Age-Dependent Dynamics of Maternally Derived Antibodies (MDAs) and Understanding MDA-Mediated Immune Tolerance in Foot-and-Mouth Disease-Vaccinated Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. ELISA for the Detection of Structural Protein (SP) Antibodies
2.3. Virus Neutralization Test (VNT)
2.4. Isotype-Specific Antibody Immunoassays
2.5. Cytokine ELISA
2.6. Statistics
3. Results
3.1. Age-Dependent Dynamics of Antibody Titers in Sows and Piglets
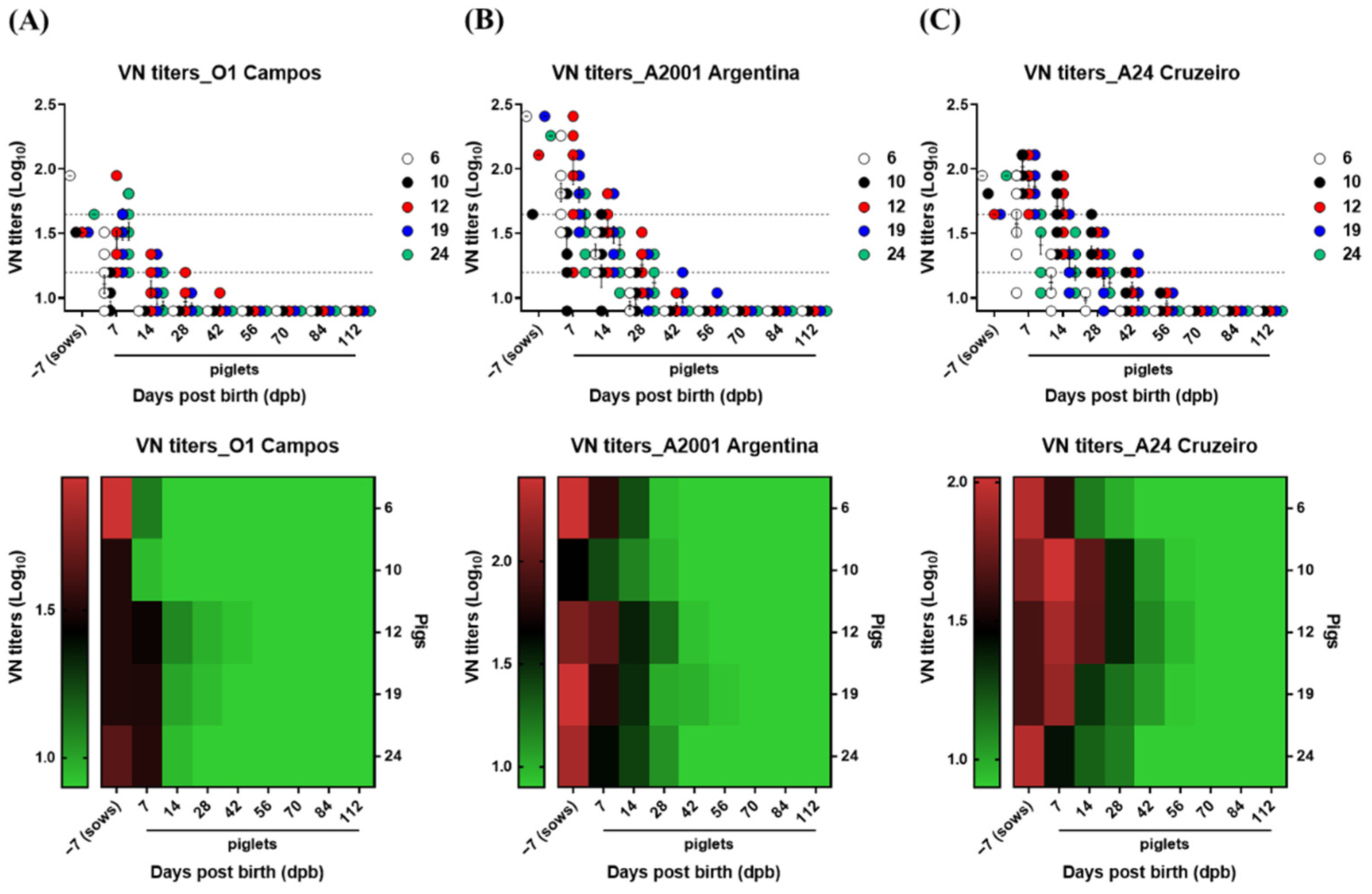
3.2. Age-Dependent Dynamics of VN Titers
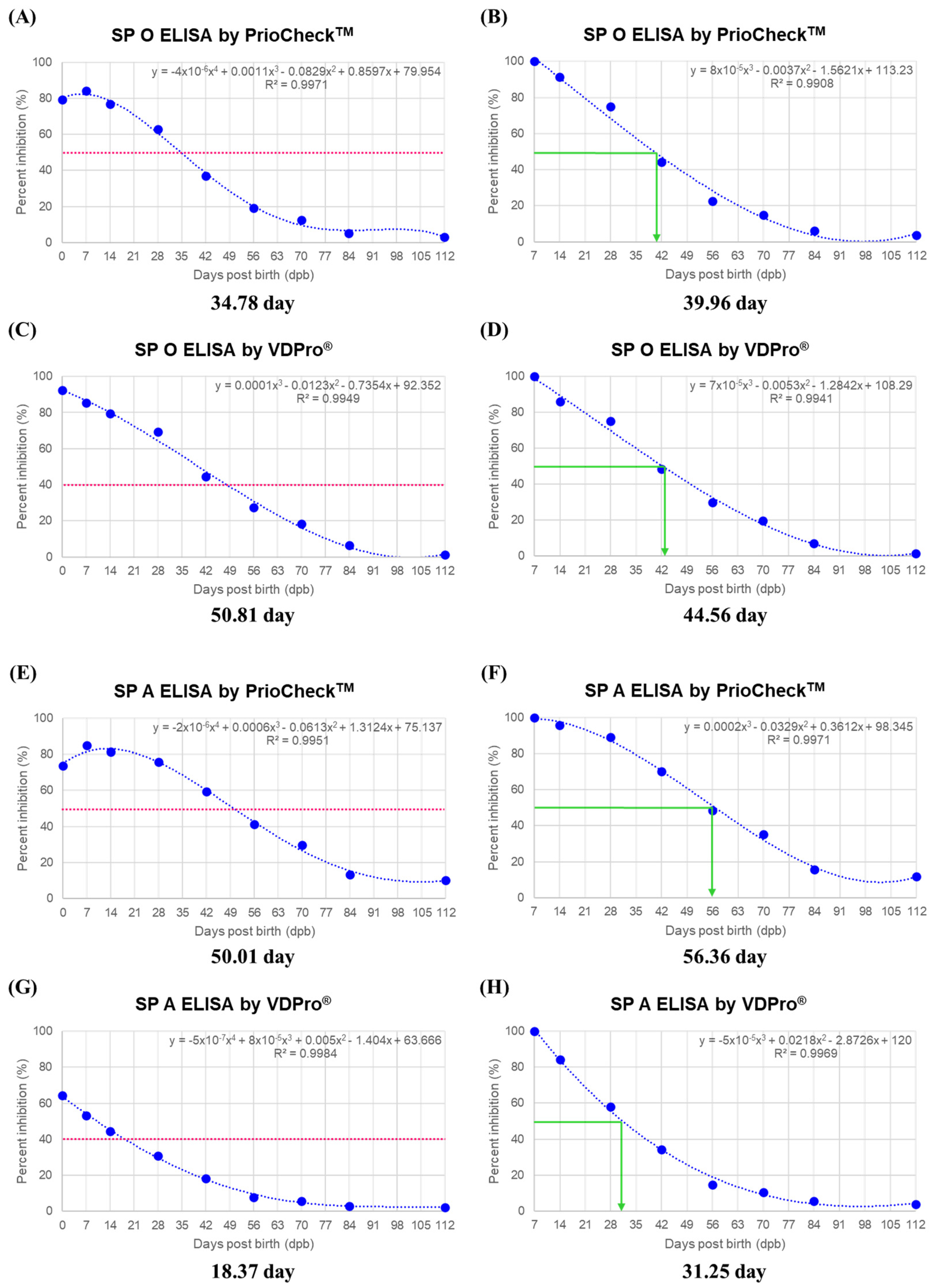
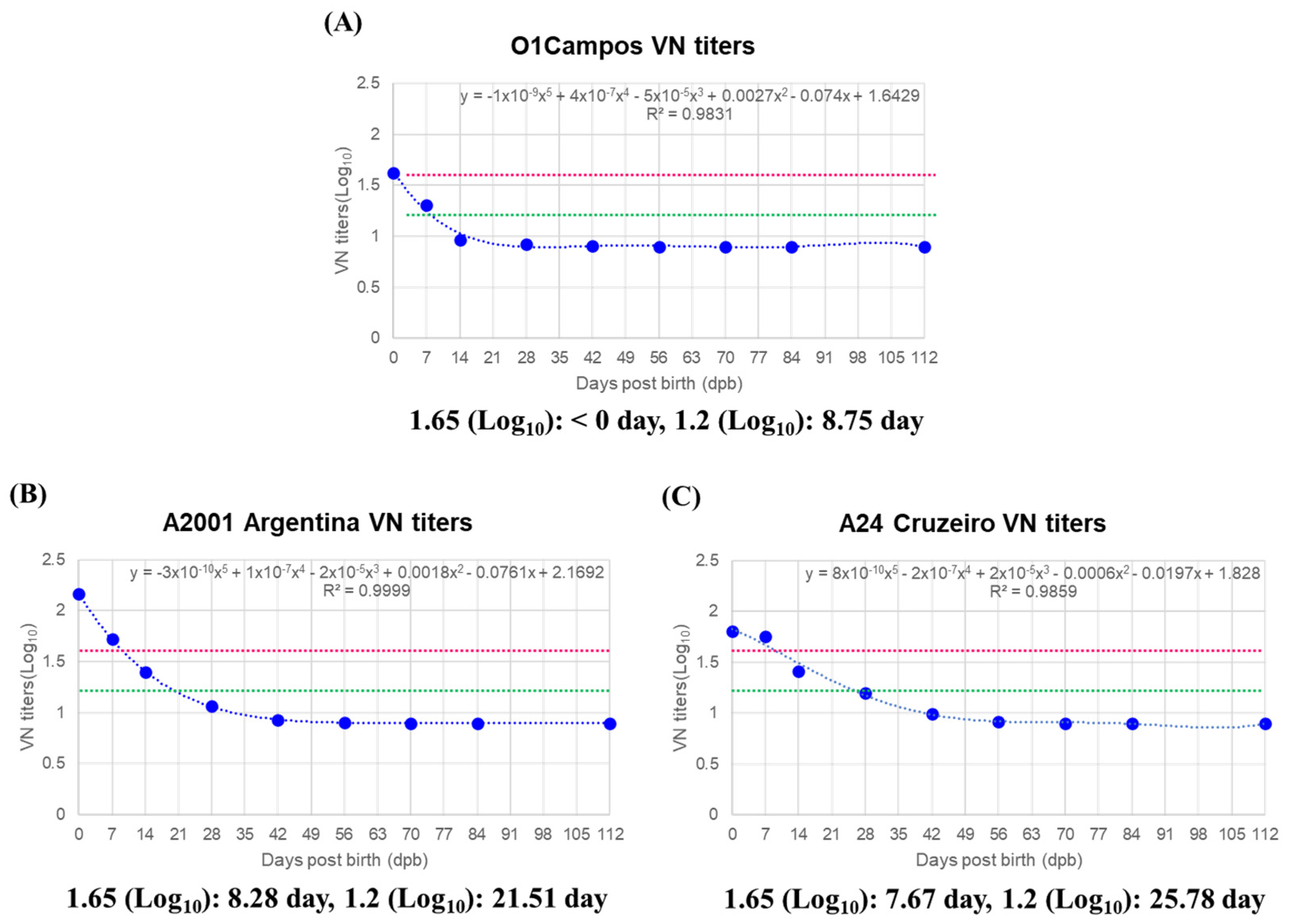
3.3. MDA Half-Life
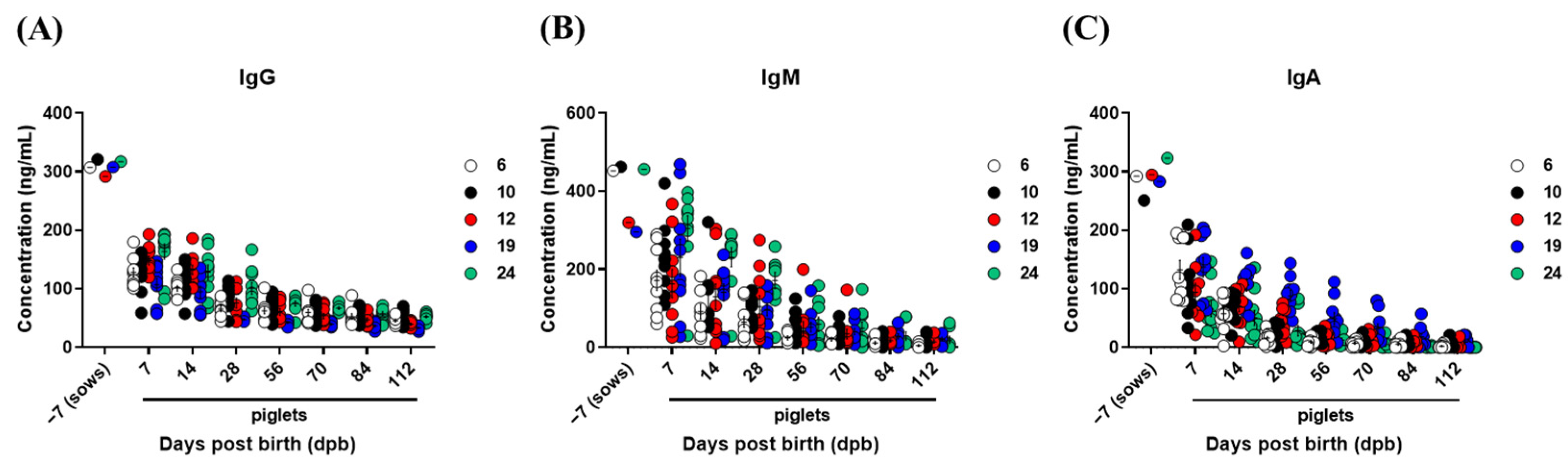
3.4. Kinetics of Total IgG, IgM, and IgA Concentrations in Sows and Piglets
3.5. The Inhibition of Vaccine-Mediated Active Immunity by MDAs and Treg Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrecht, M.; Arck, P.C. Vertically transferred immunity in neonates: Mothers, mechanisms and mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.L.A.; Noris García, E.; Fraga Guerra, J.; Vega Hurtado, Y.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef] [PubMed]
- Kitching, R.; Salt, J. The interference by maternally-derived antibody with active immunization of farm animals against foot-and-mouth disease. Vet 1995, 151, 379–389. [Google Scholar] [CrossRef]
- Elnekave, E.; Dekker, A.; Eble, P.; van Hemert-Kluitenberg, F.; Gelman, B.; Storm, N.; Klement, E. The long term effect of age and maternally derived antibodies against foot and mouth disease on the serological response following vaccination in young dairy calves. Vaccine 2016, 34, 4927–4934. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, T.; Hong, J.-K.; Lee, H.-S.; Lee, K.-N.; Jo, H.J.; Choi, J.; Choi, J.; Lee, S.H.; Lee, M.-H. The interference effect of maternally-derived antibodies on the serological performance of pigs immunized with a foot-and-mouth disease oil emulsion vaccine. Vaccine 2020, 38, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Çokçalışkan, C.; Türkoğlu, T.; Uzunlu, E.; Sareyyüpoğlu, B.; Hancı, İ.; İpek, A.; Arslan, A.; Babak, A.; İldeniz, G.; Gülyaz, V. Influence of vaccine potency and booster administration of foot-and-mouth disease vaccines on the antibody response in calves with maternal antibodies. J Vet Sci 2017, 18, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Knight-Jones, T.J.; Charleston, B.; Rodriguez, L.L.; Gay, C.G.; Sumption, K.J.; Vosloo, W. Global Foot-and-Mouth Disease Research Update and Gap Analysis: 6—Immunology. Transbound. Emerg. Dis. 2016, 63 (Suppl. 1), 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sareyyüpoğlu, B.; Gülyaz, V.; Cokçalışkan, C.; Ünal, Y.; Çökülgen, T.; Uzunlu, E.; Gürcan, S.; Ilk, O. Effect of FMD vaccination schedule of dams on the level and duration of maternally derived antibodies. Vet. Immunol. Immunopathol. 2019, 217, 109881. [Google Scholar] [CrossRef]
- Dekker, A.; Chénard, G.; Stockhofe, N.; Eblé, P.L. Proper timing of foot-and-mouth disease vaccination of piglets with maternally derived antibodies will maximize expected protection levels. Front. Vet. Sci. 2016, 3, 52. [Google Scholar] [CrossRef]
- Hu, Z.; Ni, J.; Cao, Y.; Liu, X. Newcastle disease virus as a vaccine vector for 20 years: A focus on maternally derived antibody interference. Vaccines 2020, 8, 222. [Google Scholar] [CrossRef]
- Bertran, K.; Lee, D.-H.; Criado, M.F.; Balzli, C.L.; Killmaster, L.F.; Kapczynski, D.R.; Swayne, D.E. Maternal antibody inhibition of recombinant Newcastle disease virus vectored vaccine in a primary or booster avian influenza vaccination program of broiler chickens. Vaccine 2018, 36, 6361–6372. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, A.; Vandersleyen, O.; Steensels, M.; Desloges, N.; Mast, J.; van den Berg, T.; Lambrecht, B. Stronger interference of avian influenza virus–specific than Newcastle disease virus–specific maternally derived antibodies with a recombinant NDV-H5 vaccine. Avian Dis. 2016, 60, 191–201. [Google Scholar] [CrossRef]
- Renson, P.; Fablet, C.; Andraud, M.; Normand, V.; Lebret, A.; Paboeuf, F.; Rose, N.; Bourry, O. Maternally-derived neutralizing antibodies reduce vaccine efficacy against porcine reproductive and respiratory syndrome virus infection. Vaccine 2019, 37, 4318–4324. [Google Scholar] [CrossRef] [PubMed]
- Figueras-Gourgues, S.; Fraile, L.; Segalés, J.; Hernández-Caravaca, I.; López-Úbeda, R.; García-Vázquez, F.; Gomez-Duran, O.; Grosse-Liesner, B. Effect of Porcine circovirus 2 (PCV-2) maternally derived antibodies on performance and PCV-2 viremia in vaccinated piglets under field conditions. Porcine Health Manag. 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Tassis, P.D.; Tsakmakidis, I.; Papatsiros, V.G.; Koulialis, D.; Nell, T.; Brellou, G.; Tzika, E.D. A randomized controlled study on the efficacy of a novel combination vaccine against enzootic pneumonia (Mycoplasma hyopneumoniae) and porcine Circovirus type 2 (PCV2) in the presence of strong maternally derived PCV2 immunity in pigs. BMC Vet. Res. 2017, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Rajao, D.S.; Sandbulte, M.R.; Gauger, P.C.; Kitikoon, P.; Platt, R.; Roth, J.A.; Perez, D.R.; Loving, C.L.; Vincent, A.L. Heterologous challenge in the presence of maternally-derived antibodies results in vaccine-associated enhanced respiratory disease in weaned piglets. Virology 2016, 491, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.-L.; Xiang, G.-T.; Lei, J.-L.; Du, M.; Wang, Y.; Zhou, M.; Liu, Y.; Ji, S.; Wang, Y.-L.; Luo, Y. Efficacy of the marker vaccine rAdV-SFV-E2 against classical swine fever in the presence of maternally derived antibodies to rAdV-SFV-E2 or C-strain. Vet. Microbiol. 2016, 196, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Llurba, E.; Gris, J.M. Potentiating maternal immune tolerance in pregnancy: A new challenging role for regulatory T cells. Placenta 2014, 35, 241–248. [Google Scholar] [CrossRef]
- Zenclussen, A.C. Regulatory T cells in pregnancy. Semin. Immunol. 2006, 28, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bronson, P.G.; Chang, D.; Bhangale, T.; Seldin, M.F.; Ortmann, W.; Ferreira, R.C.; Urcelay, E.; Pereira, L.F.; Martin, J.; Plebani, A.; et al. Common Variants At PVT1, Atg13-Ambra1, AHI1 and CLEC16A are Associated With Selective IgA Deficiency. Nat. Genet. 2016, 48, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, M.; Kremsner, P.G.; van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 2002, 296, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Thiermann, A.B. International standards: The World Organisation for Animal Health Terrestrial Animal Health Code. Rev. Sci. Tech. 2015, 34, 277–281. [Google Scholar] [CrossRef]
- Lee, M.J.; Jo, H.; Shin, S.H.; Kim, S.-M.; Kim, B.; Shim, H.S.; Park, J.-H. Mincle and STING-stimulating adjuvants elicit robust cellular immunity and drive long-lasting memory responses in a foot-and-mouth disease vaccine. Front. Immunol. 2019, 10, 2509. [Google Scholar] [CrossRef]
- Jo, H.; Kim, B.Y.; Park, S.H.; Kim, H.M.; Shin, S.H.; Hwang, S.Y.; Kim, S.-M.; Kim, B.; Park, J.-H.; Lee, M.J. The HSP70-fused foot-and-mouth disease epitope elicits cellular and humoral immunity and drives broad-spectrum protective efficacy. NPJ Vaccines 2021, 6, 42. [Google Scholar] [CrossRef]
- Lee, M.J.; Jo, H.; Park, S.H.; Ko, M.-K.; Kim, S.-M.; Kim, B.; Park, J.-H. Advanced foot-and-mouth disease vaccine platform for stimulation of simultaneous cellular and humoral immune responses. Vaccines 2020, 8, 254. [Google Scholar] [CrossRef]
- Swaney, L.M. A continuous bovine kidney cell line for routine assays of foot-and-mouth disease virus. Vet. Microbiol. 1988, 18, 1–14. [Google Scholar] [CrossRef]
- Fowler, V.; Knowles, N.; Paton, D.; Barnett, P. Marker vaccine potential of a foot-and-mouth disease virus with a partial VP1 GH loop deletion. Vaccine 2010, 28, 3428–3434. [Google Scholar] [CrossRef]
- Fukai, K.; Morioka, K.; Yamada, M.; Nishi, T.; Yoshida, K.; Kitano, R.; Yamazoe, R.; Kanno, T. Comparative performance of fetal goat tongue cell line ZZ-R 127 and fetal porcine kidney cell line LFBK-αvβ6 for Foot-and-mouth disease virus isolation. J. Vet. Diagn. Investig. 2015, 27, 516–521. [Google Scholar] [CrossRef]
- Vono, M.; Eberhardt, C.S.; Auderset, F.; Mastelic-Gavillet, B.; Lemeille, S.; Christensen, D.; Andersen, P.; Lambert, P.H.; Siegrist, C.A. Maternal Antibodies Inhibit Neonatal and Infant Responses to Vaccination by Shaping the Early-Life B Cell Repertoire within Germinal Centers. Cell Rep. 2019, 28, 1773–1784.e1775. [Google Scholar] [CrossRef] [PubMed]
- Baratelli, M.; Molist-Badiola, J.; Puigredon-Fontanet, A.; Pascual, M.; Boix, O.; Mora-Igual, F.X.; Woodward, M.; Lavazza, A.; Capucci, L. Characterization of the Maternally Derived Antibody Immunity against Rhdv-2 after Administration in Breeding Does of an Inactivated Vaccine. Vaccines 2020, 8, 484. [Google Scholar] [CrossRef] [PubMed]
- Chamba Pardo, F.O.; Wayne, S.; Culhane, M.R.; Perez, A.; Allerson, M.; Torremorell, M. Effect of strain-specific maternally-derived antibodies on influenza A virus infection dynamics in nursery pigs. PLoS ONE 2019, 14, e0210700. [Google Scholar] [CrossRef]
- Fablet, C.; Renson, P.; Eono, F.; Mahe, S.; Eveno, E.; Le Dimna, M.; Normand, V.; Lebret, A.; Rose, N.; Bourry, O. Maternally-derived antibodies (MDAs) impair piglets’ humoral and cellular immune responses to vaccination against porcine reproductive and respiratory syndrome (PRRS). Vet. Microbiol. 2016, 192, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Moonen, P.; Miedema, G.; Hemert-Kluitenberg, V.; Chenard, G.; Dekker, A. Comparison of FMDV neutralisation Tests Using Three Different Cell Lines: Validation of the FAO Reference Sera; Session of the research group of the European commission for the control of foot-and-mouth disease: Borovets, Bulgaria, 2000; pp. 241–246. Available online: http://www.fao.org/ag/aginfo/commissions/docs/-research_group/borovet/app30.pdf (accessed on 2 February 2022).
- Schutta, C.; Barrera, J.; Pisano, M.; Zsak, L.; Grubman, M.J.; Mayr, G.A.; Moraes, M.P.; Kamicker, B.J.; Brake, D.A.; Ettyreddy, D.; et al. Multiple efficacy studies of an adenovirus-vectored foot-and-mouth disease virus serotype A24 subunit vaccine in cattle using homologous challenge. Vaccine 2016, 34, 3214–3220. [Google Scholar] [CrossRef]
- Koch, M.A.; Reiner, G.L.; Lugo, K.A.; Kreuk, L.S.; Stanbery, A.G.; Ansaldo, E.; Seher, T.D.; Ludington, W.B.; Barton, G.M. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 2016, 165, 827–841. [Google Scholar] [CrossRef]
- De Groot, A.S.; Moise, L.; McMurry, J.A.; Wambre, E.; Van Overtvelt, L.; Moingeon, P.; Scott, D.W.; Martin, W. Activation of natural regulatory T cells by IgG Fc–derived peptide “Tregitopes”. Blood 2008, 112, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531. [Google Scholar] [CrossRef]
- Ndure, J.; Flanagan, K.L. Targeting regulatory T cells to improve vaccine immunogenicity in early life. Front. Microbiol. 2014, 5, 477. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Wong, W.I.; Wang, Y.; Hsieh, M.; Lu, C.; Liang, C.; Jui, S.; Wu, F.; Chen, P.; Yang, H. Vaccine-induced antigen-specific regulatory T cells attenuate the antiviral immunity against acute influenza virus infection. Mucosal Immunol. 2018, 11, 1239. [Google Scholar] [CrossRef]
- Clemente, A.; Caporale, R.; Sannella, A.R.; Majori, G.; Severini, C.; Fadigati, G. Plasmodium falciparum soluble extracts potentiate the suppressive function of polyclonal T regulatory cells through activation of TGFbeta-mediated signals. Cell Microbiol. 2001, 13, 1328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanra, G.; Yalcin, S.S.; Ceyhan, M.; Yurdakok, K. Clinical trial to evaluating immunogenicity and safety of inactivated hepatitis A vaccination starting at 2-monnth-old children. Turk. J. Pediatr. 2000, 42, 105. [Google Scholar] [PubMed]
- Huang, N.; Chi, H.; Qiao, J. Role of regulatory t cells in regulating fetal-maternal immune tolerance in healthy pregnancies and reproductive diseases. Front. Immunol. 2020, 11, 1023. [Google Scholar] [CrossRef]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; Satitsuksanoa, P.; Akdis, M.; Akdis, C.A. Il-10 producing T and B cells in allergy. Semin. Immunol. 2019, 44, 101326. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, S.; Li, M.O. TGF-β Control of Adaptive Immune Tolerance: A Break From Treg Cells. Bioessays 2018, 40, 1800063. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-M.; Shi, Y.-Z.; Cheng, M.; Wang, D.-F.; Fan, J.-F. Serum IL-6, IL-23 profile and Treg/Th17 peripheral cell populations in pediatric patients with inflammatory bowel disease. Pharmazie 2017, 72, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, Y.; Zhang, Z.; Li, Y. Interleukin-10-Mediated Lymphopenia Caused by Acute Infection with Foot-and-Mouth Disease Virus in Mice. Viruses 2021, 13, 2358. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Park, S.H.; Park, J.-H.; Kim, S.-M.; Lee, M.J. Age-Dependent Dynamics of Maternally Derived Antibodies (MDAs) and Understanding MDA-Mediated Immune Tolerance in Foot-and-Mouth Disease-Vaccinated Pigs. Vaccines 2022, 10, 677. https://doi.org/10.3390/vaccines10050677
Shin S, Park SH, Park J-H, Kim S-M, Lee MJ. Age-Dependent Dynamics of Maternally Derived Antibodies (MDAs) and Understanding MDA-Mediated Immune Tolerance in Foot-and-Mouth Disease-Vaccinated Pigs. Vaccines. 2022; 10(5):677. https://doi.org/10.3390/vaccines10050677
Chicago/Turabian StyleShin, Sehee, So Hui Park, Jong-Hyeon Park, Su-Mi Kim, and Min Ja Lee. 2022. "Age-Dependent Dynamics of Maternally Derived Antibodies (MDAs) and Understanding MDA-Mediated Immune Tolerance in Foot-and-Mouth Disease-Vaccinated Pigs" Vaccines 10, no. 5: 677. https://doi.org/10.3390/vaccines10050677
APA StyleShin, S., Park, S. H., Park, J.-H., Kim, S.-M., & Lee, M. J. (2022). Age-Dependent Dynamics of Maternally Derived Antibodies (MDAs) and Understanding MDA-Mediated Immune Tolerance in Foot-and-Mouth Disease-Vaccinated Pigs. Vaccines, 10(5), 677. https://doi.org/10.3390/vaccines10050677






