The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
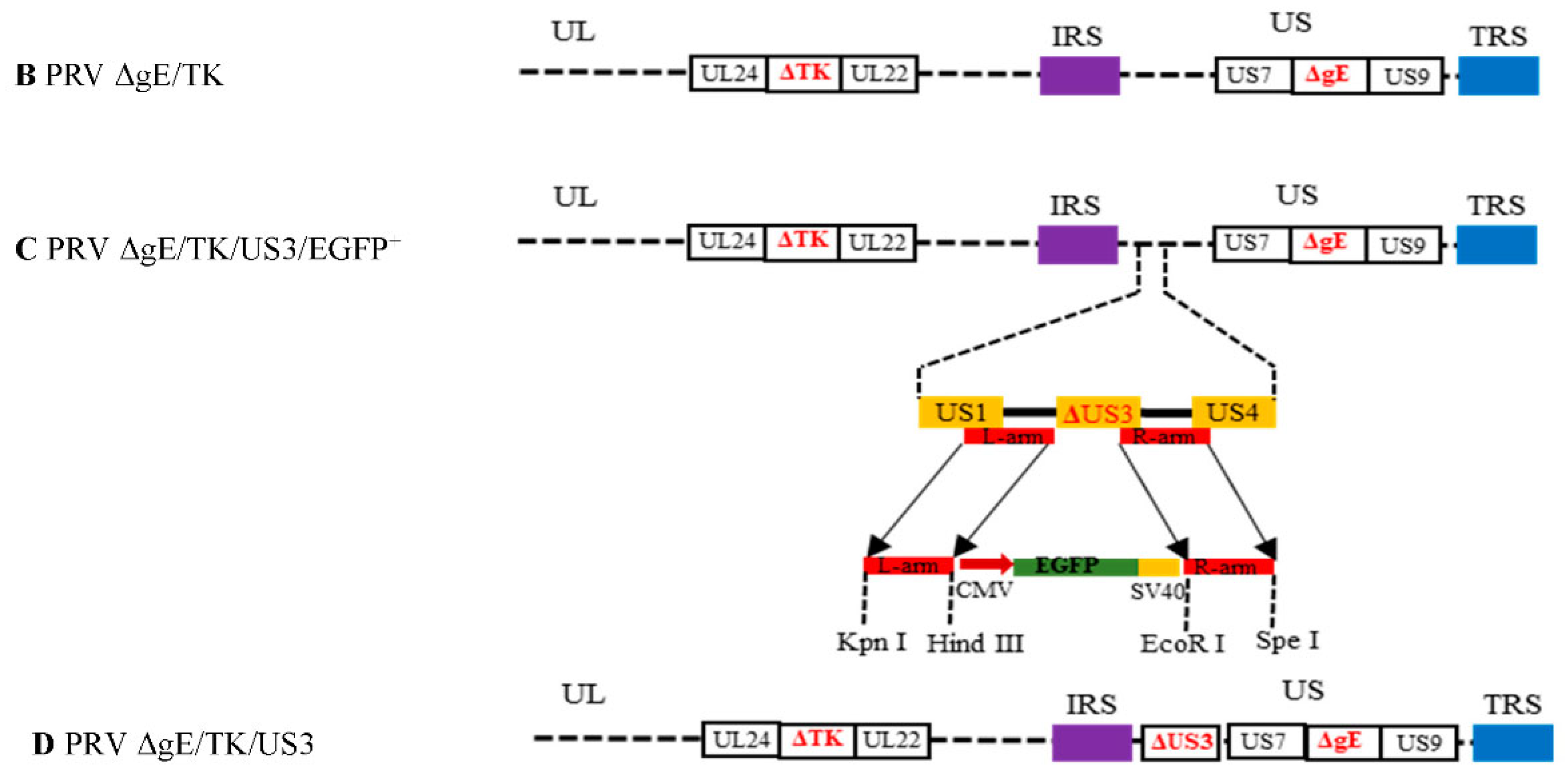
2.2. Construction of Recombinant Plasmid and Virus
2.3. Growth Characteristics of PRV ΔgE/TK/US3 Strain
2.4. Apoptosis
2.5. Swine Leukocyte Antigen Class I Molecule (SLA-I)
2.6. Pro-Inflammatory Cytokines
2.7. Pathogenic and Immunological Experiments in Mice
2.8. Statistical Analysis
3. Results
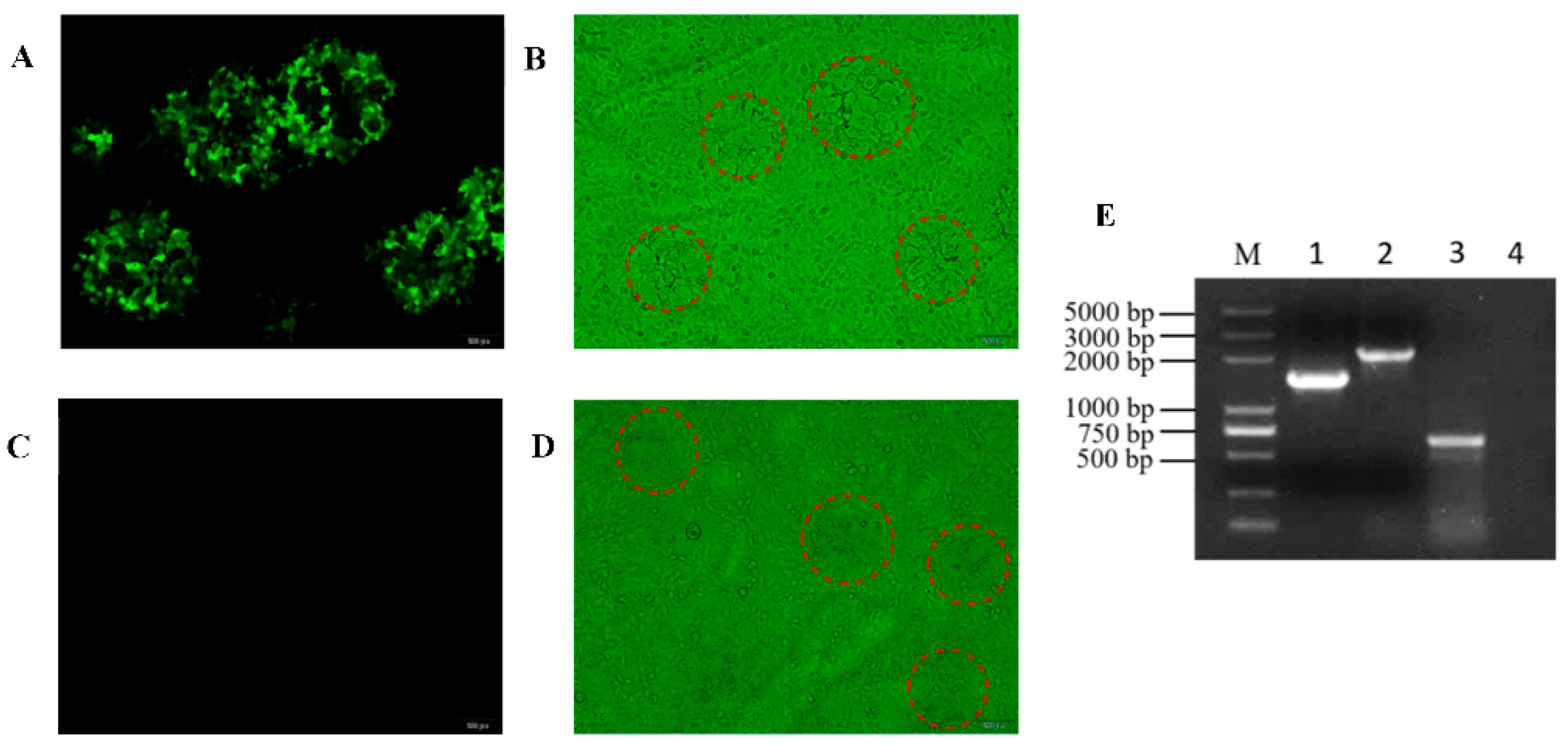
3.1. Generation of gE/TK/US3-Deleted Recombinant Virus
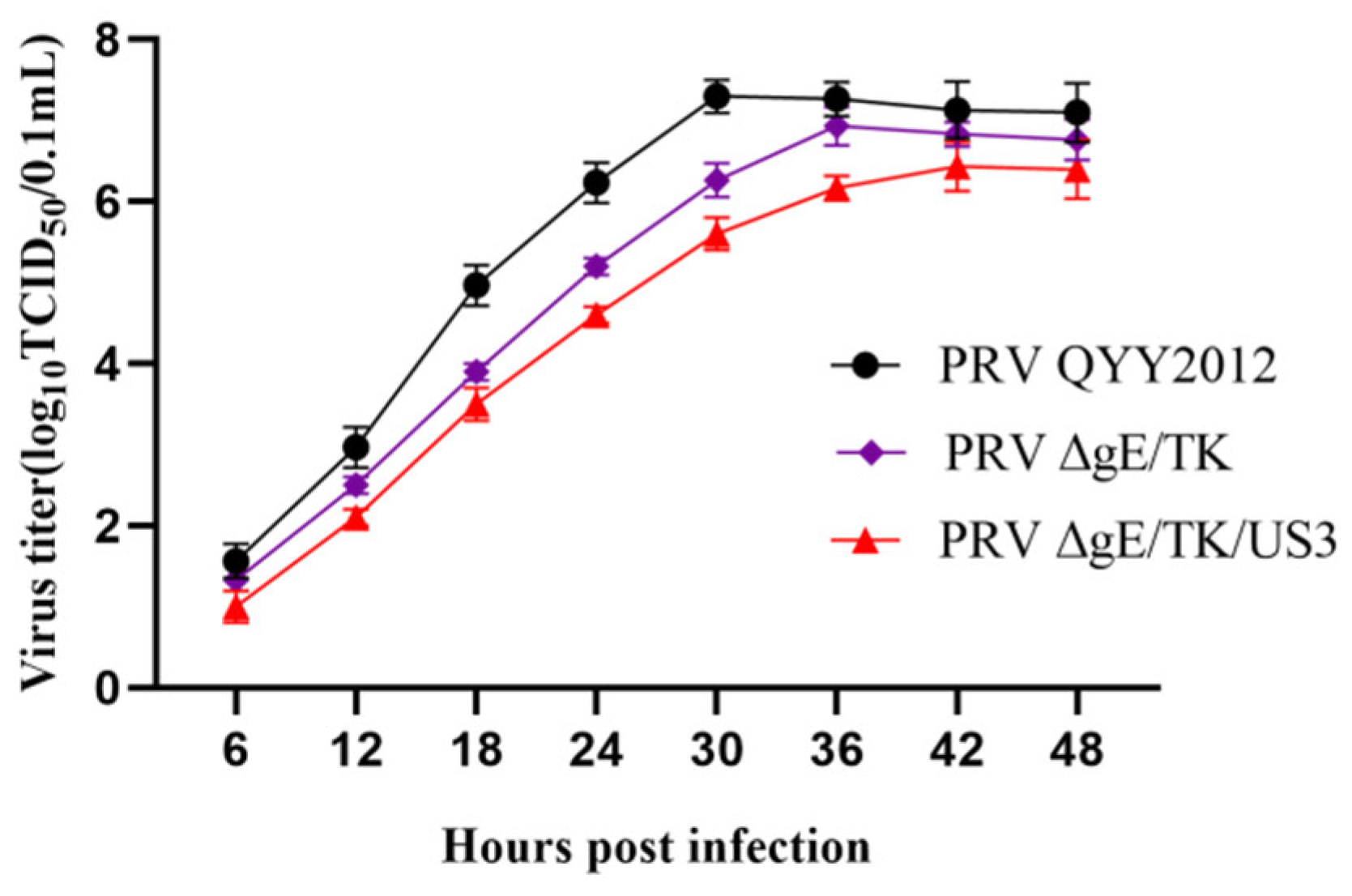
3.2. The Multiple Step Growth Curves of PRV Strains
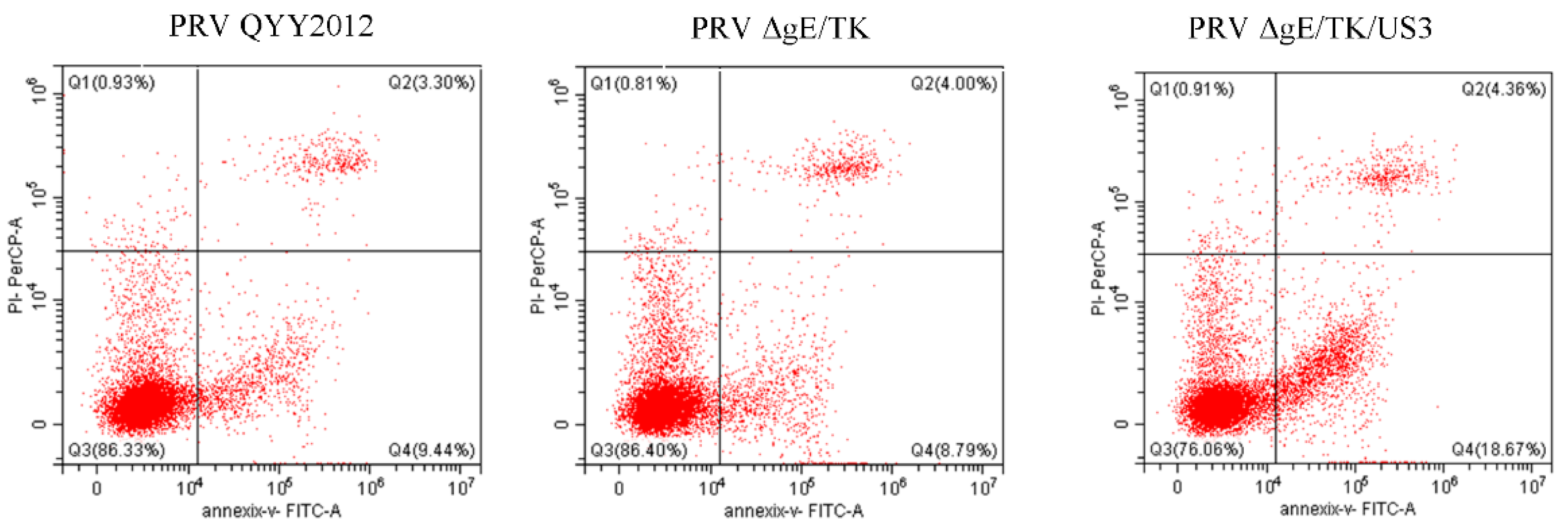
3.3. PRV ΔgE/TK/US3 Strain Promotes Apoptosis
3.4. The Deletion of US3 Gene Upregulates the Transcriptional Level of SLA-I
3.5. PRV ΔgE/TK/US3 Strain Downregulates the Levels of Pro-Inflammatory Cytokines
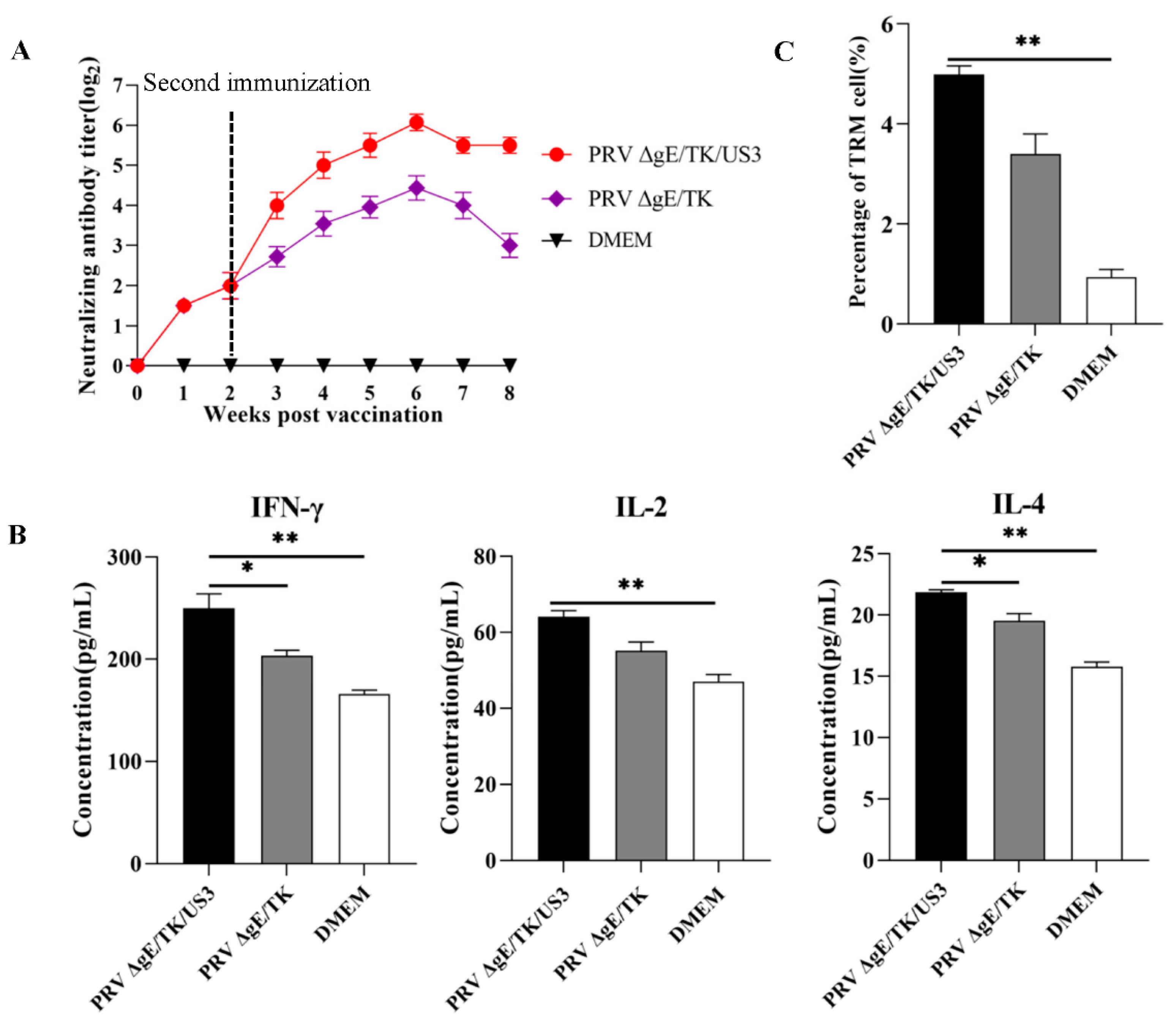
3.6. The Safety and Immunogenicity of PRV ΔgE/TK/US3 in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Freuling, C.M.; Müller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, S.; Liu, C.; Han, J.; Tang, J.; Zhou, L.; Ge, X.; Guo, X.; Yang, H. The pUL56 of pseudorabies virus variant induces downregulation of swine leukocyte antigen class I molecules through the lysosome pathway. Virus Res. 2018, 251, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Laval, K.; Enquist, L.W. The Neuropathic Itch Caused by Pseudorabies Virus. Pathogens 2020, 9, 254. [Google Scholar] [CrossRef]
- Ao, J.Q.; Wang, J.W.; Chen, X.H.; Wang, X.Z.; Long, Q.X. Expression of pseudorabies virus gE epitopes in Pichia pastoris and its utilization in an indirect PRV gE-ELISA. J. Virol. Methods 2003, 114, 145–150. [Google Scholar] [CrossRef]
- Li, W.; Zhuang, D.; Li, H.; Zhao, M.; Zhu, E.; Xie, B.; Chen, J.; Zhao, M. Recombinant pseudorabies virus with gI/gE deletion generated by overlapping polymerase chain reaction and homologous recombination technology induces protection against the PRV variant PRV-GD2013. BMC Vet. Res. 2021, 17, 164. [Google Scholar] [CrossRef]
- Wang, J.; Song, Z.; Ge, A.; Guo, R.; Qiao, Y.; Xu, M.; Wang, Z.; Liu, Y.; Zheng, Y.; Fan, H.; et al. Safety and immunogenicity of an attenuated Chinese pseudorabies variant by dual deletion of TK&gE genes. BMC Vet. Res. 2018, 14, 287. [Google Scholar] [CrossRef]
- Dong, B.; Zarlenga, D.S.; Ren, X. An overview of live attenuated recombinant pseudorabies viruses for use as novel vaccines. J. Immunol. Res. 2014, 2014, 824630. [Google Scholar] [CrossRef]
- Jansens, R.J.J.; Marmiroli, S.; Favoreel, H.W. An Unbiased Approach to Mapping the Signaling Network of the Pseudorabies Virus US3 Protein. Pathogens 2020, 9, 916. [Google Scholar] [CrossRef]
- Lamote, J.A.S.; Glorieux, S.; Nauwynck, H.J.; Favoreel, H.W. The US3 Protein of Pseudorabies Virus Drives Viral Passage across the Basement Membrane in Porcine Respiratory Mucosa Explants. J. Virol. 2016, 90, 10945–10950. [Google Scholar] [CrossRef]
- Sehl, J.; Pörtner, S.; Klupp, B.G.; Granzow, H.; Franzke, K.; Teifke, J.P.; Mettenleiter, T.C. Roles of the Different Isoforms of the Pseudorabies Virus Protein Kinase pUS3 in Nuclear Egress. J. Virol. 2020, 94, e02029-19. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Wells, J.; Klein, R.; Sylvester, T.; Sunenshine, R. Notes from the field: Outbreak of skin lesions among high school wrestlers—Arizona, 2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 559–560. [Google Scholar] [PubMed]
- Gravier, R.; Dory, D.; Rodriguez, F.; Bougeard, S.; Beven, V.; Cariolet, R.; Jestin, A. Immune and protective abilities of ubiquitinated and non-ubiquitinated pseudorabies virus glycoproteins. Acta Virol. 2007, 51, 35–45. [Google Scholar] [PubMed]
- Delva, J.L.; Nauwynck, H.J.; Mettenleiter, T.C.; Favoreel, H.W. The Attenuated Pseudorabies Virus Vaccine Strain Bartha K61: A Brief Review on the Knowledge Gathered During 60 Years of Research. Pathogens 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Miao, Y.; Xi, R.; Gao, X.; Miao, D.; Chen, H.; Jung, Y.S.; Qian, Y.; Dai, J. Emergence of a novel pathogenic recombinant virus from Bartha vaccine and variant pseudorabies virus in China. Transbound. Emerg. Dis. 2021, 68, 1454–1464. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Chen, S.; Qiao, Y.; Guo, M.; Zheng, Y.; Xu, M.; Wang, Z.; Hou, J.; Wang, J. A gD&gC-substituted pseudorabies virus vaccine strain provides complete clinical protection and is helpful to prevent virus shedding against challenge by a Chinese pseudorabies variant. BMC Vet. Res. 2019, 15, 2. [Google Scholar] [CrossRef]
- Wang, J.; Cui, X.; Wang, X.; Wang, W.; Gao, S.; Liu, X.; Kai, Y.; Chen, C. Efficacy of the Bartha-K61 vaccine and a gE(-)/gI(-)/TK(-) prototype vaccine against variant porcine pseudorabies virus (vPRV) in piglets with sublethal challenge of vPRV. Res. Vet. Sci. 2020, 128, 16–23. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Magtoto, R.; Henao-Díaz, A. Detection of pseudorabies virus antibody in swine serum and oral fluid specimens using a recombinant gE glycoprotein dual-matrix indirect ELISA. J. Vet. Diagn. Investig. 2021, 33, 1106–1114. [Google Scholar] [CrossRef]
- Lin, J.; Li, Z.; Feng, Z.; Fang, Z.; Chen, J.; Chen, W.; Liang, W.; Chen, Q. Pseudorabies virus (PRV) strain with defects in gE, gC, and TK genes protects piglets against an emerging PRV variant. J. Vet. Med. Sci. 2020, 82, 846–855. [Google Scholar] [CrossRef]
- Ferrari, M.; Gualandi, G.L.; Corradi, A.; Monaci, C.; Romanelli, M.G.; Losio, M.N.; Cantoni, A.M.; Pratelli, A. The response of pigs inoculated with a thymidine kinase-negative (TK-) pseudorabies virus to challenge infection with virulent virus. Comp. Immunol. Microbiol. Infect. Dis. 2000, 23, 15–26. [Google Scholar] [CrossRef]
- Tenser, R.B.; Ressel, S.J.; Fralish, F.A.; Jones, J.C. The role of pseudorabies virus thymidine kinase expression in trigeminal ganglion infection. J. Gen. Virol. 1983, 64 Pt 6, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.Q.; Zheng, H.H.; Yang, Y.R.; Liu, F.; Zheng, L.L.; Jin, Y.; Chen, H.Y. Construction and immunogenicity of a gE/gI/TK-deleted PRV based on porcine pseudorabies virus variant. Mol. Cell. Probes 2020, 53, 101605. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, N. Intratumoral cancer immunotherapy exploiting anti-viral immunity. J. Clin. Exp. Hematop. 2021, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Van den Broeke, C.; Grauwet, K.; Baert, K.; Claessen, C.; De Pelsmaeker, S.; Van Waesberghe, C.; Favoreel, H.W. Pseudorabies virus US3 leads to filamentous actin disassembly and contributes to viral genome delivery to the nucleus. Vet. Microbiol. 2015, 177, 379–385. [Google Scholar] [CrossRef]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef]
- Chang, C.D.; Lin, P.Y.; Liao, M.H.; Chang, C.I.; Hsu, J.L.; Yu, F.L.; Wu, H.Y.; Shih, W.L. Suppression of apoptosis by pseudorabies virus Us3 protein kinase through the activation of PI3-K/Akt and NF-κB pathways. Res. Vet. Sci. 2013, 95, 764–774. [Google Scholar] [CrossRef]
- Grauwet, K.; Vitale, M.; De Pelsmaeker, S.; Jacob, T.; Laval, K.; Moretta, L.; Parodi, M.; Parolini, S.; Cantoni, C.; Favoreel, H.W. Pseudorabies Virus US3 Protein Kinase Protects Infected Cells from NK Cell-Mediated Lysis via Increased Binding of the Inhibitory NK Cell Receptor CD300a. J. Virol. 2016, 90, 1522–1533. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, R.; Lang, Y.; Shao, A.; Xu, A.; Feng, W.; Han, J.; Wang, M.; He, W.; Yu, C.; et al. Bclaf1 critically regulates the type I interferon response and is degraded by alphaherpesvirus US3. PLoS Pathog. 2019, 15, e1007559. [Google Scholar] [CrossRef]
- Geenen, K.; Favoreel, H.W.; Olsen, L.; Enquist, L.W.; Nauwynck, H.J. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology 2005, 331, 144–150. [Google Scholar] [CrossRef]
- Deruelle, M.J.; Van den Broeke, C.; Nauwynck, H.J.; Mettenleiter, T.C.; Favoreel, H.W. Pseudorabies virus US3- and UL49.5-dependent and -independent downregulation of MHC I cell surface expression in different cell types. Virology 2009, 395, 172–181. [Google Scholar] [CrossRef][Green Version]
- Carpenter, D.; Hsiang, C.; Jiang, X.; Osorio, N.; BenMohamed, L.; Jones, C.; Wechsler, S.L. The herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) protects cells against cold-shock-induced apoptosis by maintaining phosphorylation of protein kinase B (AKT). J. Neurovirol. 2015, 21, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, X.; Chen, L.; Bi, Y.; Idris, A.; Xu, S.; Li, X.; Zhang, Y.; Feng, R. Pseudorabies Virus US3 Protein Inhibits IFN-β Production by Interacting With IRF3 to Block Its Activation. Front. Microbiol. 2021, 12, 761282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tang, J. Evasion of I Interferon-Mediated Innate Immunity by Pseudorabies Virus. Front. Microbiol. 2021, 12, 801257. [Google Scholar] [CrossRef] [PubMed]
- Ogg, P.D.; McDonell, P.J.; Ryckman, B.J.; Knudson, C.M.; Roller, R.J. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 2004, 319, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Koyanagi, N.; Ogawa, R.; Shindo, K.; Suenaga, T.; Sato, A.; Arii, J.; Kato, A.; Kiyono, H.; Arase, H.; et al. Us3 kinase encoded by herpes simplex virus 1 mediates downregulation of cell surface major histocompatibility complex class I and evasion of CD8+ T cells. PLoS ONE 2013, 8, e72050. [Google Scholar] [CrossRef]
- Orr, M.T.; Mathis, M.A.; Lagunoff, M.; Sacks, J.A.; Wilson, C.B. CD8 T cell control of HSV reactivation from latency is abrogated by viral inhibition of MHC class I. Cell Host Microbe 2007, 2, 172–180. [Google Scholar] [CrossRef]
- Cioni, M.; Mittelholzer, C.; Wernli, M.; Hirsch, H.H. Comparing effects of BK virus agnoprotein and herpes simplex-1 ICP47 on MHC-I and MHC-II expression. Clin. Dev. Immunol. 2013, 2013, 626823. [Google Scholar] [CrossRef]
- Raafat, N.; Sadowski-Cron, C.; Mengus, C.; Heberer, M.; Spagnoli, G.C.; Zajac, P. Preventing vaccinia virus class-I epitopes presentation by HSV-ICP47 enhances the immunogenicity of a TAP-independent cancer vaccine epitope. Int. J. Cancer 2012, 131, E659–E669. [Google Scholar] [CrossRef]
- Noriega, V.M.; Hesse, J.; Gardner, T.J.; Besold, K.; Plachter, B.; Tortorella, D. Human cytomegalovirus US3 modulates destruction of MHC class I molecules. Mol. Immunol. 2012, 51, 245–253. [Google Scholar] [CrossRef]
- Liu, Z.; Winkler, M.; Biegalke, B. Human cytomegalovirus: Host immune modulation by the viral US3 gene. Int. J. Biochem. Cell Biol. 2009, 41, 503–506. [Google Scholar] [CrossRef]
- Ren, C.Z.; Hu, W.Y. Establishment of inflammatory model induced by Pseudorabies virus infection in mice. J. Vet. Sci. 2021, 22, e20. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ni, L.; Wang, S.; Zheng, C. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-κB activation. J. Virol. 2014, 88, 7941–7951. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Lei, J.L.; Xia, S.L.; Wang, Y.M.; Li, Y.; Li, S.; Luo, Y.; Sun, Y.; Qiu, H.J. Pathogenicity and immunogenicity of a gE/gI/TK gene-deleted pseudorabies virus variant in susceptible animals. Vet. Microbiol. 2016, 182, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, P.; Swan, D.A.; Duke, E.; Corey, L.; Zhu, J.; Davé, V.; Spuhler, L.R.; Lund, J.M.; Prlic, M.; Schiffer, J.T. Tissue-resident T cell-derived cytokines eliminate herpes simplex virus-2-infected cells. J. Clin. Investig. 2020, 130, 2903–2919. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, T.R.; Hu, K.; Truong, N.R. The Role of Tissue Resident Memory CD4 T Cells in Herpes Simplex Viral and HIV Infection. Viruses 2021, 13, 359. [Google Scholar] [CrossRef]



| Primers | Sequence(5′→3′) | Restriction Site | Expected Product/bp |
|---|---|---|---|
| US3L-F/R | F: CGGGGTACCGCGAGTCTGCGGGATGGT | Kpn Ⅰ | 658 |
| R: CCCAAGCTTAGCGTCGAGGGCTTCTGG | Hind Ⅲ | ||
| US3R-F/R | F: CCGGAATTCCTCAACAATGAAGTGGGCAAC | EcoR Ⅰ | 778 |
| R: CGGACTAGTTCAGACTCCAGGCGGAAGAT | Spe Ⅰ | ||
| EGFP-F/R | F: CCCAAGCTTATAACTTCGTATAGCATACATTATACGAAGTTATTTCCCATAGTAACGCCAATAG | Hind Ⅲ | 1620 |
| R: CCGGAATTCATAACTTCGTATAATGTATGCTATACGAAGTTATGAAAGGACAGTGGGAGTG | EcoR Ⅰ | ||
| US3-F/R | F: GGAGATGGGTCACCAAGAGG | / | 1617 |
| R: CCGCCGTCAAAGAACCAG | / | ||
| SLA-I qF/R | F: AAGTCAAGGAAACCGCACAG | / | 113 |
| R: CAAGTAGCAGCCAAACATGC | / | ||
| TNF-α qF/R | F:TGATCCGCGACGTGGAA | / | 72 |
| R: ACCGCCTGGAGTTCTGGAA | / | ||
| IL-1β qF/R | F: ACTCCTTAGTCCTCGGCCA | / | 99 |
| R: CCATCAGAGGCAAGGAGGAA | / | ||
| IL-6 qF/R | F: GAGGATACCACTCCCAACAGACC | / | 141 |
| R: AAGTGCATCATCGTTGTTCATACA | / | ||
| β-actin qF/R | F:TCCTGCGGCA TCCACGAAAC | / | 82 |
| R: CCGTGTTGGCGTAGAGGTCCTTG | / | ||
| GAPDH qF/R | F: AGGTCGGTGTGAACGGAT | / | 123 |
| R: TGTAGACCATGTAGTTGAGG | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, M.-M.; Sun, Y.-W.; Ding, C.-M.; Xu, X.-Y.; Guo, Z.-Y.; Han, Z.-W.; Lv, C.-Z.; Qi, J.-K.; Li, Y.-T.; Yang, X.; et al. The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice. Vaccines 2022, 10, 1603. https://doi.org/10.3390/vaccines10101603
Deng M-M, Sun Y-W, Ding C-M, Xu X-Y, Guo Z-Y, Han Z-W, Lv C-Z, Qi J-K, Li Y-T, Yang X, et al. The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice. Vaccines. 2022; 10(10):1603. https://doi.org/10.3390/vaccines10101603
Chicago/Turabian StyleDeng, Meng-Meng, Ya-Wei Sun, Chen-Meng Ding, Xi-Ya Xu, Zi-Yi Guo, Zi-Wei Han, Chen-Zhe Lv, Jiang-Kun Qi, Yong-Tao Li, Xia Yang, and et al. 2022. "The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice" Vaccines 10, no. 10: 1603. https://doi.org/10.3390/vaccines10101603
APA StyleDeng, M.-M., Sun, Y.-W., Ding, C.-M., Xu, X.-Y., Guo, Z.-Y., Han, Z.-W., Lv, C.-Z., Qi, J.-K., Li, Y.-T., Yang, X., Yu, L.-Y., & Chen, L. (2022). The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice. Vaccines, 10(10), 1603. https://doi.org/10.3390/vaccines10101603





