Efficacy and Effectiveness of the Meningococcal Conjugate Group A Vaccine MenAfriVac® in Preventing Recurrent Meningitis Epidemics in Sub-Saharan Africa
Abstract
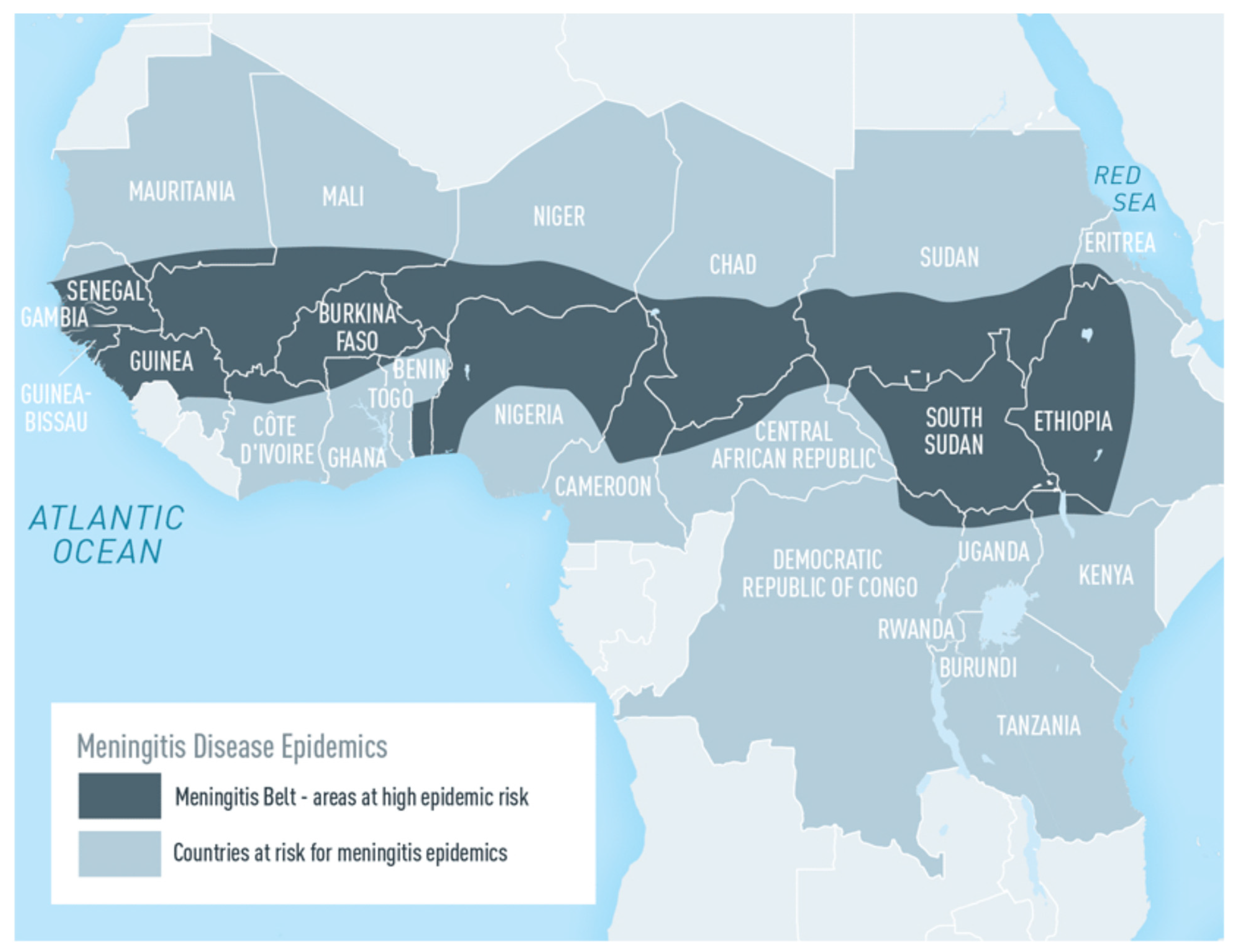
1. Introduction
2. Implementation of the Meningitis Vaccine Project (MVP), the Organization, the Scientific and Technical Partnerships, and Collaboration
3. Technical and Scientific Strategy Pursued to Develop a Vaccine with High Efficacy and Effectiveness for Preventing Meningitis Epidemics Caused by Neisseria Meningitidis Group A
3.1. MenAfriVac® Efficacy
3.2. MenAfriVac® Effectivness
4. Conclusions and Future Direction
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, B. Manson lecture. Meningococcal meningitis in Africa. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 341–353. [Google Scholar] [CrossRef]
- Greenwood, B. Editorial: 100 years of epidemic meningitis in West Africa—has anything changed? Trop. Med. Int. Health 2006, 11, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Lapeyssonie, L. La méningite cérébrospinale en Afrique. Bull. World Health Organ. 1963, 28, 3–114. [Google Scholar]
- World Health Organization. Epidemic meningitis in Africa, 1997. Wkly. Epidemiol. Rec. 1997, 72, 313–315. [Google Scholar]
- Colombini, A.; Bationo, F.; Zongo, S.; Ouattara, F.; Badolo, O.; Jaillard, P.; Seini, E.; Gessner, B.D.; Da Silva, A. Costs for households and community perception of meningitis epidemics in Burkina Faso. Clin. Infect. Dis. 2009, 49, 1520–1525. [Google Scholar] [CrossRef]
- Campagne, G.; Schuchat, A.; Djibo, S.; Ousséini, A.; Cissé, L.; Chippaux, J.P. Epidemiology of bacterial meningitis in Niamey, Niger, 1981–1996. Bull. World Health Organ. 1999, 77, 499–508. [Google Scholar]
- Greenwood, B.M.; Blakebrough, I.S.; Bradley, A.K.; Wali, S.; Whittle, H.C. Meningococcal disease and season in sub-Saharan Africa. Lancet 1984, 1, 1339–1342. [Google Scholar] [CrossRef]
- Gotschlich, E.C.; Goldschneider, I.; Artenstein, M.S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J. Exp. Med. 1969, 129, 1367–1384. [Google Scholar] [CrossRef]
- Wahdan, M.H.; Rizk, F.; el-Akkad, A.M.; el-Ghoroury, A.A.; Hablas, R.; Girgis, N.I.; Amer, A.; Boctar, W.; Sippel, J.E.; Gotschlich, E.C.; et al. A controlled field trial of a serogroup A meningococcal polysaccharide vaccine. Bull World Health Organ. 1973, 48, 667–673. [Google Scholar]
- Erwa, H.H.; Haseeb, M.A.; Idris, A.A.; Lapeyssonnie, L.; Sanborn, W.R.; Sippel, J.E. A serogroup A meningococcal polysaccharide vaccine: Studies in the Sudan to combat cerebrospinal meningitis caused by Neisseria meningitidis group A. Bull. World Health Organ. 1973, 49, 301–305. [Google Scholar]
- Saliou, P.; Stoeckel, P.; Lafaye, A.; Rey, J.L.; Renaudet, J. Controlled tests of anti-meningococcal polysaccharide A vaccine in the African Sahel area (Upper Volta and Mali). Dev. Biol. Stand. 1978, 41, 97–108. (In French) [Google Scholar] [PubMed]
- Control of Epidemic Meningococcal Disease: WHO Practical Guidelines (WHO/ EMC/BAC/98.3), 2nd ed.; World Health Organization: Geneva, Switzerland, 1998.
- Greenwood, B.M.; Hassan-King, M.; Whittle, H.C. Prevention of secondary cases of meningococcal disease in household contacts by vaccination. Br. Med. J. 1978, 1, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Hassan-King, M.K.; Wall, R.A.; Greenwood, B.M. Meningococcal carriage, meningococcal disease and vaccination. J. Infect. 1988, 16, 55–59. [Google Scholar] [CrossRef]
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef]
- Heath, P.T. Haemophilus influenzae type b conjugate vaccines: 249 a review of efficacy data. Pediatr. Infect. Dis. J. Sep. 1998, 17 (Suppl. S9), S117–S122. [Google Scholar] [CrossRef]
- Peltola, H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century. Global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of the conjugates. Clin. Microbiol. Rev. 2000, 13, 302–317. [Google Scholar] [CrossRef]
- Global Programme for Vaccines and Immunization. The WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly. Epidemiol. Rec. 1998, 73, 64–68. [Google Scholar]
- Campagne, G.; Garba, G.; Fabre, P.; Schuchat, A.; Ryall, R.; Boulanger, D.; Bybel, M.; Carlone, G.; Briantais, P.; Ivanoff, B.; et al. Safety and immunogenicity of three doses of a Neisseria meningitidis A+C diphtheria conjugate vaccine in infants from Niger. Pedriatr. Infect. Dis. J. 2000, 19, 144–150. [Google Scholar] [CrossRef][Green Version]
- Twumasi, P.A.; Kumah, S.; Leach, A.; O’Dempsey, T.J.; Ceesay, S.J.; Todd, J.; Broome, C.V.; Carlone, G.M.; Pais, L.B.; Holder, P.K.; et al. A trial of a group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J. Infect. Dis. 1995, 171, 632–638. [Google Scholar] [CrossRef]
- Aguado, M.T.; Jodar, L.; Granoff, D.; Rabinovich, R.; Ceccarini, C.; Perkin, G.W. From Epidemic Meningitis Vaccines for Africa to the Meningitis Vaccine Project. Clin. Infect. Dis. 2015, 61 (Suppl. S5), S391–S395. [Google Scholar] [CrossRef][Green Version]
- Joint Recommendations for the Development and Introduction of Conjugate Vaccines against Meningococcal Disease in the African and Eastern Mediterranean Regions. In World Health Organization Meeting Report; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151686-0.
- Package Insert-Menactra. Available online: https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert---Menactra (accessed on 12 April 2022).
- Jodar, L.; LaForce, F.M.; Ceccarini, C.; Aguado, T.; Granoff, D.M. Meningococcal conjugate vaccine for Africa: A model for development of new vaccines for the poorest countries. Lancet 2003, 361, 1902–1904. [Google Scholar] [CrossRef]
- LaForce, F.M.; Ravenscroft, N.; Djingarey, M.; Viviani, S. Epidemic meningitis due to group A Neisseria meningitidis in the African meningitis belt: A persistent problem with an imminent solution. Vaccine 2009, 27 (Suppl. S2), B13–B19. [Google Scholar] [CrossRef] [PubMed]
- LaForce, F.M.; Konde, K.; Viviani, S.; Préziosi, M.P. The Meningitis Vaccine Project. Vaccine 2007, 25 (Suppl. S1), A97–A100. [Google Scholar] [CrossRef] [PubMed]
- LaForce, F.M.; Djingarey, M.; Viviani, S.; Preziosi, M.P. Lessons from the Meningitis Vaccine Project. Viral Immunol. 2018, 31, 109–113. [Google Scholar] [CrossRef]
- Schlesinger, Y.; Granoff, D.M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA 1992, 267, 1489–1494. [Google Scholar] [CrossRef]
- Frasch, C.E.; Kapre, S.V.; Lee, C.-H.; Préaud, J.M. Technical Development of a New Meningococcal Conjugate Vaccine. Clin. Infect. Dis. 2015, 61 (Suppl. S5), S404–S409. [Google Scholar] [CrossRef][Green Version]
- Richmond, P.; Borrow, R.; Miller, E.; Clark, S.; Sadler, F.; Fox, A.; Begg, N.; Morris, R.; Cartwright, K. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis. 1999, 179, 1569–1572. [Google Scholar] [CrossRef]
- Maclennan, J.; Obaro, S.; Deeks, J.; Lake, D.; Elie, C.; Carlone, G.; Moxon, E.R.; Greenwood, B. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J. Infect. Dis. 2001, 183, 97–104. [Google Scholar] [CrossRef]
- Richmond, P.; Borrow, R.; Goldblatt, D.; Findlow, J.; Martin, S.; Morris, R.; Cartwright, K.; Miller, E. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 2001, 183, 160–163. [Google Scholar] [CrossRef]
- Miller, E.; Salisbury, D.; Ramsay, M. Planning, registration, and implementation of an immunization campaign against meningococcal serogroup C disease in the UK: A success story. Vaccine 2001, 20 (Suppl. S1), S58–S67. [Google Scholar] [CrossRef]
- Goldschneider, I.; Gotschlich, E.C.; Artenstein, M.S. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1969, 129, 1307–1326. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, M.E.; Andrews, N.; Kaczmarski, E.B.; Miller, E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 2001, 357, 195–196. [Google Scholar] [CrossRef]
- Maiden, M.C.; Stuart, J.M. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 2002, 359, 1829–1831. [Google Scholar] [CrossRef]
- Ramsay, M.E.; Andrews, N.J.; Trotter, C.L.; Kaczmarski, E.B.; Miller, E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: Database analysis. BMJ 2003, 326, 365–366. [Google Scholar] [CrossRef]
- Trotter, C.L.; Andrews, N.J.; Kaczmarski, E.B.; Miller, E.; Ramsay, M.E. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004, 364, 365–367. [Google Scholar] [CrossRef]
- Borrow, R.; Andrews, N.; Goldblatt, D.; Miller, E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: A re-evaluation of correlates of protection. Infect. Immun. 2001, 69, 1568–1573. [Google Scholar] [CrossRef]
- Andrews, N.; Borrow, R.; Miller, E. Validation of serological correlate of protection for meningococcal C conjugate vaccine using efficacy estimates from post-licensure surveillance in England. Clin. Diagn. Lab. Immunol. 2003, 10, 780–786. [Google Scholar]
- Kshirsagar, N.; Mur, N.; Thatte, U.; Gogtay, N.; Viviani, S.; Préziosi, M.P.; Elie, C.; Findlow, H.; Carlone, G.; Borrow, R. Safety, immunogenicity, and antibody persistence of a new meningococcal group A conjugate vaccine in healthy Indian adults. Vaccine 2007, 25 (Suppl. S1), A101–A107. [Google Scholar] [CrossRef]
- Sow, S.O.; Okoko, B.J.; Diallo, A.; Viviani, S.; Borrow, R.; Carlone, G.; Tapia, M.; Akinsola, A.K.; Arduin, P.; Findlow, H.; et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N. Engl. J. Med. 2011, 364, 2293–2304. [Google Scholar] [CrossRef]
- Vann, W.F.; Liu, T.Y.; Robbins, J.B. Bacillus pumilus polysaccharide cross-reactive with meningococcal group A polysaccharide. Infect. Immun. 1976, 13, 1654–1662. [Google Scholar] [CrossRef]
- Guirguis, N.; Schneerson, R.; Bax, A.; Egan, W.; Robbins, J.B.; Shiloach, J.; Orskov, I.; Orskov, F.; el Kholy, A. Escherichia coli K51 and K93 capsular polysaccharides are cross reactive with the group A capsular polysaccharide of Neisseria meningitidis. Immunochemical, biological, and epidemiological studies. J. Exp. Med. 1985, 162, 1837–1851. [Google Scholar] [CrossRef]
- World Health Organization. Group A and C meningococcal vaccines, WHO position paper. Wkly Epidemiol Rec 1999, 74, 297–304. [Google Scholar]
- Meningococcal vaccines: WHO position paper-November 2011. Wkly. Epidemiol. Rec. 2011, 86, 521–540.
- Tapia, M.D.; Sow, S.O.; Haidara, F.C.; Diallo, F.; Doumbia, M.; Enwere, G.C.; Paranjape, G.; Hervé, J.; Bouma, E.; Parulekar, V.; et al. A Phase 3, Double-Blind, Randomized, Active Controlled Study to Evaluate the Safety of MenAfriVac in Healthy Malians. Clin. Infect. Dis. 2015, 61 (Suppl. S5), S507–S513. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Meningococcal A conjugate vaccine: Updated guidance, February 2015. Wkly. Epidemiol. Rec. 2015, 90, 57–62. [Google Scholar]
- Djingarey, M.H.; Noazin, S.; Preziosi, M.P.; Tiendrebeogo, S.; Toure, K.; Kairo, K.K.; Perea, W.; Bertherat, E.; Kandolo, D.; Konde, K.; et al. A twenty year retrospective analysis of meningitis surveillance data from Burkina Faso, Mali and Niger. In Proceedings of the Abstract P166, 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands, 7–12 September 2008; pp. 232–233. [Google Scholar]
- Lingani, C.; Bergeron-Caron, C.; Stuart, J.M.; Fernandez, K.; Djingarey, M.H.; Ronveaux, O.; Schnitzler, J.C.; Perea, W.A. Meningococcal Meningitis Surveillance in the African Meningitis Belt, 2004–2013. Clin. Infect. Dis. 2015, 61 (Suppl. S5), S410–S415. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Evaluation of meningitis surveillance before introduction of serogroup A meningococcal conjugate vaccine-Burkina Faso and Mali. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 1025–1028. [Google Scholar]
- WHO. Meningococcal disease, African meningitis belt. WHO Wkly. Epidemiol. Rec. 2009, 84, 117–118. [Google Scholar]
- Cibrelus, L.; Lingani, C.; Fernandez, K.; Djingarey, M.H.; Perea, W.A.; Hugonnet, S. Risk assessment and meningococcal a conjugate vaccine introduction in africa: The district prioritization tool. Clin. Infect. Dis. 2015, 61 (Suppl. S5), S442–S450. [Google Scholar] [CrossRef]
- Djingarey, M.H.; Diomandé, F.V.; Barry, R.; Kandolo, D.; Shirehwa, F.; Lingani, C.; Novak, R.T.; Tevi-Benissan, C.; Perea, W.; Preziosi, M.P.; et al. Introduction and Rollout of a New Group A Meningococcal Conjugate Vaccine (PsA-TT) in African Meningitis Belt Countries, 2010–2014. Clin. Infect. Dis. 2015, 61 (Suppl. S5), S434–S441. [Google Scholar] [CrossRef]
- Bwaka, A.; Bita, A.; Lingani, C.; Durupt, A.; Mwenda, J.M.; Mihigo, R.; Djingarey, M.H.; Ronveaux, O.; Preziosi, M.P. Status of the Rollout of the Meningococcal Serogroup A Conjugate Vaccine in African Meningitis Belt Countries in 2018. J. Infect. Dis. 2019, 220 (Suppl. S4), S140–S147. [Google Scholar] [CrossRef] [PubMed]
- Novak, R.T.; Kambou, J.L.; Diomande, F.V.; Tarbangdo, T.F.; Ouédraogo-Traoré, R.; Sangaré, L.; Lingani, C.; Martin, S.W.; Hatcher, C.; Mayer, L.W.; et al. Serogroup A meningococcal conjugate vaccination in Burkina Faso: Analysis of national surveillance data. Lancet Infect. Dis. 2012, 12, 757–764. [Google Scholar] [CrossRef]
- Daugla, D.; Gami, J.; Gamougam, K.; Naibei, N.; Mbainadji, L.; Narbé, M.; Toralta, J.; Kodbesse, B.; Ngadoua, C.; Coldiron, M.E.; et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: A community trial. Lancet 2014, 383, 40–47. [Google Scholar] [CrossRef]
- Trotter, C.L.; Lingani, C.; Fernandez, K.; Cooper, L.V.; Bita, A.; Tevi-Benissan, C.; Ronveaux, O.; Préziosi, M.P.; Stuart, J.M. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–2015: An analysis of surveillance data. Lancet Infect. Dis. 2017, 17, 867–872. [Google Scholar] [CrossRef]
- Soeters, H.M.; Diallo, A.O.; Bicaba, B.W.; Kadadé, G.; Dembélé, A.Y.; Acyl, M.A.; Nikiema, C.; Sadji, A.Y.; Poy, A.N.; Lingani, C.; et al. MenAfriNet Consortium. Bacterial meningitis epidemiology in 5 countries in the meningitis belt of sub-Saharan Africa, 2015–2017. J. Infect. Dis. 2019, 220 (Suppl. S4), S165–S174. [Google Scholar] [CrossRef]
- Diallo, A.O.; Soeters, H.M.; Yameogo, I.; Sawadogo, G.; Aké, F.; Lingani, C.; Wang, X.; Bita, A.; Fall, A.; Sangaré, L.; et al. MenAfriNet Consortium. Bacterial meningitis epidemiology and return of Neisseria meningitidis serogroup A cases in Burkina Faso in the five years following MenAfriVac mass vaccination campaign. PLoS ONE 2017, 12, e0187466. [Google Scholar] [CrossRef]
- Kristiansen, P.A.; Diomandé, F.; Ba, A.K.; Sanou, I.; Ouédraogo, A.S.; Ouédraogo, R.; Sangaré, L.; Kandolo, D.; Aké, F.; Saga, I.M.; et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin. Infect. Dis. 2013, 56, 354–363. [Google Scholar] [CrossRef]
- Mbaeyi, S.; Sampo, E.; Dinanibè, K.; Yaméogo, I.; Congo-Ouédraogo, M.; Tamboura, M.; Sawadogo, G.; Ouattara, K.; Sanou, M.; Kiemtoré, T.; et al. Meningococcal carriage 7 years after introduction of a serogroup A meningococcal conjugate vaccine in Burkina Faso: Results from four cross-sectional carriage surveys. Lancet Infect. Dis. 2020, 20, 1418–1425. [Google Scholar] [CrossRef]
- Fernandez, K.; Lingani, C.; Aderinola, O.M.; Goumbi, K.; Bicaba, B.; Edea, Z.A.; Glèlè, C.; Sarkodie, B.; Tamekloe, A.; Ngomba, A.; et al. Meningococcal meningitis outbreaks in the African meningitis belt after meningococcal serogroup A conjugate vaccine introduction, 2011–2017. J. Infect. Dis. 2019, 220 (Suppl. S4), S225–S232. [Google Scholar] [CrossRef]
- WHO. Weekly epidemiological record 3 APRIL 2020, 95th YEAR Nos. 14/15. Wkly. Epidemiol. Rec. 2020, 95, 133–144. [Google Scholar]
- Meningitis. WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/meningitis (accessed on 22 March 2022).
- Bolgiano, B.; Moran, E.; Beresford, N.J.; Gao, F.; Care, R.; Desai, T.; Nordgren, I.K.; Rudd, T.R.; Feavers, I.M.; Bore, P.; et al. Evaluation of Critical Quality Attributes of a Pentavalent (A, C, Y, W, X) Meningococcal Conjugate Vaccine for Global Use. Pathogens 2021, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.D.; Sow, S.O.; Naficy, A.; Diallo, F.; Haidara, F.C.; Chaudhari, A.; Martellet, L.; Traore, A.; Townsend-Payne, K.; Borrow, R.; et al. Meningococcal Serogroup ACWYX Conjugate Vaccine in Malian Toddlers. N. Engl. J. Med. 2021, 384, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Alderson, M.R.; LaForce, M.F.; Sobanjo-ter Meulen, A.; Hwang, A.; Preziosi, M.P.; Klugman, K.P. Eliminating meningococcal epidemics from the African meningitis belt: The case for advanced prevention and control using next generation meningococcal conjugate vaccines. J. Infect. Dis. 2019, 220 (Suppl. S4), S274–S278. [Google Scholar] [CrossRef] [PubMed]
- Shermana, A.C.; Stephens, D.S. Serogroup a meningococcal conjugate vaccines: Building sustainable and equitable vaccine strategies. Expert Rev. Vaccines 2020, 19, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.M.; Harrison, L.H. Vaccine prevention of meningococcal disease in Africa: Major advances, remaining challenges. Hum. Vaccines Immunother. 2018, 14, 1107–1115. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viviani, S. Efficacy and Effectiveness of the Meningococcal Conjugate Group A Vaccine MenAfriVac® in Preventing Recurrent Meningitis Epidemics in Sub-Saharan Africa. Vaccines 2022, 10, 617. https://doi.org/10.3390/vaccines10040617
Viviani S. Efficacy and Effectiveness of the Meningococcal Conjugate Group A Vaccine MenAfriVac® in Preventing Recurrent Meningitis Epidemics in Sub-Saharan Africa. Vaccines. 2022; 10(4):617. https://doi.org/10.3390/vaccines10040617
Chicago/Turabian StyleViviani, Simonetta. 2022. "Efficacy and Effectiveness of the Meningococcal Conjugate Group A Vaccine MenAfriVac® in Preventing Recurrent Meningitis Epidemics in Sub-Saharan Africa" Vaccines 10, no. 4: 617. https://doi.org/10.3390/vaccines10040617
APA StyleViviani, S. (2022). Efficacy and Effectiveness of the Meningococcal Conjugate Group A Vaccine MenAfriVac® in Preventing Recurrent Meningitis Epidemics in Sub-Saharan Africa. Vaccines, 10(4), 617. https://doi.org/10.3390/vaccines10040617





