Opinion Polls and Antibody Response Dynamics of Vaccination with COVID-19 Booster Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design and Participants
2.2. Opinion Polls Design
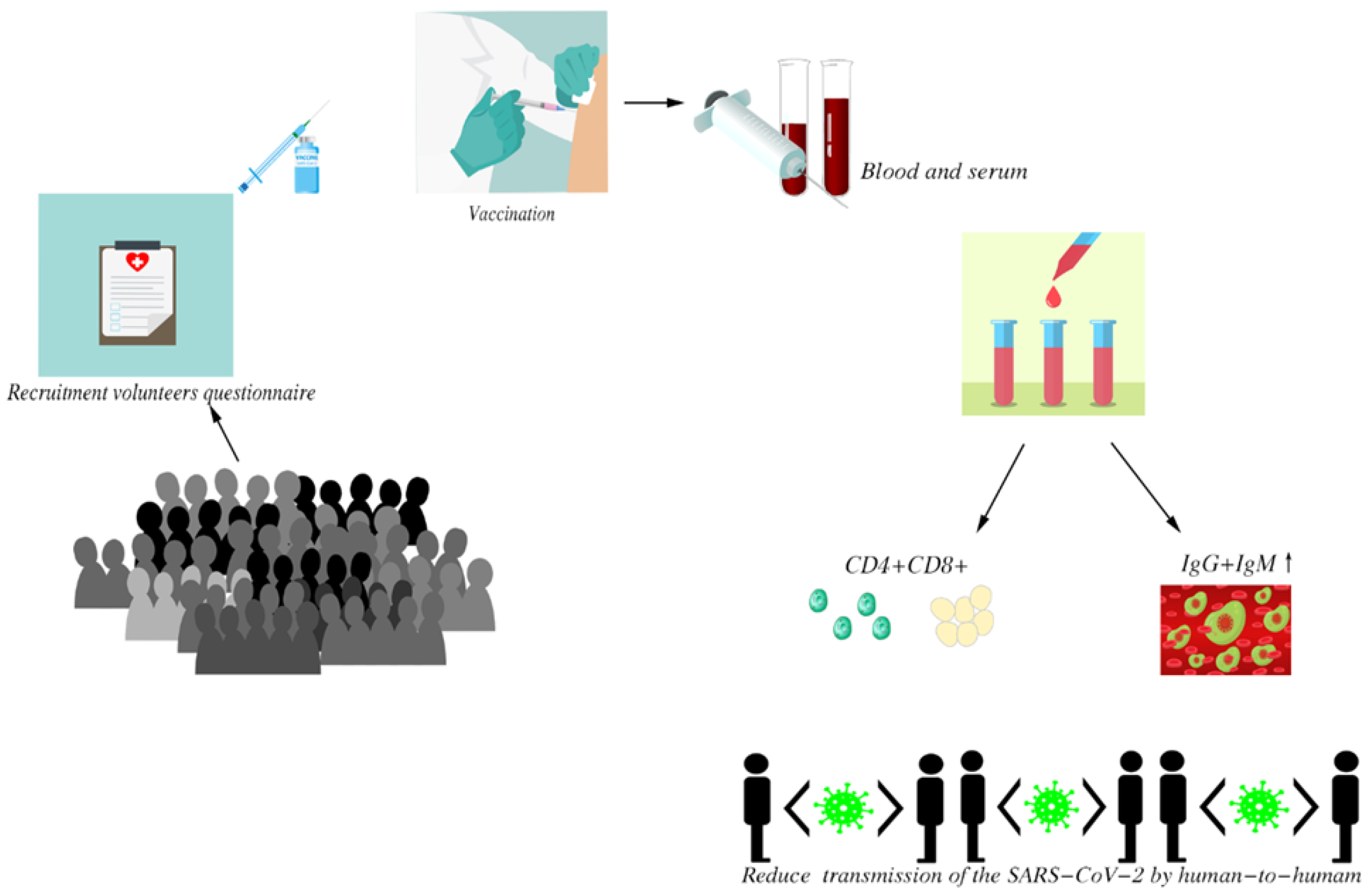
2.3. Specimen Collection
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
3.1. Research Process Flow
3.2. Respondent Characteristics
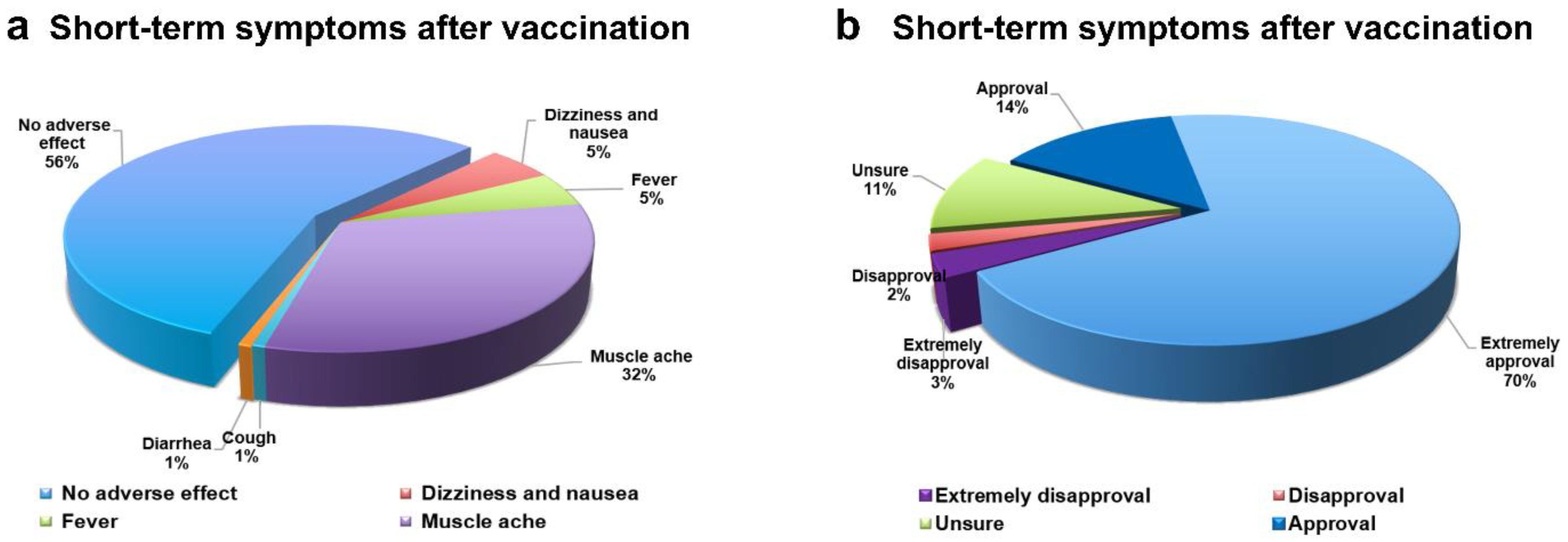
3.3. Estimation of Preferences and Heterogeneity
3.4. Dynamic Serosurvey of SARS-CoV-2 S-specific Binding Antibodies Concentration
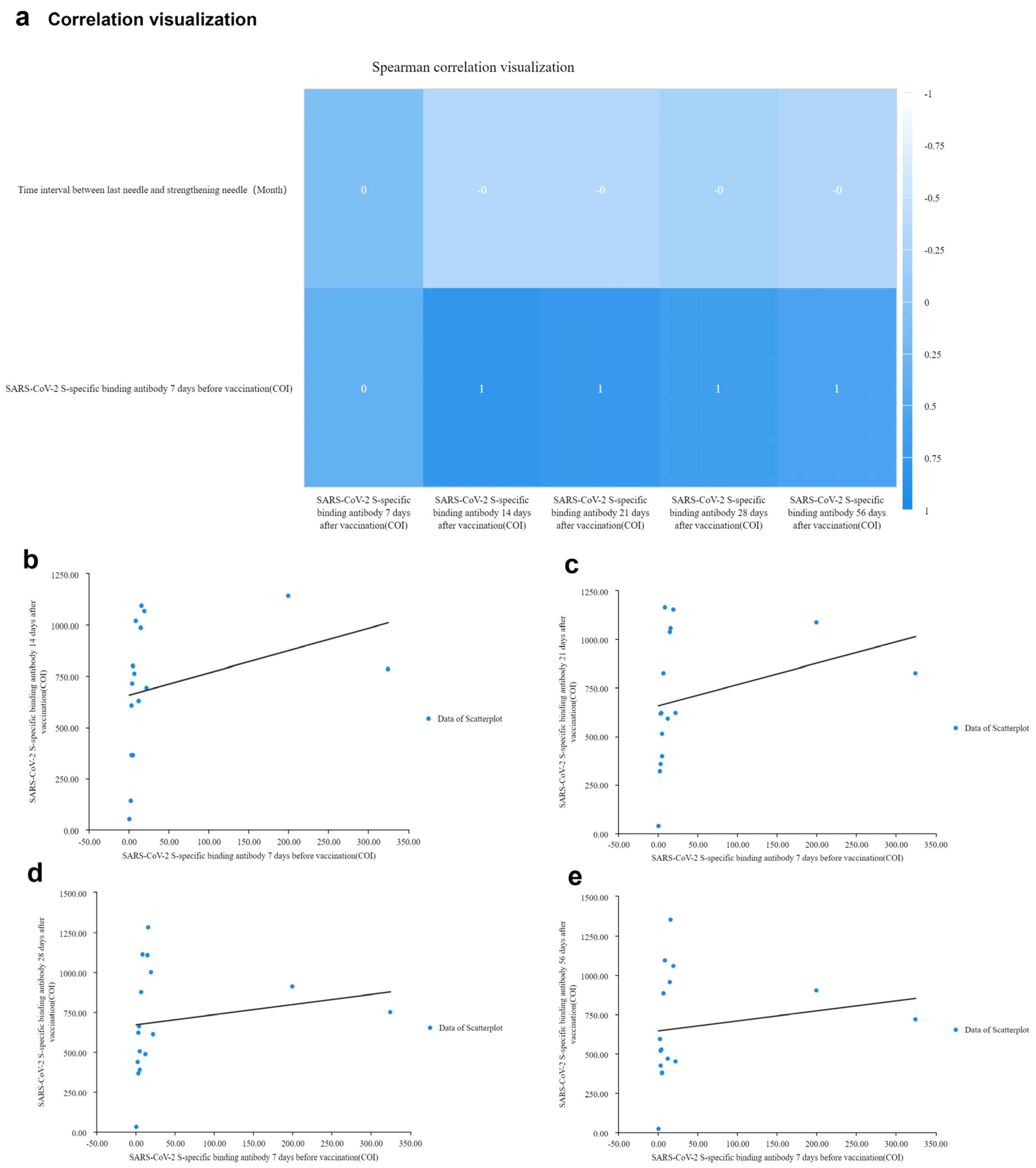
3.5. Correlation Analysis of SARS-CoV-2 S-specific Binding Antibodies Concentration
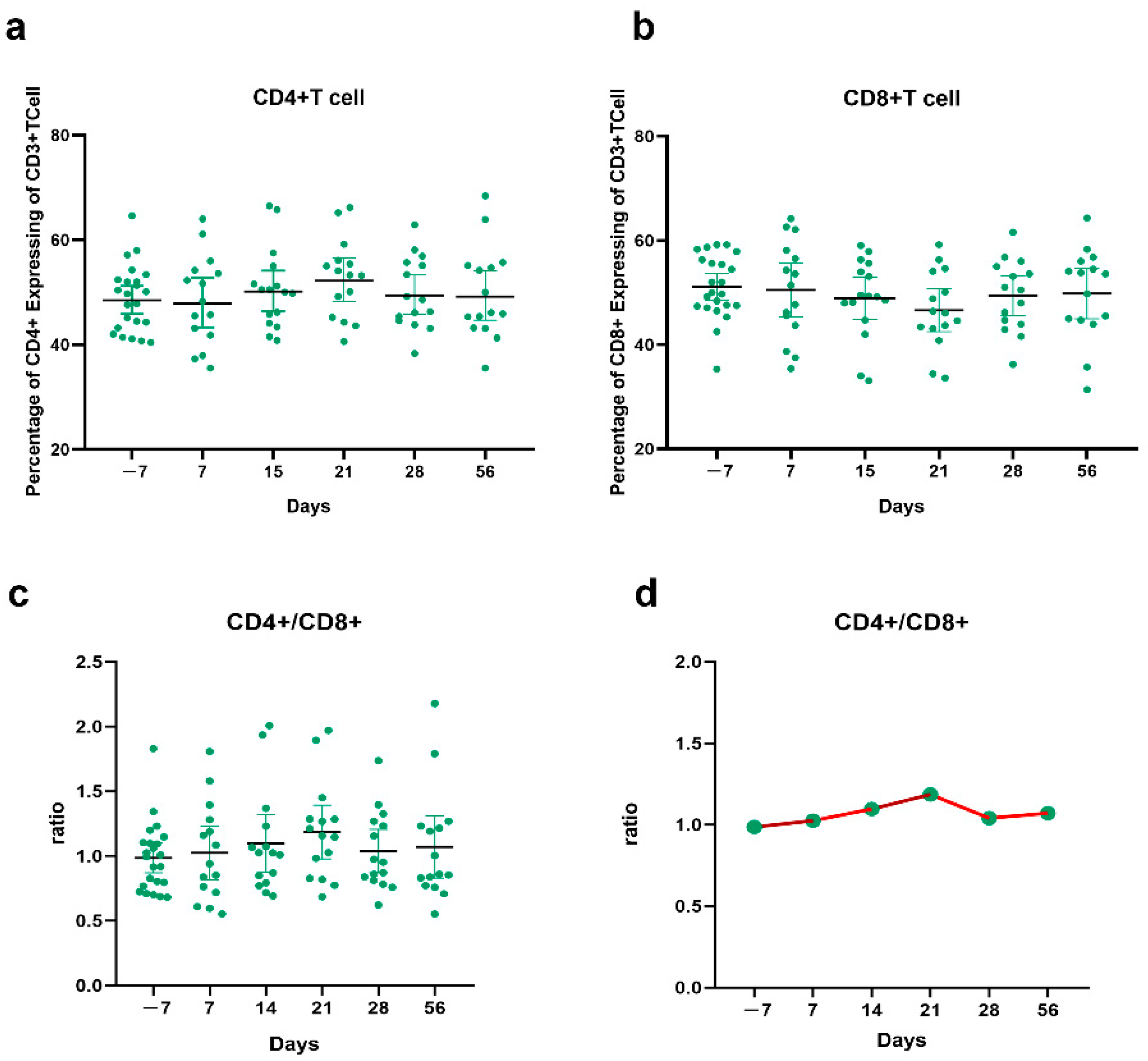
3.6. CD4+ T Cell and CD8+ T Cell Responses before and after the Third Vaccination
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, A.C.K.; Morling, J.R. COVID-19 vaccine dilemmas. Public Health 2022, 202, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Ji, M.; Pei, F.; Zhao, Q.; Zhou, Y.; Hong, Y.; Han, S.; Wang, J.; Wang, Q.; et al. Transmission Routes Analysis of SARS-CoV-2: A Systematic Review and Case Report. Front. Cell Dev. Biol. 2020, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.; Cotton, D.; Moyer, D.V. COVID-19 Vaccine: Promoting Vaccine Acceptance. Ann. Intern. Med. 2021, 174, 252–253. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Harris, T. An Effective COVID-19 Vaccine Needs to Engage T Cells. Front. Immunol. 2020, 11, 581807. [Google Scholar] [CrossRef] [PubMed]
- Slifka, M.K.; Amanna, I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine 2014, 32, 2948–2957. [Google Scholar] [CrossRef] [Green Version]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef] [Green Version]
- Lustig, Y.; Zuckerman, N.; Nemet, I.; Atari, N.; Kliker, L.; Regev-Yochay, G.; Sapir, E.; Mor, O.; Alroy-Preis, S.; Mendelson, E. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill. 2021, 26, 2100557. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.S.; McElrath, M.J. COVID-19 and the Path to Immunity. JAMA 2020, 324, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Röst, G.; Moghadas, S.M. Delay in booster schedule as a control parameter in vaccination dynamics. J. Math. Biol. 2019, 79, 2157–2182. [Google Scholar] [CrossRef] [Green Version]
- Mösbauer, K.; Fritsch, V.N.; Adrian, L.; Bernhardt, J.; Gruhlke, M.C.; Slusarenko, A.J.; Niemeyer, D.; Antelmann, H. The Effect of Allicin on the Proteome of SARS-CoV-2 Infected Calu-3 Cells. Front. Microbiol. 2021, 12, 746795. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.B.; Malani, P.N. Booster Vaccination to Prevent COVID-19 in the Era of Omicron: An Effective Part of a Layered Public Health Approach. JAMA 2022, 327, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Slifka, M.K. The immunostimulatory power of acute viral infection. Immunity 2008, 28, 604–606. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, R.N.; Hay, C.M.; Stapleton, J.T.; Marbury, T.C.; Wagner, E.; Kreitmeir, E.; Röesch, S.; Von Krempelhuber, A.; Young, P.; Nichols, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Trial Investigating the Safety and Immunogenicity of Modified Vaccinia Ankara Smallpox Vaccine (MVA-BN®) in 56-80-Year-Old Subjects. PLoS ONE 2016, 11, e0157335. [Google Scholar] [CrossRef]
- Challenger, J.D.; Foo, C.Y.; Wu, Y.; Yan, A.W.C.; Marjaneh, M.M.; Liew, F.; Thwaites, R.S.; Okell, L.C.; Cunnington, A.J. Modelling upper respiratory viral load dynamics of SARS-CoV-2. BMC Med. 2022, 20, 25. [Google Scholar] [CrossRef]
- Nakanishi, T.; Pigazzini, S.; Degenhardt, F.; Cordioli, M.; Butler-Laporte, G.; Maya-Miles, D.; Bujanda, L.; Bouysran, Y.; Niemi, M.E.; Palom, A.; et al. Age-dependent impact of the major common genetic risk factor for COVID-19 on severity and mortality. J. Clin. Invest. 2021, 131, e152386. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Prévost, J.; Nayrac, M.; Beaudoin-Bussières, G.; Benlarbi, M.; Gasser, R.; Brassard, N.; Laumaea, A.; Gong, S.Y.; Bourassa, C.; et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 Spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021, 2, 100290. [Google Scholar] [CrossRef] [PubMed]
- Mahara, G.; Liang, J.; Zhang, Z.; Ge, Q.; Zhang, J. Associated Factors of Suboptimal Health Status Among Adolescents in China: A Cross-Sectional Study. J. Multidiscip. Healthc. 2021, 14, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Merriam-Webster. Definition of HEALTH. Dictionary. Available online: https://www.merriam-webster.com/dictionary/health (accessed on 21 March 2019).
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Yin, Y.; Jie, Q.I.; Yaping, D.A.; Fanwei, Z.E.; Hao, P.E.; Jun, W.A. The CD4+/CD8+ Ratio in Pulmonary Tuberculosis: Systematic and Meta-Analysis Article. Iran. J. Public Health 2015, 44, 185–193. [Google Scholar]
- Caserotti, M.; Girardi, P.; Rubaltelli, E.; Tasso, A.; Lotto, L.; Gavaruzzi, T. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Soc. Sci. Med. 2021, 272, 113688. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lin, C.-C.; Hsieh, C.-Y.; Lin, C.-Y.; Chen, T.-T.; Wu, P.-C.; Liu, D.-H.; Tou, S.-I.; Yen, H.-R. The Willingness of Elderly Taiwanese Individuals to Accept COVID-19 Vaccines after the First Local Outbreak. Vaccines 2022, 10, 520. [Google Scholar] [CrossRef]
- Macchia, A.; Ferrante, D.; Angeleri, P.; Biscayart, C.; Mariani, J.; Esteban, S.; Tablado, M.R.; de Quirós, F.G.B. Evaluation of a COVID-19 Vaccine Campaign and SARS-CoV-2 Infection and Mortality Among Adults Aged 60 Years and Older in a Middle-Income Country. JAMA Netw. Open 2021, 4, e2130800. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes. Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Kreps, S.; Dasgupta, N.; Brownstein, J.S.; Hswen, Y.; Kriner, D.L. Public Attitudes toward COVID-19 Vaccination: The Role of Vaccine Attributes, Incentives, and Misinformation. NPJ Vaccines 2021, 6, 73. [Google Scholar] [CrossRef]
- Aguiar, M.; Dosi, G.; Knopoff, D.A.; Virgillito, M.E. A multiscale network-based model of contagion dynamics: Heterogeneity, spatial distancing and vaccination. Math. Models Methods Appl. Sci. 2021, 31, 2425–2454. [Google Scholar] [CrossRef]
- Jiang, S.; Lechler, R.I. CD4+CD25+ regulatory T-cell therapy for allergy, autoimmune disease and transplant rejection. Inflamm. Allergy Drug Targets 2006, 5, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, S.; Adachi, T.; Saito, K.; Tanaka, Y. Role of P-glycoprotein on CD69(+) CD4(+) cells in the pathogenesis of proliferative lupus nephritis and non-responsiveness to immunosuppressive therapy. RMD Open 2017, 3, e000423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef]
- Ma, C.; Chen, X.; Mei, F.; Xiong, Q.; Liu, Q.; Dong, L.; Liu, C.; Zou, W.; Zhan, F.; Hu, B.; et al. Drastic decline in sera neutralization against SARS-CoV-2 Omicron variant in Wuhan COVID-19 convalescents. Emerg. Microbes Infect. 2022, 11, 567–572. [Google Scholar] [CrossRef]
- Loubet, P.; Laureillard, D.; Martin, A.; Larcher, R.; Sotto, A. Why promoting a COVID-19 vaccine booster dose? Anaesth. Crit. Care Pain Med. 2021, 40, 100967. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- García-Pérez, B.E.; González-Rojas, J.A.; Salazar, M.I.; Torres-Torres, C.; Castrejón-Jiménez, N.S. Taming the Autophagy as a Strategy for Treating COVID-19. Cells 2020, 9, 2679. [Google Scholar] [CrossRef]


| Characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 170 | 43.04 |
| Female | 225 | 56.96 |
| Age | ||
| Age12–18 | 5 | 1.27 |
| Age18–25 | 80 | 20.23 |
| Age25–40 | 163 | 41.27 |
| Age40–60 | 145 | 36.71 |
| Age60–65 | 2 | 0.51 |
| Education | ||
| Bachelor’s degree | 174 | 44.05 |
| Bachelor’s degree below | 76 | 19.24 |
| Master’s degree or above | 145 | 36.71 |
| Occupation | ||
| Student | 108 | 27.34 |
| Medical personnel | 22 | 5.57 |
| Personnel of state organs and institutions | 83 | 21.01 |
| Professional and technical personnel (teachers, lawyers, engineers and technicians, etc.) | 73 | 18.48 |
| Company employees | 37 | 9.37 |
| Self-employed person | 15 | 3.8 |
| Retirees | 26 | 6.58 |
| Freelancer | 23 | 5.82 |
| Others | 8 | 2.03 |
| Health | ||
| Good | 348 | 88.1 |
| General | 43 | 10.89 |
| Poor | 4 | 1.01 |
| Basic illness | ||
| CVD | 12 | 3.04 |
| Chronic tumor | 5 | 1.27 |
| Chronic respiratory disease | 11 | 1.78 |
| Immune deficiency diseases | 3 | 0.76 |
| Other chronic diseases | 21 | 5.32 |
| COVID-19 | 0 | 0 |
| Vaccination of booster COVID-19 Vaccine | 138 | 34.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, H.; Wang, Y.; Huang, P.; Xu, Y.; Xu, M.; Zhao, Q.; Zhou, Y.; Wang, J.; Ji, M.; et al. Opinion Polls and Antibody Response Dynamics of Vaccination with COVID-19 Booster Vaccines. Vaccines 2022, 10, 647. https://doi.org/10.3390/vaccines10050647
Wu Y, Li H, Wang Y, Huang P, Xu Y, Xu M, Zhao Q, Zhou Y, Wang J, Ji M, et al. Opinion Polls and Antibody Response Dynamics of Vaccination with COVID-19 Booster Vaccines. Vaccines. 2022; 10(5):647. https://doi.org/10.3390/vaccines10050647
Chicago/Turabian StyleWu, Yufei, Huanjie Li, Yangyang Wang, Ping Huang, Yihui Xu, Mingjie Xu, Qianqian Zhao, Yunying Zhou, Jun Wang, Mingyu Ji, and et al. 2022. "Opinion Polls and Antibody Response Dynamics of Vaccination with COVID-19 Booster Vaccines" Vaccines 10, no. 5: 647. https://doi.org/10.3390/vaccines10050647
APA StyleWu, Y., Li, H., Wang, Y., Huang, P., Xu, Y., Xu, M., Zhao, Q., Zhou, Y., Wang, J., Ji, M., & Wang, Y. (2022). Opinion Polls and Antibody Response Dynamics of Vaccination with COVID-19 Booster Vaccines. Vaccines, 10(5), 647. https://doi.org/10.3390/vaccines10050647






