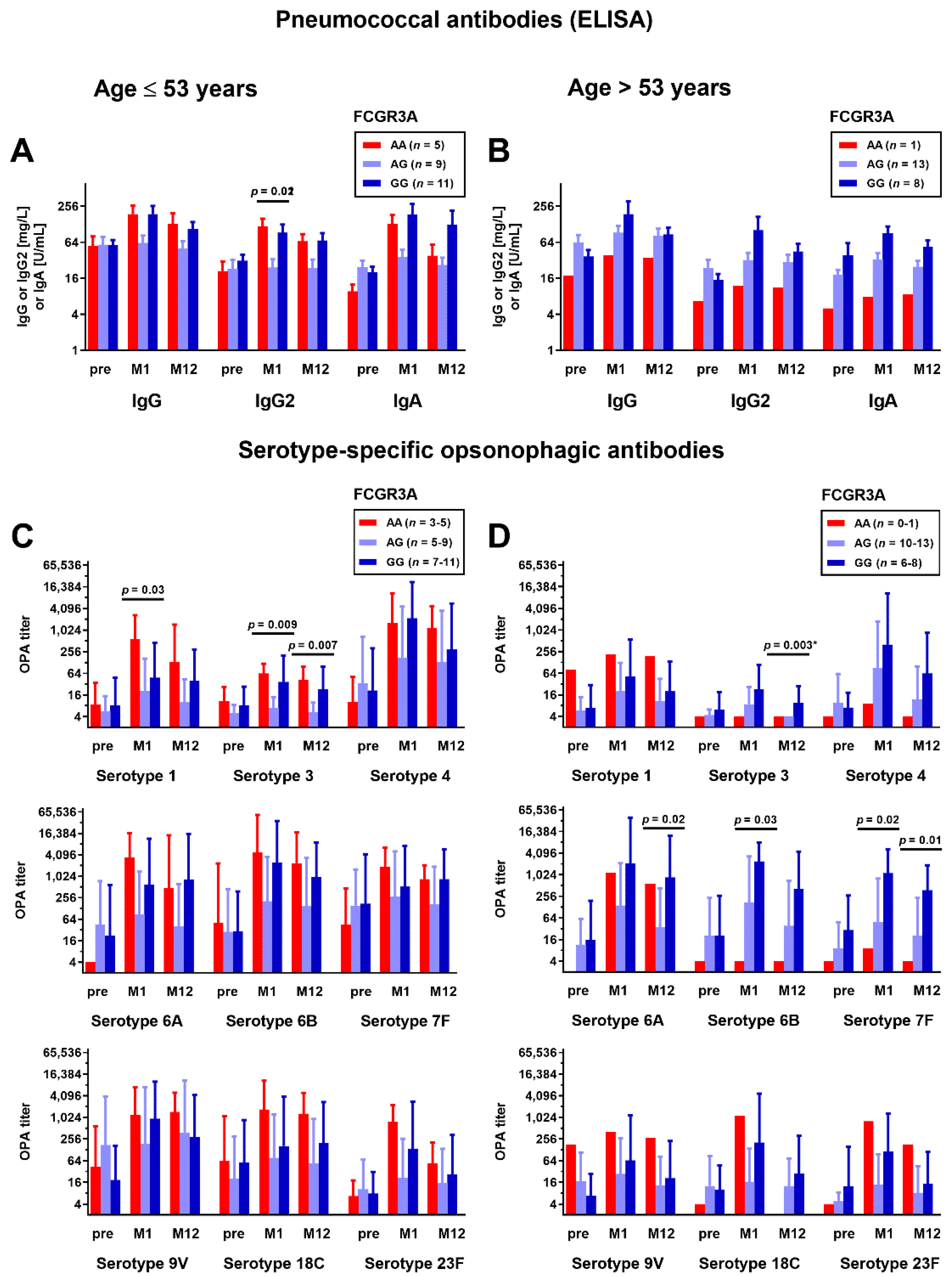
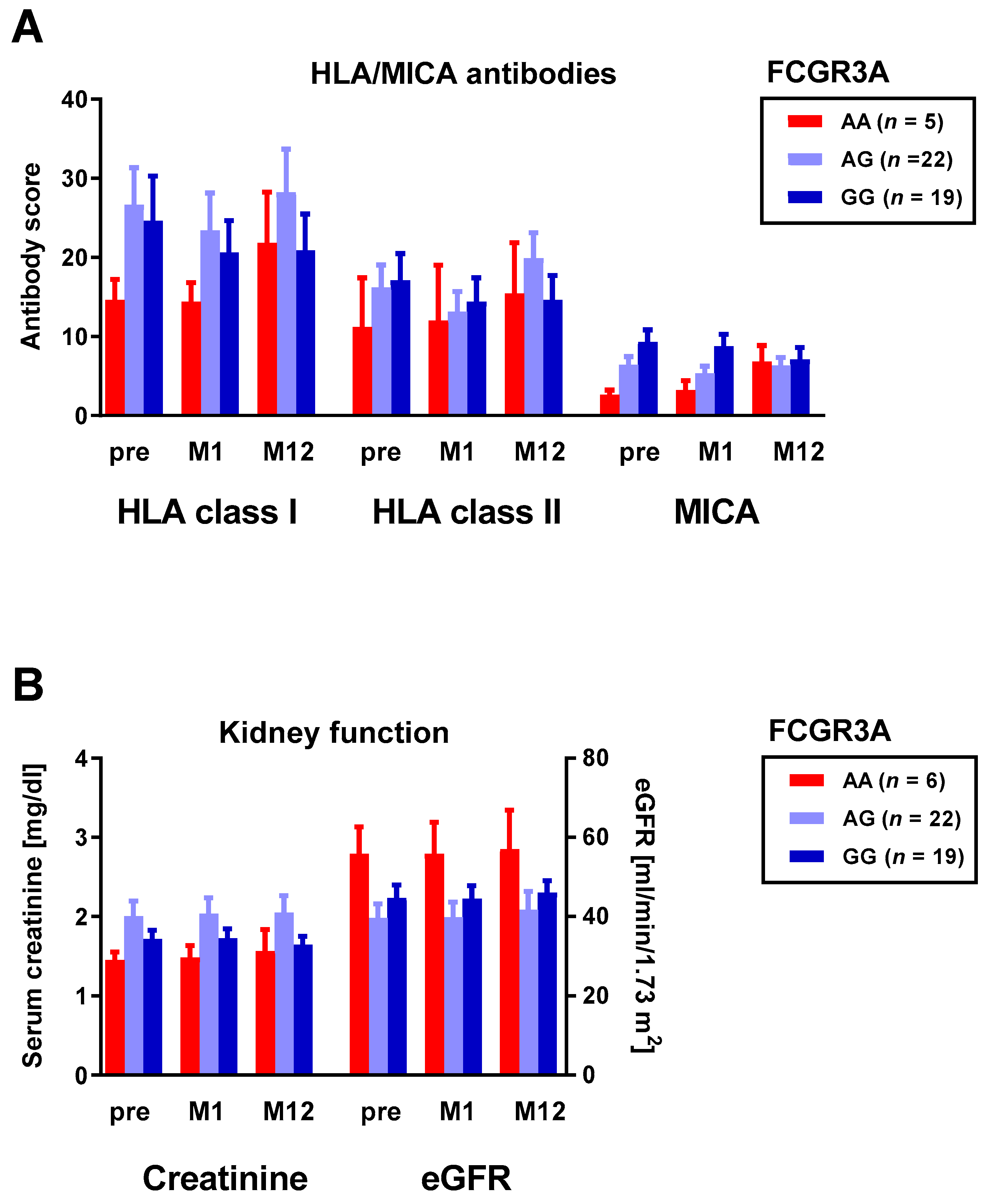
3.3. Correlation between Fc Receptor Polymorphisms and Kidney Fucntion
3.4. Correlation between Fc Receptor Polymorphisms and Immunosuppressive Treatment
3.5. Correlation between Kidney Function and Pneumococcal Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, W.K.; Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003, 21, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; Veenhoven, R.H.; Walraven, V.; Uiterwaal, C.S.; Schilder, A.G.; Rijkers, G.T.; Sanders, E.A. Pneumococcal vaccine efficacy for mucosal pneumococcal infections depends on Fcgamma receptor IIa polymorphism. Vaccine 2006, 24, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Simell, B.; Auranen, K.; Kayhty, H.; Goldblatt, D.; Dagan, R.; O’Brien, K.L.; Pneumococcal Carriage, G. The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klugman, K.P.; Chien, Y.W.; Madhi, S.A. Pneumococcal pneumonia and influenza: A deadly combination. Vaccine 2009, 27 (Suppl. 3), C9–C14. [Google Scholar] [CrossRef]
- National Vaccine Program Office. Adult Immunization Plans. Available online: http://www.hhs.gov/nvpo/national-adult-immunization-plan/ (accessed on 14 February 2022).
- Arora, S.; Kipp, G.; Bhanot, N.; Sureshkumar, K.K. Vaccinations in kidney transplant recipients: Clearing the muddy waters. World J. Transplant. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Use of 13-Valent Pneumococcal Conjugate Vaccine and 23-Valent Pneumococcal Polysaccharide Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2012, 61, 816–819. [Google Scholar]
- Robert-Koch-Institut. Wissenschaftliche Begründung für die Aktualisierung der Empfehlungen zur Indikationsimpfung gegen Pneumokokken für Risikogruppen. Epid. Bull. 2016, 37, 385–406. [Google Scholar]
- Bonten, M.J.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.L.; Fuernrohr, B.G.; Weiss, K.M.; Harre, U.; Wiesener, M.S.; Spriewald, B.M. Association of a coding polymorphism in Fc gamma receptor 2A and graft survival in re-transplant candidates. Hum. Immunol. 2015, 76, 759–764. [Google Scholar] [CrossRef]
- Ozkayin, N.; Mir, S.; Afig, B. The role of fcgamma receptor gene polymorphism in pediatric renal transplant rejections. Transplant. Proc. 2008, 40, 3367–3374. [Google Scholar] [CrossRef]
- Pawlik, A.; Florczak, M.; Bak, L.; Domanski, L.; Rozanski, J.; Dabrowska-Zamojcin, E.; Machalinski, B.; Gawronska-Szklarz, B. The Fc gamma RIIa polymorphism in patients with acute kidney graft rejection. Ann. Transplant. 2003, 8, 24–26. [Google Scholar] [PubMed]
- Pawlik, A.; Florczak, M.; Bak, L.; Dabrowska-Zamojcin, E.; Rozanski, J.; Domanski, L.; Gawronska-Szklarz, B. The FcgammaRIIa polymorphism in patients with chronic kidney graft rejection. Transplant. Proc. 2004, 36, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.F.; Watson, N.; Sullivan, J.S.; Biffin, S.; Moses, J.; Geczy, A.F.; Chapman, J.R. Association of Fc gamma receptor IIA polymorphisms with acute renal-allograft rejection. Transplantation 2004, 78, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.L.; Kainz, A.; Hidalgo, L.G.; Eskandary, F.; Kozakowski, N.; Wahrmann, M.; Haslacher, H.; Oberbauer, R.; Heilos, A.; Spriewald, B.M.; et al. Functional Fc gamma receptor gene polymorphisms and donor-specific antibody-triggered microcirculation inflammation. Am. J. Transplant. 2018, 18, 2261–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, V.; Franco, R.F.; Porcher, R.; Bittencourt, H.; Silva, W.A., Jr.; Latouche, A.; Devergie, A.; Esperou, H.; Ribaud, P.; Socie, G.; et al. Host defense and inflammatory gene polymorphisms are associated with outcomes after HLA-identical sibling bone marrow transplantation. Blood 2002, 100, 3908–3918. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daeron, M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- Koene, H.R.; Kleijer, M.; Algra, J.; Roos, D.; von dem Borne, A.E.; de Haas, M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997, 90, 1109–1114. [Google Scholar] [CrossRef] [Green Version]
- Salmon, J.E.; Edberg, J.C.; Brogle, N.L.; Kimberly, R.P. Allelic polymorphisms of human Fc gamma receptor IIA and Fc gamma receptor IIIB. Independent mechanisms for differences in human phagocyte function. J. Clin. Investig. 1992, 89, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Edberg, J.C.; Redecha, P.B.; Bansal, V.; Guyre, P.M.; Coleman, K.; Salmon, J.E.; Kimberly, R.P. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J. Clin. Investig. 1997, 100, 1059–1070. [Google Scholar] [CrossRef]
- Rosales, C. Fcgamma Receptor Heterogeneity in Leukocyte Functional Responses. Front. Immunol. 2017, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Oesterreich, S.; Lindemann, M.; Goldblatt, D.; Horn, P.A.; Wilde, B.; Witzke, O. Humoral response to a 13-valent pneumococcal conjugate vaccine in kidney transplant recipients. Vaccine 2020, 38, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Oesterreich, S.; Wilde, B.; Eisenberger, U.; Muelling, N.; Horn, P.A.; Heinemann, F.M.; Witzke, O. Sex-Specific Differences in HLA Antibodies after Pneumococcal Vaccination in Kidney Transplant Recipients. Vaccines 2019, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindemann, M.; Heinemann, F.M.; Horn, P.A.; Witzke, O. Vaccination against Streptococcus pneumoniae does not induce antibodies against HLA or MICA in clinically stable kidney transplant recipients. Hum. Immunol. 2013, 74, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Hurst, F.P.; Lee, J.J.; Jindal, R.M.; Agodoa, L.Y.; Abbott, K.C. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2011, 6, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Scharpe, J.; Evenepoel, P.; Maes, B.; Bammens, B.; Claes, K.; Osterhaus, A.D.; Vanrenterghem, Y.; Peetermans, W.E. Influenza vaccination is efficacious and safe in renal transplant recipients. Am. J. Transplant. 2008, 8, 332–337. [Google Scholar] [CrossRef]
- Cainelli, F.; Vento, S. Infections and solid organ transplant rejection: A cause-and-effect relationship? Lancet Infect. Dis. 2002, 2, 539–549. [Google Scholar] [CrossRef]
- Connolly, J.; Douglas, J.; Kumar, R.; Middleton, D.; McEvoy, J.; Nelson, S.; McGeown, M.G. Letter: Influenza virus vaccination and renal transplant rejection. Br. Med. J. 1974, 1, 638. [Google Scholar] [CrossRef] [Green Version]
- Candon, S.; Thervet, E.; Lebon, P.; Suberbielle, C.; Zuber, J.; Lima, C.; Charron, D.; Legendre, C.; Chatenoud, L. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am. J. Transplant. 2009, 9, 2346–2354. [Google Scholar] [CrossRef]
- Dendle, C.; Stuart, R.L.; Polkinghorne, K.R.; Balloch, A.; Kanellis, J.; Ling, J.; Kummrow, M.; Moore, C.; Thursky, K.; Buttery, J.; et al. Seroresponses and safety of 13-valent pneumococcal conjugate vaccination in kidney transplant recipients. Transpl. Infect. Dis. 2018, 20, e12866. [Google Scholar] [CrossRef]
- Arnold, M.L.; Bach, C.; Heinemann, F.M.; Horn, P.A.; Ziemann, M.; Lachmann, N.; Muhlbacher, A.; Dick, A.; Ender, A.; Thammanichanond, D.; et al. Anti-HLA alloantibodies of the IgA isotype in re-transplant candidates part II: Correlation with graft survival. Int. J. Immunogenet. 2018, 45, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, A.; Shimokawa, T.; Moriyama, M.; Komine, F.; Amaki, S.; Arakawa, Y.; Ra, C. Genetic variants of the IgA Fc receptor (FcalphaR, CD89) promoter in chronic hepatitis C patients. Immunogenetics 2006, 58, 937–946. [Google Scholar] [CrossRef] [PubMed]
- David-Neto, E.; Triboni, A.H.; Ramos, F.; Agena, F.; Galante, N.Z.; Altona, M.; Lemos, F.B.; Sapienza, M.T.; Nahas, W.C. Evaluation of MDRD4, CKD-EPI, BIS-1, and modified Cockcroft-Gault equations to estimate glomerular filtration rate in the elderly renal-transplanted recipients. Clin. Transplant. 2016, 30, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Yu, X.; Sidhu, M.; Nahm, M.H.; Fernsten, P.; Jansen, K.U. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine 2011, 29, 7207–7211. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.T.; Yu, X.; Jones, T.R.; Kirch, C.; Harris, S.; Hildreth, S.W.; Madore, D.V.; Quataert, S.A. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin Diagn Lab Immunol. 2005, 12, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Fleck, R.A.; Romero-Steiner, S.; Nahm, M.H. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol. 2005, 12, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Dendle, C.; Stuart, R.L.; Mulley, W.R.; Holdsworth, S.R. Pneumococcal vaccination in adult solid organ transplant recipients: A review of current evidence. Vaccine 2018, 36, 6253–6261. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Teillaud, J.L.; Bouchard, C.; Teillaud, C.; Astier, A.; Tartour, E.; Galon, J.; Mathiot, C.; Sautes, C. Soluble Fc gamma receptors. J. Leukoc. Biol. 1993, 54, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B.L. Geometric Means and Measures of Dispersion. Biometrics 1979, 35, 908–909. [Google Scholar]
- Mocsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Mejorada, G.; Rosales, C. Signal transduction by immunoglobulin Fc receptors. J. Leukoc. Biol. 1998, 63, 521–533. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bournazos, S.; DiLillo, D.J.; Ravetch, J.V. The role of Fc-FcgammaR interactions in IgG-mediated microbial neutralization. J. Exp. Med. 2015, 212, 1361–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincetic, A.; Bournazos, S.; DiLillo, D.J.; Maamary, J.; Wang, T.T.; Dahan, R.; Fiebiger, B.M.; Ravetch, J.V. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat. Immunol. 2014, 15, 707–716. [Google Scholar] [CrossRef] [PubMed]
- van de Winkel, J.G.; Capel, P.J. Human IgG Fc receptor heterogeneity: Molecular aspects and clinical implications. Immunol. Today 1993, 14, 215–221. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.Y.; Kim, H.G.; Kwak, M.W.; Kang, T.H. Fc Receptor Variants and Disease: A Crucial Factor to Consider in the Antibody Therapeutics in Clinic. Int. J. Mol. Sci. 2021, 22, 9489. [Google Scholar] [CrossRef]
- Zakeri, S.; Mashhadi, R.; Mehrizi, A.A.; Djadid, N.D. Analysis of Fcgamma receptor IIa (cd32) gene polymorphism and anti-malarial IgG subclass antibodies to asexual blood-stage antigen of Plasmodium falciparum in an unstable malaria endemic area of Iran. Exp. Parasitol. 2013, 134, 115–121. [Google Scholar] [CrossRef]
- Warmerdam, P.A.; van de Winkel, J.G.; Vlug, A.; Westerdaal, N.A.; Capel, P.J. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J. Immunol. 1991, 147, 1338–1343. [Google Scholar]
- Sanders, L.A.; Feldman, R.G.; Voorhorst-Ogink, M.M.; de Haas, M.; Rijkers, G.T.; Capel, P.J.; Zegers, B.J.; van de Winkel, J.G. Human immunoglobulin G (IgG) Fc receptor IIA (CD32) polymorphism and IgG2-mediated bacterial phagocytosis by neutrophils. Infect. Immun. 1995, 63, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Jansen, W.T.; Breukels, M.A.; Snippe, H.; Sanders, L.A.; Verheul, A.F.; Rijkers, G.T. Fcgamma receptor polymorphisms determine the magnitude of in vitro phagocytosis of Streptococcus pneumoniae mediated by pneumococcal conjugate sera. J Infect. Dis. 1999, 180, 888–891. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transplant. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Ternant, D.; Buchler, M.; Thibault, G.; Ohresser, M.; Watier, H.; Lebranchu, Y.; Paintaud, G. Influence of FcgammaRIIIA genetic polymorphism on T-lymphocyte depletion induced by rabbit antithymocyte globulins in kidney transplant patients. Pharm. Genom. 2014, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
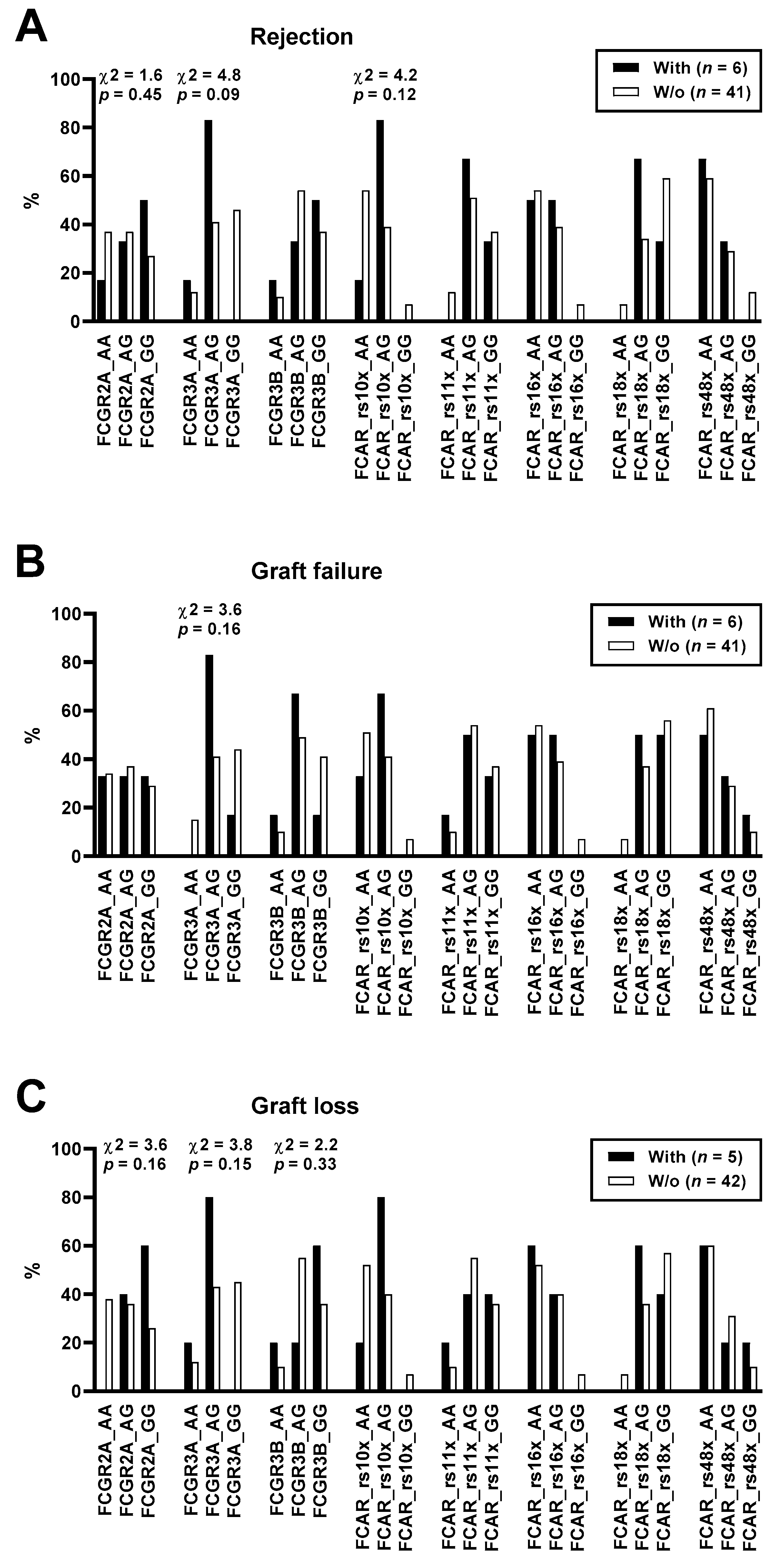
| SNP | Genotype | Model 1 | OR | p | ||
|---|---|---|---|---|---|---|
| FCGR2A rs1801274 | Rejection (n = 6) | No Rejection (n = 41) | ||||
| AA | 1 (17%) | 15 (37%) | Dominant | 0.6 | 0.65 | |
| AG | 2 (33%) | 15 (37%) | Recessive | 0.4 | 0.34 | |
| GG | 3 (50%) | 11 (27%) | ||||
| Graft Failure (n = 6) | No Graft Failure (n = 41) | |||||
| AA | 2 (33%) | 14 (34%) | Dominant | 1.0 | 1.00 | |
| AG | 2 (33%) | 15 (37%) | Recessive | 0.8 | 1.00 | |
| GG | 2 (33%) | 12 (29%) | ||||
| Graft Loss (n = 5) | No Graft Loss (n = 42) | |||||
| AA | 0 (0%) | 16 (38%) | Dominant | 0.0 | 0.15 | |
| AG | 2 (40%) | 15 (36%) | Recessive | 0.2 | 0.15 | |
| GG | 3 (60%) | 11 (26%) | ||||
| FCGR3A rs396991 | Rejection (n = 6) | No Rejection (n = 41) | ||||
| GG | 0 (0%) | 19 (46%) | Dominant | 0.0 | 0.07 | |
| AG | 5 (83%) | 17 (41%) | Recessive | 0.7 | 1.00 | |
| AA | 1 (17%) | 5 (12%) | ||||
| Graft Failure (n = 6) | No Graft Failure (n = 41) | |||||
| GG | 1 (17%) | 18 (44%) | Dominant | 0.3 | 0.38 | |
| AG | 5 (83%) | 17 (41%) | Recessive | Infinite | 1.00 | |
| AA | 0 (0%) | 6 (15%) | ||||
| Graft Loss (n = 5) | No Graft Loss (n = 42) | |||||
| GG | 0 (0%) | 19 (45%) | Dominant | 0.0 | 0.07 | |
| AG | 4 (80%) | 18 (43%) | Recessive | 0.5 | 0.51 | |
| AA | 1 (20%) | 5 (12%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnold, M.-L.; Heinemann, F.M.; Oesterreich, S.; Wilde, B.; Gäckler, A.; Goldblatt, D.; Spriewald, B.M.; Horn, P.A.; Witzke, O.; Lindemann, M. Correlation of Fc Receptor Polymorphisms with Pneumococcal Antibodies in Vaccinated Kidney Transplant Recipients. Vaccines 2022, 10, 725. https://doi.org/10.3390/vaccines10050725
Arnold M-L, Heinemann FM, Oesterreich S, Wilde B, Gäckler A, Goldblatt D, Spriewald BM, Horn PA, Witzke O, Lindemann M. Correlation of Fc Receptor Polymorphisms with Pneumococcal Antibodies in Vaccinated Kidney Transplant Recipients. Vaccines. 2022; 10(5):725. https://doi.org/10.3390/vaccines10050725
Chicago/Turabian StyleArnold, Marie-Luise, Falko M. Heinemann, Simon Oesterreich, Benjamin Wilde, Anja Gäckler, David Goldblatt, Bernd M. Spriewald, Peter A. Horn, Oliver Witzke, and Monika Lindemann. 2022. "Correlation of Fc Receptor Polymorphisms with Pneumococcal Antibodies in Vaccinated Kidney Transplant Recipients" Vaccines 10, no. 5: 725. https://doi.org/10.3390/vaccines10050725
APA StyleArnold, M.-L., Heinemann, F. M., Oesterreich, S., Wilde, B., Gäckler, A., Goldblatt, D., Spriewald, B. M., Horn, P. A., Witzke, O., & Lindemann, M. (2022). Correlation of Fc Receptor Polymorphisms with Pneumococcal Antibodies in Vaccinated Kidney Transplant Recipients. Vaccines, 10(5), 725. https://doi.org/10.3390/vaccines10050725






