From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review
Abstract
1. Introduction
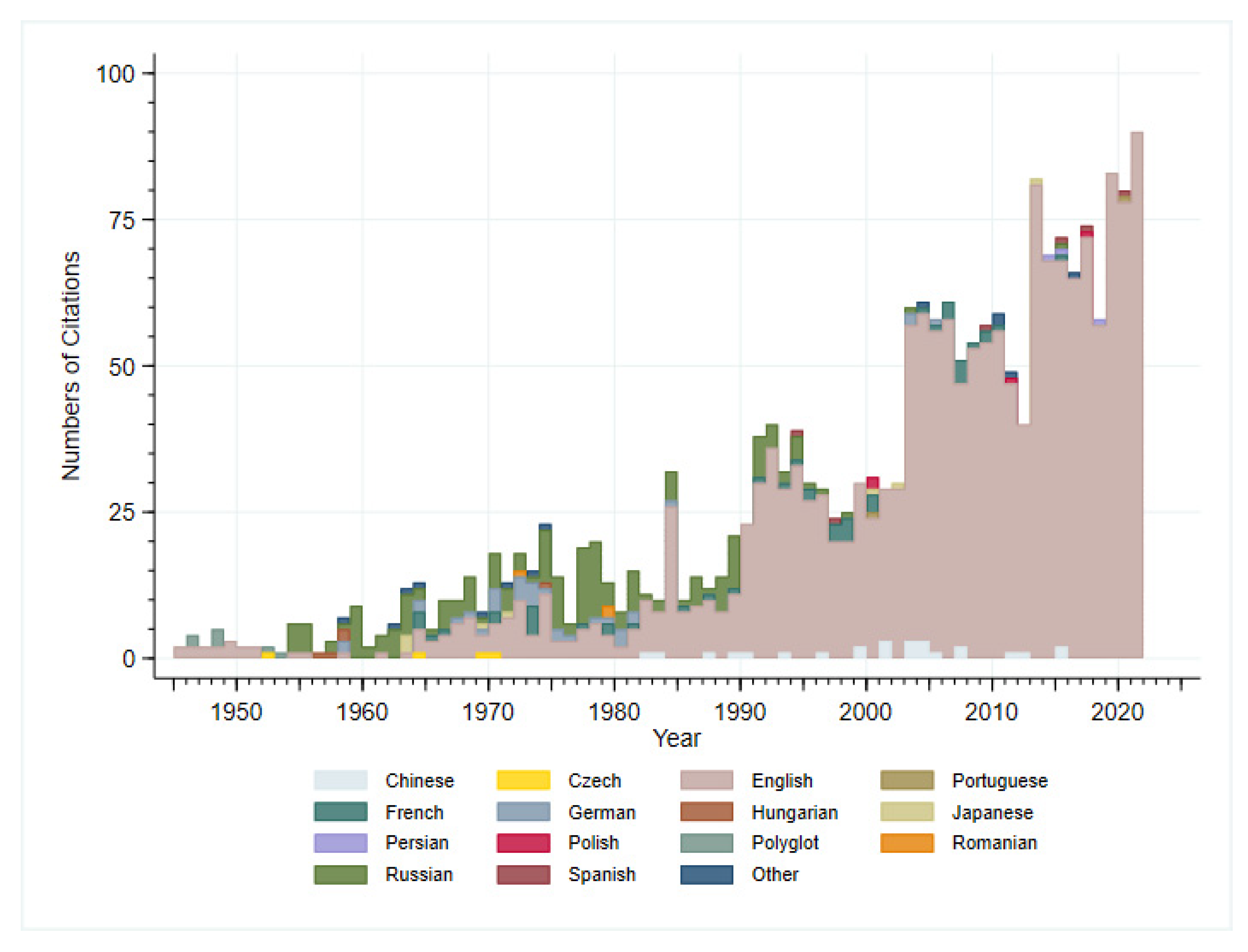
2. Methods
3. Results
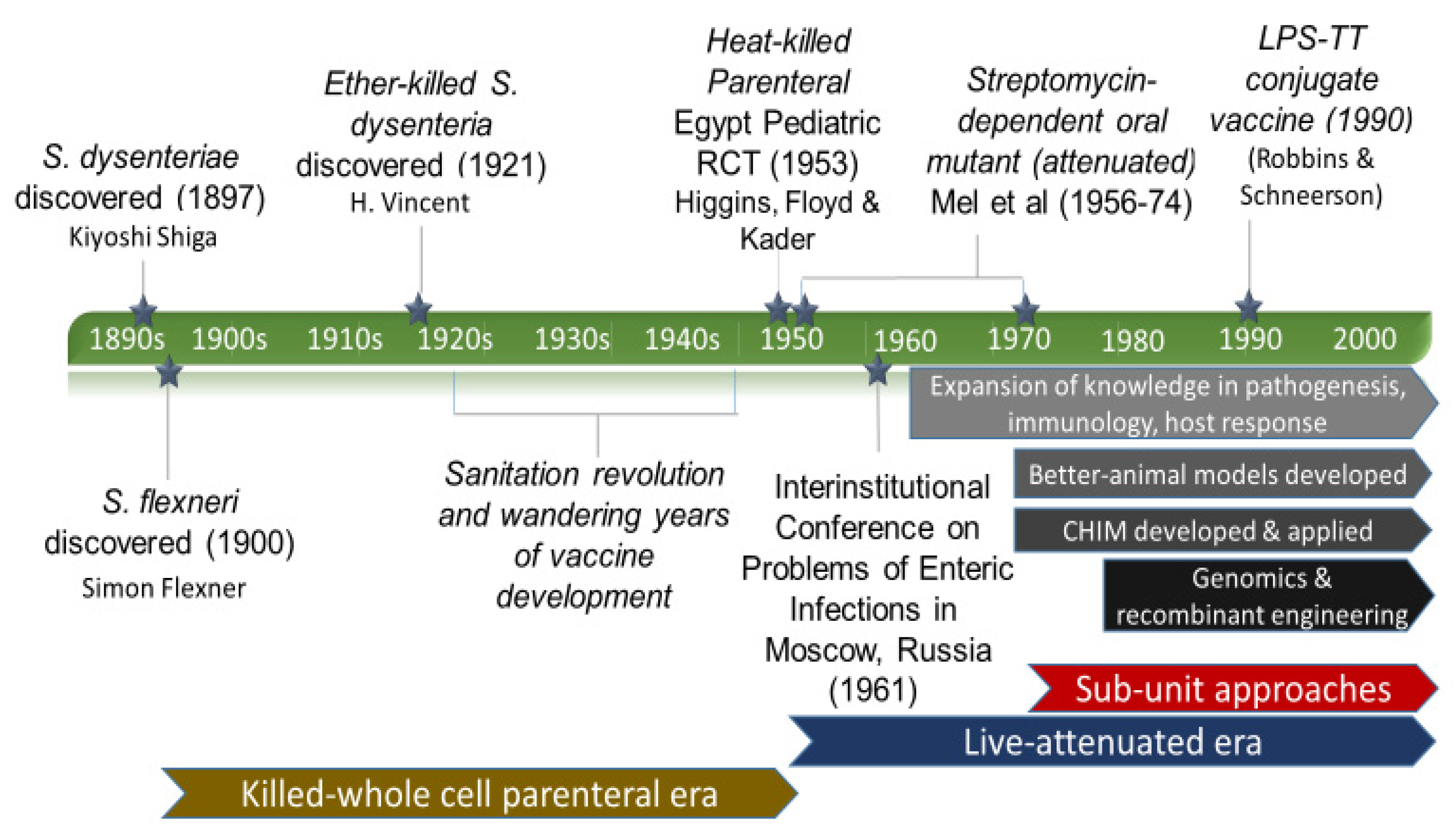
3.1. Discovery and the First Vaccine Attempt
3.2. Eras of Development
3.2.1. The Empirical Vaccine Era
3.2.2. The Wandering Years
3.2.3. Live-Attenuated Vaccines Emerge
3.2.4. On the Origins of the Shigella Controlled Human Infection Model
3.2.5. The Modern Approach Era
3.3. A Glimpse into the Future
4. Conclusions
“The discovery of the dysentery bacillus stirred my young heart with hopes of eradicating the disease. Many thousands still suffer from this disease every year, and the light of hope that once burned so brightly has faded as a dream of a summer night. This sacred fire must not burn out.”
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. Preferred Product Characteristics for Vaccines against Shigella. Available online: https://www.who.int/publications/i/item/who-preferred-product-characteristics-for-vaccines-against-shigella (accessed on 14 January 2022).
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- The College of Physicians of Philadelphia. History of Vaccines—Rotavirus. Available online: https://historyofvaccines.org/content/articles/rotavirus (accessed on 17 April 2022).
- Lampel, K.A.; Formal, S.B.; Maurelli, A.T. A Brief History of Shigella. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Trofa, A.F.; Ueno-Olsen, H.; Oiwa, R.; Yoshikawa, M. Dr. Kiyoshi Shiga: Discoverer of the Dysentery Bacillus. Clin. Infect. Dis. 1999, 29, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef]
- Vincent, H. Vaccination Against Bacillary Dysentery with Ethero-Vaccine. J. State Med. 1921, 29, 54–57. [Google Scholar]
- Gradmann, C.; Harrison, M.; Rasmussen, A. Typhoid and the Military in the Early 20th Century. Clin. Infect. Dis. 2019, 69 (Suppl. S5), S385–S387. [Google Scholar] [CrossRef]
- Eckels, K.H.; Harrison, V.R.; Hetrick, F.M. Chikungunya Virus Vaccine Prepared by Tween-Ether Extraction. Appl. Microbiol. 1970, 19, 321–325. [Google Scholar] [CrossRef]
- Pawar, S.D.; Murtadak, V.B.; Kale, S.D.; Shinde, P.V.; Parkhi, S.S. Evaluation of different inactivation methods for high and low pathogenic avian influenza viruses in egg-fluids for antigen preparation. J. Virol. Methods 2015, 222, 28–33. [Google Scholar] [CrossRef]
- Shiga, K. The Trend of Prevention, Therapy and Epidemiology of Dysentery since the Discovery of Its Causative Organism. N. Engl. J. Med. 1936, 215, 1205–1211. [Google Scholar] [CrossRef]
- Riddle, M.S.; Savarino, S.J.; Sanders, J.W. Gastrointestinal Infections in Deployed Forces in the Middle East Theater: An Historical 60 Year Perspective. Am. J. Trop. Med. Hyg. 2015, 93, 912–917. [Google Scholar] [CrossRef]
- Higgins, A.R.; Floyd, T.M.; Kader, M.A. Studies in shigellosis. III. A controlled evaluation of a monovalent Shigella vaccine in a highly endemic environment. Am. J. Trop. Med. Hyg. 1955, 4, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Mel, D.; Gangarosa, E.J.; Radovanovic, M.L.; Arsic, B.L.; Litvinjenko, S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull. World Health Organ. 1971, 45, 457–464. [Google Scholar] [PubMed]
- Mel, D.M.; Cvjetanović, B.; Felsenfeld, O. Studies on vaccination against bacillary dysentery. 5. Studies in Erythrocebus patas. Bull. World Health Organ. 1970, 43, 431–437. [Google Scholar]
- Freter, R. Experimental Enteric Shigella and Vibrio Infections in Mice and Guinea Pigs. J. Exp. Med. 1956, 104, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Mel, D.M.; Arsić, B.L.; Nikolić, B.D.; Radovanić, M.L. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull. World Health Organ. 1968, 39, 375–380. [Google Scholar] [PubMed]
- Mel, D.M.; Terzin, A.L.; Vuksić, L. Studies on vaccination against bacillary dysentery. 3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull. World Health Organ. 1965, 32, 647–655. [Google Scholar]
- Mel, D.M.; Papo, R.G.; Terzin, A.L.; Vuksić, L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull. World Health Organ. 1965, 32, 637–645. [Google Scholar]
- Mel, D.M.; Terzin, A.L.; Vuksić, L. Studies on vaccination against bacillary dysentery. 1. Immunization of mice against experimental Shigella infection. Bull. World Health Organ. 1965, 32, 633–636. [Google Scholar]
- Cvjetanović, B.; Mel, D.M.; Felsenfeld, O. Study of live typhoid vaccine in chimpanzees. Bull. World Health Organ. 1970, 42, 499–507. [Google Scholar]
- Mel, D.M.; Arsic, B.L.; Radovanovic, M.L.; Litvinjenko, S.A. Live oral Shigella vaccine: Vaccination schedule and the effect of booster dose. Acta Microbiol. Acad. Sci. Hung. 1974, 21, 109–114. [Google Scholar]
- Mel, D.M.; Arsic, B.L.; Radovanovic, M.L.; Kaljalovic, R.; Litvinjenko, S. Safety tests in adults and children with live oral typhoid vaccine. Acta Microbiol. Acad. Sci. Hung. 1974, 21, 161–166. [Google Scholar] [PubMed]
- Mel, D.M. Modern aspects in the prevention and control of cholera. Glas. Srp. Akad. Nauka Umet. Odeljenje Med. Nauka 1981, 33, 105–112. [Google Scholar]
- Herzberg, M.; Elberg, S.S. Immunization against brucella infection. J. Bacteriol. 1955, 69, 432–435. [Google Scholar] [CrossRef]
- Mitchison, D.A. Development of Streptomycin Resistant Strains of Tubercle Bacilli in Pulmonary Tuberculosis: Results of Simultaneous Sensitivity Tests in Liquid and on Solid Media. Thorax 1950, 5, 144–161. [Google Scholar] [CrossRef]
- Foley, G.E.; Shwachman, H. Some observations on a streptomycin-dependent strain of Staphylococcus aureus. J. Gen. Microbiol. 1950, 4, 141–149. [Google Scholar] [CrossRef]
- Levine, M.M.; Kotloff, K.L.; Barry, E.M.; Pasetti, M.F.; Sztein, M.B. Clinical trials of Shigella vaccines: Two steps forward and one step back on a long, hard road. Nat. Rev. Microbiol. 2007, 5, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Church, J.A.; Parker, E.P.; Kosek, M.N.; Kang, G.; Grassly, N.C.; Kelly, P.; Prendergast, A.J. Exploring the relationship between environmental enteric dysfunction and oral vaccine responses. Future Microbiol. 2018, 13, 1055–1070. [Google Scholar] [CrossRef]
- Shaughnessy, H.J. Experimental human bacillary dysentery. J. Am. Med. Assoc. 1946, 132, 362. [Google Scholar] [CrossRef]
- Jamrozik, E.; Selgelid, M.J. History of Human Challenge Studies. In Human Challenge Studies in Endemic Settings; Springer: Berlin/Heidelberg, Germany, 2021; pp. 9–23. [Google Scholar] [CrossRef]
- Levine, M.M.; Kaper, J.B.; Black, R.E.; Clements, M.L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 1983, 47, 510–550. [Google Scholar] [CrossRef]
- Keusch, G.T. The rediscovery of Shiga toxin and its role in clinical disease. Jpn. J. Med. Sci. Biol. 1998, 51 (Suppl. S1), S5–S22. [Google Scholar] [CrossRef]
- Melton-Celsa, A.R. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol. Spectr. 2014, 2, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Youmans, A.S.; Youmans, G.P. Immunogenic Activity of a Ribosomal Fraction Obtained from Mycobacterium tuberculosis. J. Bacteriol. 1965, 89, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Levenson, V.I.; Egorova, T.P.; Belkin, Z.P.; Fedosova, V.G.; Subbotina, J.L.; Rukhadze, E.Z.; Dzhikidze, E.K.; Stassilevich, Z.K. Protective ribosomal preparation from Shigella sonnei as a parenteral candidate vaccine. Infect. Immun. 1991, 59, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.H.; Chang, S.Y.; Park, S.M.; Jang, H.; Carbis, R.; Czerkinsky, C.; Uematsu, S.; Akira, S.; Kweon, M.N. Immunogenicity and protective efficacy offered by a ribosomal-based vaccine from Shigella flexneri 2a. Vaccine 2007, 25, 4828–4836. [Google Scholar] [CrossRef]
- Labrec, E.H.; Schneider, H.; Magnani, T.J.; Formal, S.B. Epithelial cell penetration as an essential step in the pathogenesis of bacillary dysentery. J. Bacteriol. 1964, 88, 1503–1518. [Google Scholar] [CrossRef]
- Falkow, S.; Schneider, H.; Baron, L.S.; Formal, S.B. Virulence of Escherichia-shigella genetic hybrids for the guinea pig. J. Bacteriol. 1963, 86, 1251–1258. [Google Scholar] [CrossRef]
- Kopecko, D.J.; Washington, O.; Formal, S.B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect. Immun. 1980, 29, 207–214. [Google Scholar] [CrossRef]
- Sansonetti, P.J.; Kopecko, D.J.; Formal, S.B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 1982, 35, 852–860. [Google Scholar] [CrossRef]
- Mills, J.A.; Venkatesan, M.M.; Baron, L.S.; Buysse, J.M. Spontaneous insertion of an IS1-like element into the virF gene is responsible for avirulence in opaque colonial variants of Shigella flexneri 2a. Infect. Immun. 1992, 60, 175–182. [Google Scholar] [CrossRef]
- DuPont, H.L.; Hornick, R.B.; Snyder, M.J.; Libonati, J.P.; Formal, S.B.; Gangarosa, E.J. Immunity in Shigellosis. II. Protection Induced by Oral Live Vaccine or Primary Infection. J. Infect. Dis. 1972, 125, 12–16. [Google Scholar] [CrossRef]
- DuPont, H.L.; Hornick, R.B.; Dawkins, A.T.; Snyder, M.J.; Formal, S.B. The Response of Man to Virulent Shigella flexneri 2a. J. Infect. Dis. 1969, 119, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Formal, S.B.; Baron, L.S.; Kopecko, D.J.; Washington, O.; Powell, C.; Life, C.A. Construction of a potential bivalent vaccine strain: Introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect. Immun. 1981, 34, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Formal, S.B.; Hale, T.L.; Kapfer, C.; Cogan, J.P.; Snoy, P.J.; Chung, R.; Wingfield, M.E.; Elisberg, B.L.; Baron, L.S. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect. Immun. 1984, 46, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Newland, J.W.; Hale, T.L.; Formal, S.B. Genotypic and phenotypic characterization of an aroD deletion-attenuated Escherichia coli K12-Shigella flexneri hybrid vaccine expressing S. flexneri 2a somatic antigen. Vaccine 1992, 10, 766–776. [Google Scholar] [CrossRef]
- Coster, T.S.; Hoge, C.W.; VanDeVerg, L.L.; Hartman, A.B.; Oaks, E.V.; Venkatesan, M.M.; Cohen, D.; Robin, G.; Fontaine-Thompson, A.; Sansonetti, P.J.; et al. Vaccination against Shigellosis with Attenuated Shigella flexneri 2a Strain SC602. Infect. Immun. 1999, 67, 3437–3443. [Google Scholar] [CrossRef]
- Rahman, K.M.; El Arifeen, S.; Zaman, K.; Rahman, M.; Raqib, R.; Yunus, M.; Begum, N.; Islam, M.S.; Sohel, B.M.; Rahman, M.; et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine 2011, 29, 1347–1354. [Google Scholar] [CrossRef]
- Simon, J.K.; Wahid, R.; Maciel, M., Jr.; Picking, W.L.; Kotloff, K.L.; Levine, M.M.; Sztein, M.B. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine 2009, 27, 565–572. [Google Scholar] [CrossRef]
- Meitert, T.; Pencu, E.; Ciudin, L.; Tonciu, M. Vaccine strain S. flexneri T32-Istrati. Studies in animals and in volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen (S. flexneri T32-Istrati). Arch. Roum. Pathol. Exp. Microbiol. 1984, 43, 251–278. [Google Scholar]
- Robbins, J.B.; Schneerson, R. Polysaccharide-Protein Conjugates: A New Generation of Vaccines. J. Infect. Dis. 1990, 161, 821–832. [Google Scholar] [CrossRef]
- Avery, O.T.; Goebel, W.F. Chemo-immunological studies on conjugated carbohydrate-proteins. J. Exp. Med. 1929, 50, 533–550. [Google Scholar] [CrossRef]
- Robbins, J.B.; Chu, C.; Watson, D.C.; Szu, S.C.; Daniels, E.M.; Lowe, C.U.; Schneerson, R. O-Specific Side-Chain Toxin-Protein Conjugates as Parenteral Vaccines for the Prevention of Shigellosis and Related Diseases. Clin. Infect. Dis. 1991, 13 (Suppl. S4), S362–S365. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.N.; Trofa, A.C.; Sadoff, J.; Chu, C.; Bryla, D.; Shiloach, J.; Cohen, D.; Ashkenazi, S.; Lerman, Y.; Egan, W. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect. Immun. 1993, 61, 3678–3687. [Google Scholar] [CrossRef]
- Cohen, D.; Ashkenazi, S.; Green, M.; Lerman, Y.; Slepon, R.; Robin, G.; Orr, N.; Taylor, D.N.; Sadoff, J.C.; Chu, C.; et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect. Immun. 1996, 64, 4074–4077. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Passwell, J.H.; Ashkenazi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine 2010, 28, 2231–2235. [Google Scholar] [CrossRef]
- Ravenscroft, N.; Haeuptle, M.A.; Kowarik, M.; Fernandez, F.S.; Carranza, P.; Brunner, A.; Steffen, M.; Wetter, M.; Keller, S.; Ruch, C.; et al. Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli. Glycobiology 2015, 26, 51–62. [Google Scholar] [CrossRef]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. EBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Barel, L.A.; Mulard, L.A. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: From concept to efficacy in human. Hum. Vaccines Immunother. 2019, 15, 1338–1356. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Hartman, A.B.; Oaks, E.V. Isolation and Characterization of a Shigella flexneri Invasin Complex Subunit Vaccine. Infect. Immun. 2000, 68, 6624–6632. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Clarkson, K.A.; Vortherms, A.R.; Oaks, E.V.; Kaminski, R.W. Assembly, Biochemical Characterization, Immunogenicity, Adjuvanticity, and Efficacy of Shigella Artificial Invaplex. mSphere 2018, 3, e00583-17. [Google Scholar] [CrossRef]
- Berlanda Scorza, F.; Colucci, A.M.; Maggiore, L.; Sanzone, S.; Rossi, O.; Ferlenghi, I.; Pesce, I.; Caboni, M.; Norais, N.; Di Cioccio, V.; et al. High Yield Production Process for Shigella Outer Membrane Particles. PLoS ONE 2012, 7, e35616. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, W. Vaccines don’t save lives. Vaccinations save lives. Hum. Vaccines Immunother. 2019, 15, 2786–2789. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Kotloff, K.L.; Nataro, J.P.; Muhsen, K. The Global Enteric Multicenter Study (GEMS): Impetus, Rationale, and Genesis. Clin. Infect. Dis. 2012, 55 (Suppl. S4), S215–S224. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.M.; Chavez, C.B.; Flores, J.T.; Olotegui, M.P.; Pinedo, S.R.; Trigoso, D.R.; Vasquez, A.O.; Ahmed, I.; Alam, D.; Ali, A.; et al. The MAL-ED Study: A Multinational and Multidisciplinary Approach to Understand the Relationship between Enteric Pathogens, Malnutrition, Gut Physiology, Physical Growth, Cognitive Development, and Immune Responses in Infants and Children Up to 2 Years of Age in Resource-Poor Environments. Clin. Infect. Dis. 2014, 59 (Suppl. S4), S193–S206. [Google Scholar] [CrossRef]
- Wilkins, L. Immunization of children against Flexner dysentery. JAMA J. Am. Med. Assoc. 1924, 82, 1599. [Google Scholar] [CrossRef]
- Munro, R. Prophylaxis against dysentery by oral vaccines. Brit. Med. J. 1930, 1, 1001. [Google Scholar]
- Paddle, K. Vaccination against asylum dysentery. JAMA J. Am. Med. Assoc. 1938, 110, 380. [Google Scholar]
- Caldwell, W.A.; Hardwick, S.W. Observations on Flexner dysentery. Br. Med. Bull. 1943, 1, 42. [Google Scholar] [CrossRef][Green Version]
- Johns, E.P.; Chalk, S.G. Prophylactic oral vaccine in bacillary dysentery: A preliminary report. Can. Med. Assoc. J. 1933, 29, 40–43. [Google Scholar]
- Klimentova, A.A. Subcutaneous anavaccine VIEM for dysentery. Am. Rev. Sov. Med. 1945, 3, 135–139. [Google Scholar]
- Hardy, A.V.; Watt, J. Studies of the Acute Diarrheal Diseases: XVIII. Epidemiology. Public Health Rep. 1948, 63, 363. [Google Scholar] [CrossRef] [PubMed]
- Thale, T.; Opper, L. Factors Affecting the Susceptibility to Bacillary Dysentery. Am. J. Public Health Nation’s Health 1946, 36, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, C.M.; Schmitt, J.S.; Chowdhry, E.I.; Riddle, M.S. From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review. Vaccines 2022, 10, 645. https://doi.org/10.3390/vaccines10050645
Herrera CM, Schmitt JS, Chowdhry EI, Riddle MS. From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review. Vaccines. 2022; 10(5):645. https://doi.org/10.3390/vaccines10050645
Chicago/Turabian StyleHerrera, Crystal M., Jessicia S. Schmitt, Erum I. Chowdhry, and Mark S. Riddle. 2022. "From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review" Vaccines 10, no. 5: 645. https://doi.org/10.3390/vaccines10050645
APA StyleHerrera, C. M., Schmitt, J. S., Chowdhry, E. I., & Riddle, M. S. (2022). From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review. Vaccines, 10(5), 645. https://doi.org/10.3390/vaccines10050645





