Prolonged Protective Immunity Induced by Mild SARS-CoV-2 Infection of K18-hACE2 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Animal Experiments
2.3. ELISA
2.4. ELISpot Assays
2.5. Determination of the Viral Load in Organs
2.6. Plaque Reduction Neutralization Test
2.7. Quantitative Real-Time RT-PCR
2.8. Statistical Analysis
3. Results
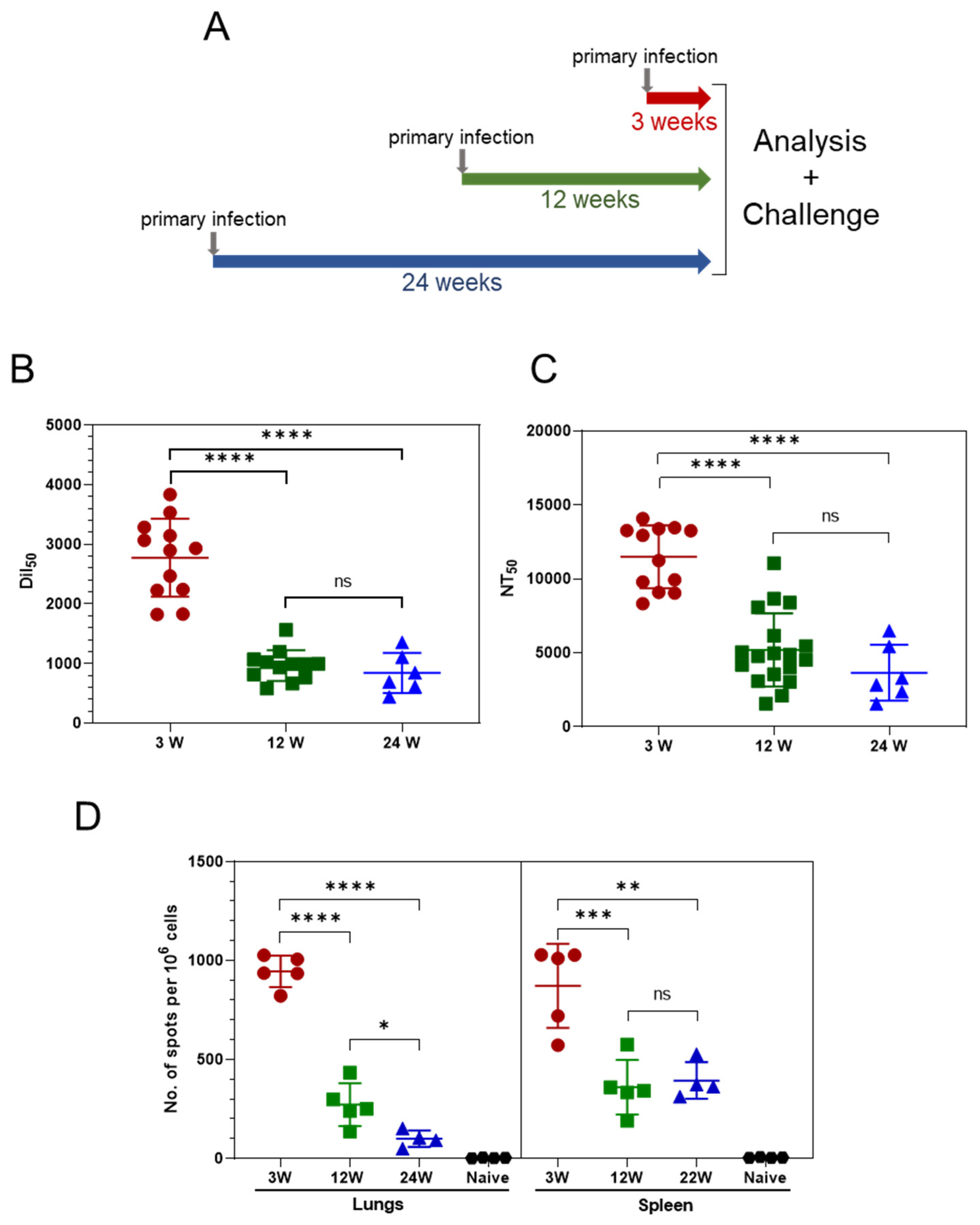
3.1. Persistence of the Immune Response Post Asymptomatic SARS-CoV-2 Infection
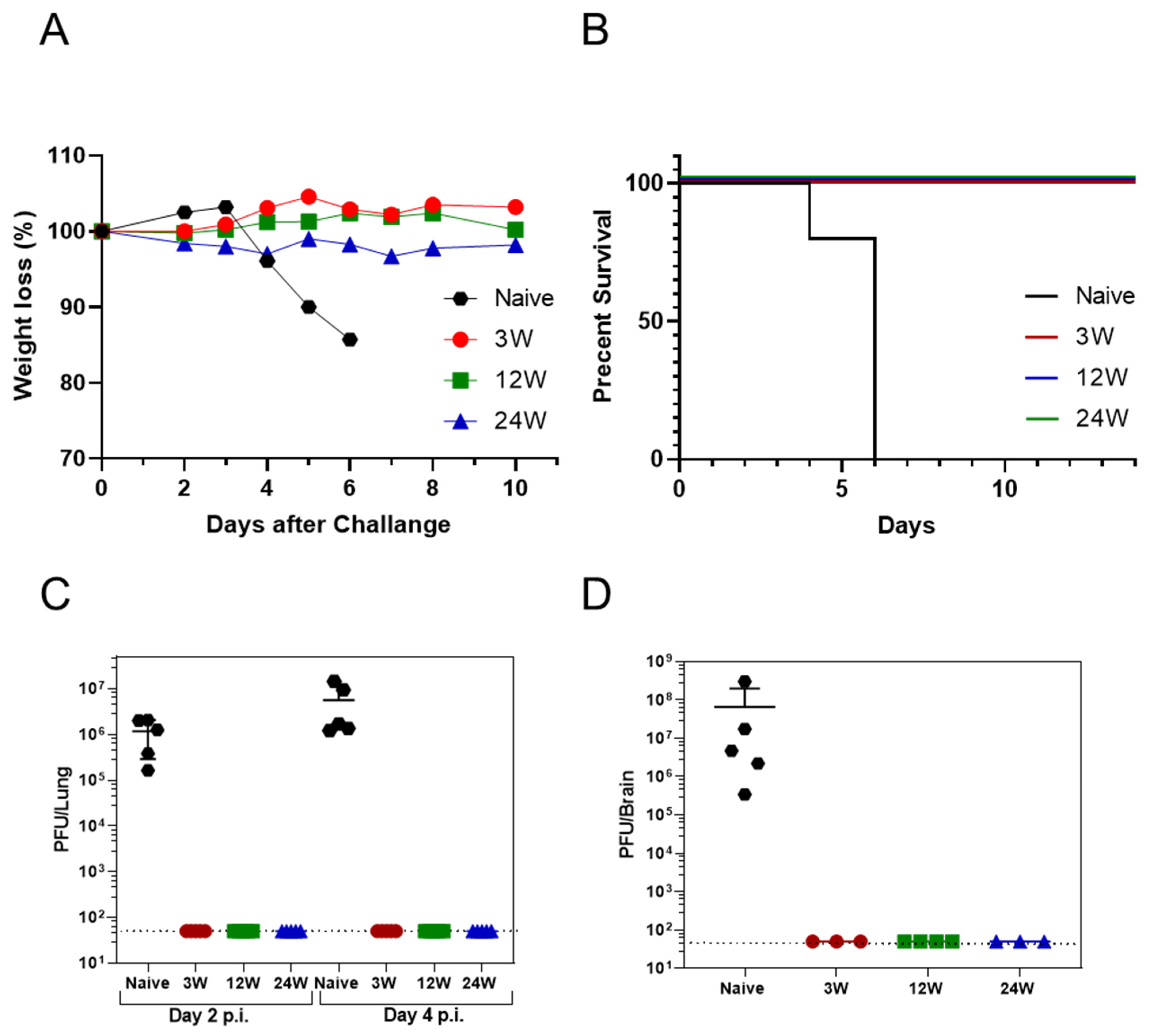
3.2. Primary SARS-CoV-2 Infection Protects K18-hACE2 Mice against Reinfection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamayoshi, S.; Yasuhara, A.; Ito, M.; Akasaka, O.; Nakamura, M.; Nakachi, I.; Koga, M.; Mitamura, K.; Yagi, K.; Maeda, K.; et al. Antibody titers against SARS-CoV-2 decline, but do not disappear for several months. EClinicalMedicine 2021, 32, 100734. [Google Scholar] [CrossRef] [PubMed]
- Grau-Expósito, J.; Sánchez-Gaona, N.; Massana, N.; Suppi, M.; Astorga-Gamaza, A.; Perea, D.; Rosado, J.; Falco, A.; Kirkegaard, C.; Torrella, A.; et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021, 12, 3010. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 2021, 184, 169–183. [Google Scholar] [CrossRef]
- Long, Q.X.; Jia, Y.J.; Wang, X.; Deng, H.J.; Cao, X.X.; Yuan, J.; Fang, L.; Cheng, X.-R.; Luo, C.; He, A.-R.; et al. Immune memory in convalescent patients with asymptomatic or mild COVID-19. Cell Discov. 2021, 7, 18. [Google Scholar] [CrossRef]
- Havervall, S.; Jernbom Falk, A.; Klingström, J.; Ng, H.; Greilert-Norin, N.; Gabrielsson, L.; Salomonsson, A.-C.; Isaksson, E.; Rudberg, A.-S.; Hellstrom, C.; et al. SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. PLoS ONE 2022, 17, e0262169. [Google Scholar] [CrossRef]
- Yinda, C.K.; Port, J.R.; Bushmaker, T.; Owusu, I.O.; Purushotham, J.N.; Avanzato, V.A.; Fischer, R.J.; Schulz, J.E.; Holbrook, M.G.; Hebner, M.J.; et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021, 17, e1009195. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Moreau, G.B.; Burgess, S.L.; Sturek, J.M.; Donlan, A.N.; Petri, W.A., Jr.; Mann, B.J. Evaluation of K18-hACE2 mice as a model of SARS-CoV-2 infection. Am. J. Trop. Med. Hyg. 2020, 103, 1215. [Google Scholar] [CrossRef]
- Dong, W.; Mead, H.; Tian, L.; Park, J.-G.; Garcia, J.I.; Jaramillo, S.; Barr, T.; Kollath, D.S.; Coyne, V.K.; Stone, N.E.; et al. The K18-hACE2 Transgenic Mouse Model Recapitulates Non-Severe and Severe COVID-19 in Response to Infectious Dose of SARS-CoV-2 Virus. J. Virol. 2021, 96, e00964-21. [Google Scholar]
- Rosenfeld, R.; Noy-Porat, T.; Mechaly, A.; Makdasi, E.; Levy, Y.; Alcalay, R.; Falach, R.; Aftalion, M.; Epstein, E.; Gur, D.; et al. Post-exposure protection of SARS-CoV-2 lethal infected K18-hACE2 transgenic mice by neutralizing human monoclonal antibody. Nat. Commun. 2021, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.M.; Jessop, F.; Wehrly, T.D.; Bosio, C.M. Cutting Edge: Lung-Resident T Cells Elicited by SARS-CoV-2 Do Not Mediate Protection against Secondary Infection. J. Immunol. 2021, 207, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Israelow, B.; Mao, T.; Klein, J.; Song, E.; Menasche, B.; Omer, S.B.; Iwasaki, A. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci. Immunol. 2021, 6, 4509. [Google Scholar] [CrossRef] [PubMed]
- Achdout, H.; Vitner, E.B.; Politi, B.; Melamed, S.; Yahalom-Ronen, Y.; Tamir, H.; Erez, N.; Avraham, R.; Weiss, S.; Cherry, L.; et al. Increased lethality in Influenza and SARS-CoV-2 co-infection is prevented by influenza immunity but not SARS-CoV-2 immunity. Nat. Commun. 2021, 12, 5819. [Google Scholar] [CrossRef] [PubMed]
- Falach, R.; Bar-On, L.; Lazar, S.; Kadar, T.; Mazor, O.; Aftalion, M.; Gur, D.; Evgy, Y.; Shifman, O.; Aminov, T.; et al. Mice with induced pulmonary morbidities display severe lung inflammation and mortality following exposure to SARS-CoV-2. JCI Insight 2021, 6, e145916. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K. SARS-CoV-2: A new song recalls an old melody. Cell Host Microbe 2020, 27, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Van Braeckel-Budimir, N.; Abboud, G.; Varga, S.M.; Salek-Ardakani, S.; Harty, J.T. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2017, 2, eaag2031. [Google Scholar] [CrossRef] [Green Version]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.; Ajithdoss, D.; Copin, R.; Zhou, A.; Lanza, K.; Negron, N.; Ni, M.; Wei, Y.; Mohammadi, K.; Musser, B.; et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020, 370, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Messer, R.J.; Dittmer, U.; Peterson, K.E.; Hasenkrug, K.J. Essential role for virus-neutralizing antibodies in sterilizing immunity against Friend retrovirus infection. Proc. Nat. Acad. Sci. USA 2004, 101, 12260–12265. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bar-On, L.; Aftalion, M.; Makdasi, E.; Gur, D.; Alcalay, R.; Cohen, H.; Beth-Din, A.; Rosenfeld, R.; Achdout, H.; Bar-Haim, E.; et al. Prolonged Protective Immunity Induced by Mild SARS-CoV-2 Infection of K18-hACE2 Mice. Vaccines 2022, 10, 613. https://doi.org/10.3390/vaccines10040613
Bar-On L, Aftalion M, Makdasi E, Gur D, Alcalay R, Cohen H, Beth-Din A, Rosenfeld R, Achdout H, Bar-Haim E, et al. Prolonged Protective Immunity Induced by Mild SARS-CoV-2 Infection of K18-hACE2 Mice. Vaccines. 2022; 10(4):613. https://doi.org/10.3390/vaccines10040613
Chicago/Turabian StyleBar-On, Liat, Moshe Aftalion, Efi Makdasi, David Gur, Ron Alcalay, Hila Cohen, Adi Beth-Din, Ronit Rosenfeld, Hagit Achdout, Erez Bar-Haim, and et al. 2022. "Prolonged Protective Immunity Induced by Mild SARS-CoV-2 Infection of K18-hACE2 Mice" Vaccines 10, no. 4: 613. https://doi.org/10.3390/vaccines10040613
APA StyleBar-On, L., Aftalion, M., Makdasi, E., Gur, D., Alcalay, R., Cohen, H., Beth-Din, A., Rosenfeld, R., Achdout, H., Bar-Haim, E., Falach, R., Chitlaru, T., & Cohen, O. (2022). Prolonged Protective Immunity Induced by Mild SARS-CoV-2 Infection of K18-hACE2 Mice. Vaccines, 10(4), 613. https://doi.org/10.3390/vaccines10040613





