Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change
Abstract
1. Introduction
2. The Immune Response Assuring an Effective Vaccine Response
3. What Are the Changes That Are Commonly Considered to Alter the Vaccine Response with Ageing?
4. New Evidence from Experimental Data on Vaccine Response in Old Age
5. How Does the ageing Immune System Respond to Various Existing Vaccines and How Do the Vaccine Modifications Improve the Response?
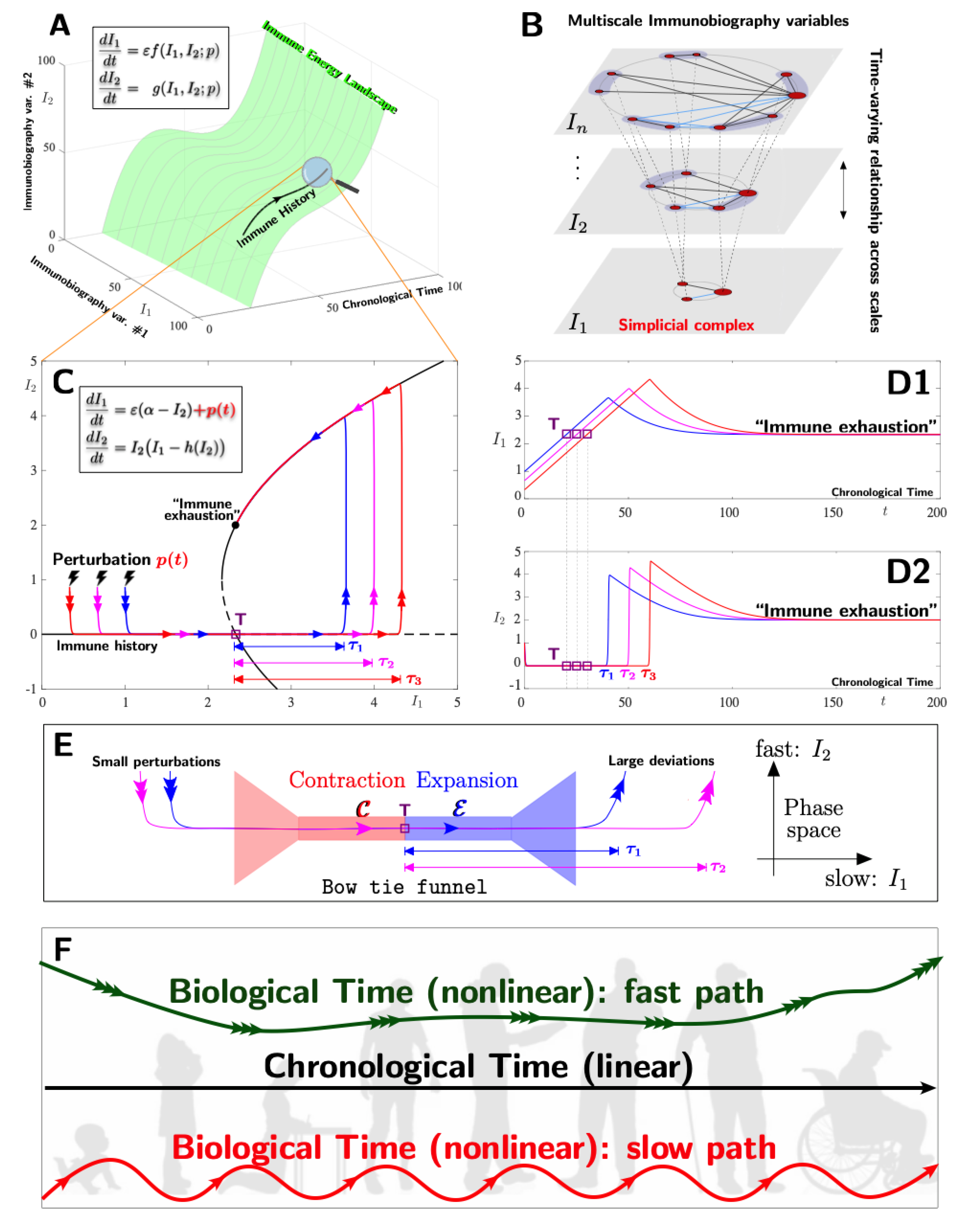
6. Perspective on Mathematical Modelling, Illustrating the Role of Immunobiography in Vaccine Efficiency
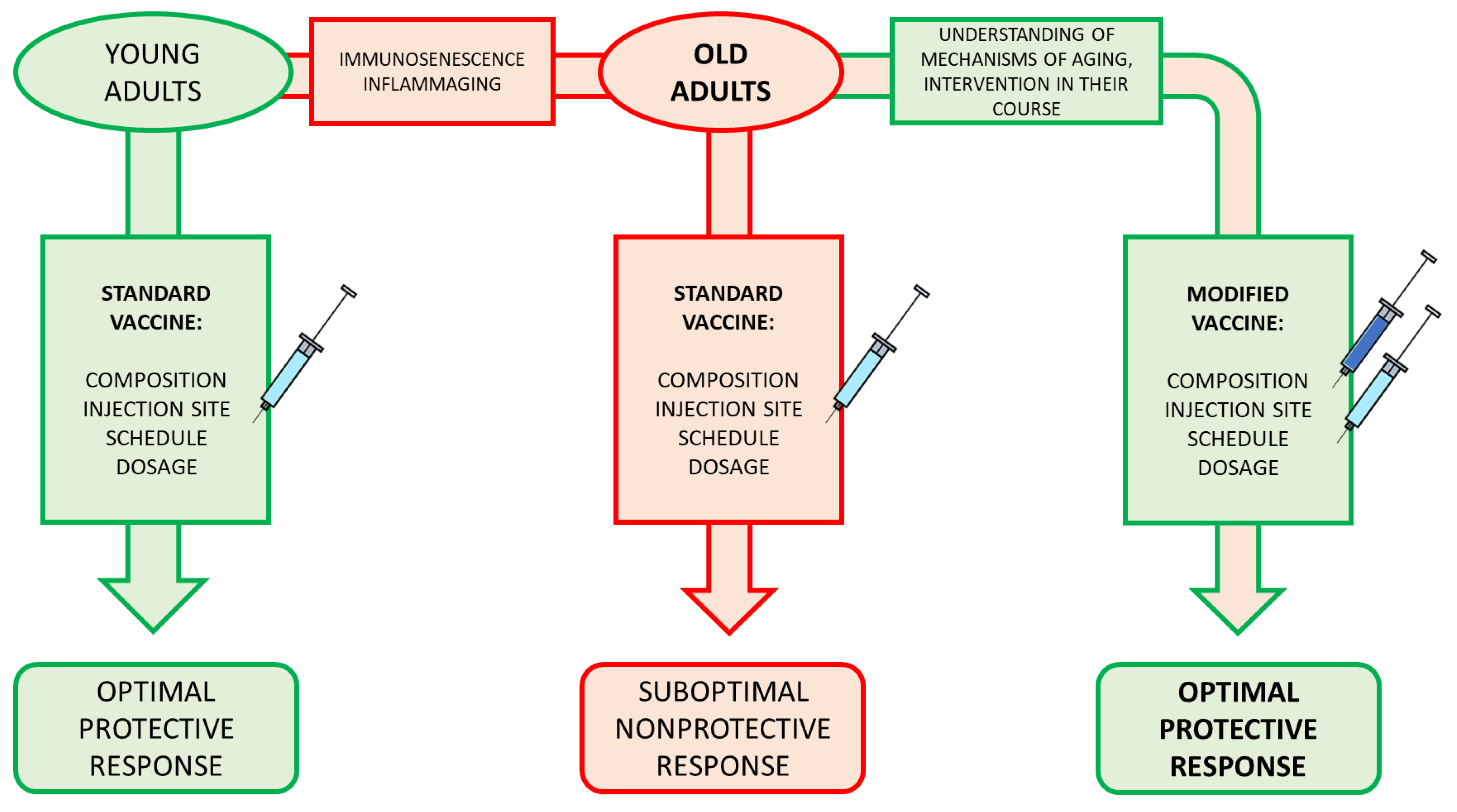
7. What Is the Future?
8. Conclusions and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Weinberger, B. Vaccines to Prevent Infectious Diseases in the Older Population: Immunological Challenges and Future Perspectives. Front. Immunol. 2020, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; McIntyre, P.; Subbarao, K.; Booy, R.; Levin, M.J. Vaccines for older adults. BMJ 2021, 372, n188. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B. Vaccination of older adults: Influenza, pneumococcal disease, herpes zoster, COVID-19 and beyond. Immun. Ageing 2021, 18, 38. [Google Scholar] [CrossRef]
- Mallapaty, S. The coronavirus is most deadly if you are older and male—New data reveal the risks. Nature 2020, 585, 16–17. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Akbar, A.N.; Gilroy, D.W. Aging immunity may exacerbate COVID-19. Science 2020, 369, 256–257. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S.J. Age-related susceptibility to coronavirus infections: Role of impaired and dysregulated host immunity. Clin. Invest. 2020, 130, 6204–6213. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Jangravi, Z.; Sahraei, H.; Bahari, Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflamm. Res. 2020, 69, 825–839. [Google Scholar] [CrossRef]
- Deans, G.D.; Stiver, H.G.; McElhaney, J.E. Influenza vaccines provide diminished protection but are cost-saving in older adults. J. Intern. Med. 2010, 267, 220–227. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Effros, R.B. Immunosenescence: What does it mean to health outcomes in older adults? Curr. Opin. Immunol. 2009, 21, 418–424. [Google Scholar] [CrossRef]
- Jackson, L.A.; Neuzil, K.M.; Yu, O.; Benson, P.; Barlow, W.E.; Adams, A.L.; Hanson, C.A.; Mahoney, L.D.; Shay, D.K.; Thompson, W.W. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N. Engl. J. Med. 2003, 348, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Di Benedetto, S.; Pawelec, G. The Immune System and Its Dysregulation with Aging. Subcell Biochem. 2019, 91, 21–43. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; De Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front. Immunol. 2017, 8, 982. [Google Scholar] [CrossRef]
- Andrew, M.K.; Shinde, V.; Ye, L.; Hatchette, T.; Haguinet, F.; Dos Santos, G.; E McElhaney, J.; Ambrose, A.; Boivin, G.; Bowie, W.; et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J. Infect. Dis. 2017, 216, 405–414. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Zhou, X.; Talbot, H.K.; Soethout, E.; Bleackley, R.C.; Granville, D.J.; Pawelec, G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012, 30, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Canouï, E.; Launay, O. History and principles of vaccination. Rev. Mal. Respir. 2019, 36, 74–81. [Google Scholar] [CrossRef]
- Zepp, F. Principles of Vaccination. Methods Mol. Biol. 2016, 1403, 57–84. [Google Scholar] [CrossRef]
- Cakala-Jakimowicz, M.; Kolodziej-Wojnar, P.; Puzianowska-Kuznicka, M. Aging-Related Cellular, Structural and Functional Changes in the Lymph Nodes: A Significant Component of Immunosenescence? An Overview. Cells 2021, 10, 3148. [Google Scholar] [CrossRef]
- Moser, M.; Leo, O. Key concepts in immunology. Vaccine 2010, 28 (Suppl. 3), C2–C13. [Google Scholar] [CrossRef]
- Lanzavecchia, A.; Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 2001, 106, 263–266. [Google Scholar] [CrossRef]
- Mempel, T.R.; Henrickson, S.E.; Von Adrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 2004, 427, 154–159. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Medzhitov, R.; Shaw, A.C. Triggering TLR signaling in vaccination. Trends Immunol. 2006, 27, 49–55. [Google Scholar] [CrossRef]
- Pulendran, B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004, 199, 227–250. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef]
- Lanzavecchia, A.; Sallusto, F. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 2005, 17, 326–332. [Google Scholar] [CrossRef]
- Jelley-Gibbs, D.M.; Strutt, T.M.; McKinstry, K.K.; Swain, S.L. Influencing the fates of CD4 T cells on the path to memory: Lessons from influenza. Immunol. Cell Biol. 2008, 86, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Paul, W.E. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol. Rev. 2013, 252, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Nakayamada, S.; Takahashi, H.; Kanno, Y.; O’Shea, J.J. Helper T cell diversity and plasticity. Curr. Opin. Immunol. 2012, 24, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Jenkins, M.K. Origins of CD4(+) effector and central memory T cells. Nat. Immunol. 2011, 12, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pettini, E.; Medaglini, D. CD4(+) T Cell Priming as Biomarker to Study Immune Response to Preventive Vaccines. Front. Immunol. 2013, 4, 421. [Google Scholar] [CrossRef]
- Gasper, D.J.; Tejera, M.M.; Suresh, M. CD4 T-cell memory generation and maintenance. Crit. Rev. Immunol. 2014, 34, 121–246. [Google Scholar] [CrossRef]
- Allen, C.D.; Cyster, J.G. Follicular dendritic cell networks of primary follicles and germinal centers: Phenotype and function. Semin. Immunol. 2008, 20, 14–25. [Google Scholar] [CrossRef]
- Eibel, H.; Kraus, H.; Sic, H.; Kienzler, A.-K.; Rizzi, M. B cell biology: An overview. Curr. Allergy Asthma Rep. 2014, 14, 434. [Google Scholar] [CrossRef]
- Samji, T.; Khanna, K.M. Understanding memory CD8+ T cells. Immunol. Lett. 2017, 185, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Martin-Ruiz, C.M.; Takayama, M.; Abe, Y.; Takebayashi, T.; Koyasu, S.; Suematsu, M.; Hirose, N.; von Zglinicki, T. Inflammation, But Not Telomere Length, Predicts Successful Ageing at Extreme Old Age: A Longitudinal Study of Semi-supercentenarians. EBioMedicine 2015, 2, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, J.M.; Fulop, T.; Bryl, E. Immunosenescence and COVID-19. Mech. Ageing Dev. 2022, 204, 111672. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Fahy, G.M.; Brooke, R.T.; Watson, J.P.; Good, Z.; Vasanawala, S.S.; Maecker, H.; Leipold, M.D.; Lin, D.T.S.; Kobor, M.S.; Horvath, S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019, 18, e13028. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D.; et al. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021. [Google Scholar] [CrossRef]
- Diebel, L.W.M.; Rockwood, K. Determination of Biological Age: Geriatric Assessment vs. Biological Biomarkers. Curr. Oncol. Rep. 2021, 23, 104. [Google Scholar] [CrossRef]
- Dhillon, B.K.; Smith, M.; Baghela, A.; Lee, A.H.Y.; Hancock, R.E.W. Systems Biology Approaches to Understanding the Human Immune System. Front. Immunol. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Lambert, N.D.; Ovsyannikova, I.G.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Understanding the immuneresponse to seasonal influenza vaccination in older adults: A systems biology approach. Expert Rev. Vaccines 2012, 11, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Davis, M.M. The science and medicine of humanimmunology. Science 2020, 369, eaay4014. [Google Scholar] [CrossRef] [PubMed]
- Goudsmit, J.; Biggelaar, A.H.J.v.D.; Koudstaal, W.; Hofman, A.; Koff, W.C.; Schenkelberg, T.; Alter, G.; Mina, M.J.; Wu, J.W. Immune age and biological age as determinants of vaccine responsiveness among elderly populations: The Human Immunomics Initiative research program. Eur. J. Epidemiol. 2021, 36, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Žugich, J.; Bradshaw, C.M.; Uhrlaub, J.L.; Watanabe, M. Immunity to acute virus infections with advanced age. Curr. Opin. Virol. 2021, 46, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Kasama, T.; Miyachi, Y.; Kanoh, T. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: Cross-sectional and longitudinal studies. Life Sci. 1989, 44, 1655–1664. [Google Scholar] [CrossRef]
- Wenisch, C.; Patruta, S.; Daxböck, F.; Krause, R.; Hörl, W. Effect of age on human neutrophil function. J. Leukoc. Biol. 2000, 67, 40–45. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Role of Dendritic Cells in Inflammation and Loss of Tolerance in the Elderly. Front. Immunol. 2017, 8, 896. [Google Scholar] [CrossRef]
- Plowden, J.; Renshaw-Hoelscher, M.; Engleman, C.; Katz, J.; Sambhara, S. Innate immunity in aging: Impact on macrophage function. Aging Cell 2004, 3, 161–167. [Google Scholar] [CrossRef]
- Campos, C.; Pera, A.; Lopez-Fernandez, I.; Alonso, C.; Tarazona, R.; Solana, R. Proinflammatory status influences NK cells subsets in the elderly. Immunol. Lett. 2014, 162, 298–302. [Google Scholar] [CrossRef]
- Mace, E.M.; Orange, J.S. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol. Rev. 2019, 287, 202–225. [Google Scholar] [CrossRef]
- Fülöp, T.; Larbi, A.; Witkowski, J.M. Human Inflammaging. Gerontology 2019, 65, 495–504. [Google Scholar] [CrossRef]
- Solana, R.; Tarazona, R.; Gayoso, I.; Lesur, O.; Dupuis, G.; Fulop, T. Innate immunosenescence: Effect of aging on cells and receptors of the innateimmunesystem in humans. Semin. Immunol. 2012, 24, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Lesur, O.; Fulop, T., Jr. Effects of TREM-1 activation in human neutrophils: Activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int. Immunol. 2007, 19, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, T.U.; Cubas, R.A.; Ghneim, K.; Cartwright, M.J.; Van Grevenynghe, J.; Richner, J.M.; Olagnier, D.; Wilkinson, P.A.; Cameron, M.J.; Park, B.S.; et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015, 14, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [CrossRef]
- Gupta, S. Role of dendriticn cells in innate and adaptive immune response in humanaging. Exp. Gerontol. 2014, 54, 47–52. [Google Scholar] [CrossRef]
- Borges, R.C.; Hohmann, M.S.; Borghi, S.M. Dendritic cells in COVID-19 immunopathogenesis: Insights for a possible role in determining disease outcome. Int. Rev. Immunol. 2021, 40, 108–125. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Gupta, S. Dendritic cells in human aging. Exp. Gerontol. 2007, 42, 421–426. [Google Scholar] [CrossRef]
- Hadamitzky, C.; Spohr, H.; Debertin, A.S.; Guddat, S.; Tsokos, M.; Pabst, R. Age-dependent histoarchitectural changes in human lymph nodes: An underestimated process with clinical relevance? J. Anat. 2010, 216, 556–562. [Google Scholar] [CrossRef]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef]
- Briceño, O.; Lissina, A.; Wanke, K.; Afonso, G.; Von Braun, A.; Ragon, K.; Miquel, T.; Gostick, E.; Papagno, L.; Stiasny, K.; et al. Reduced naïve CD8(+) T-cell priming efficacy in elderly adults. Aging Cell 2016, 15, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lazuardi, L.; Jenewein, B.; Wolf, A.M.; Pfister, G.; Tzankov, A.; Grubeck-Loebenstein, B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology 2005, 114, 37–43. [Google Scholar] [CrossRef]
- Czesnikiewicz-Guzik, M.; Lee, W.-W.; Cui, D.; Hiruma, Y.; Lamar, D.L.; Yang, Z.-Z.; Ouslander, J.G.; Weyand, C.M.; Goronzy, J.J. T cell subset-specific susceptibility to aging. Clin. Immunol. 2008, 127, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Whiting, C.C.; Siebert, J.; Newman, A.M.; Du, H.-W.; Alizadeh, A.A.; Goronzy, J.; Weyand, C.M.; Krishnan, E.; Fathman, C.G.; Maecker, H.T. Large-Scale and Comprehensive Immune Profiling and Functional Analysis of Normal Human Aging. PLoS ONE 2015, 10, e0133627. [Google Scholar] [CrossRef]
- Hakim, F.T.; Gress, R.E. Immunosenescence: Deficits in adaptive immunity in the elderly. Tissue Antigens 2007, 70, 179–189. [Google Scholar] [CrossRef]
- Zhang, H.; Weyand, C.M.; Goronzy, J.J.; Gustafson, C.E. Understanding T cell aging to improve anti-viral immunity. Curr. Opin. Virol. 2021, 51, 127–133. [Google Scholar] [CrossRef]
- Dugan, H.L.; Henry, C.; Wilson, P.C. Aging and influenza vaccine-induced immunity. Cell Immunol. 2020, 348, 103998. [Google Scholar] [CrossRef] [PubMed]
- Chidrawar, S.; Khan, N.; Wei, W.; McLarnon, A.; Smith, N.; Nayak, L.; Moss, P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 2009, 155, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef]
- Longo, D.M.; Louie, B.; Putta, S.; Evensen, E.; Ptacek, J.; Cordeiro, J.; Wang, E.; Pós, Z.; Hawtin, R.E.; Marincola, F.M.; et al. Single-cell network profiling of peripheral blood mononuclear cells from healthy donors reveals age- and race-associated differences in immune signaling pathway activation. J. Immunol. 2012, 188, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Shen-Orr, S.S.; Furman, D.; Kidd, B.; Hadad, F.; Lovelace, P.; Huang, Y.-W.; Rosenberg-Hasson, Y.; Mackey, S.; Grisar, F.A.G.; Pickman, Y.; et al. Defective Signaling in the JAK-STAT Pathway Tracks with Chronic Inflammation and Cardiovascular Risk in Aging Humans. Cell Syst. 2016, 3, 374–384.e4. [Google Scholar] [CrossRef]
- Li, G.; Ju, J.; Weyand, C.M.; Goronzy, J.J. Age-Associated Failure to Adjust Type I IFN Receptor Signaling Thresholds after T Cell Activation. J. Immunol. 2015, 195, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Mogilenko, D.A.; Shpynov, O.; Andhey, P.S.; Arthur, L.; Swain, A.; Esaulova, E.; Brioschi, S.; Shchukina, I.; Kerndl, M.; Bambouskova, M.; et al. Comprehensive Profiling of an Aging Immune System Reveals Clonal GZMK+ CD8+ T Cells as Conserved Hallmark of Inflammaging. Immunity 2021, 54, 99–115.e12. [Google Scholar] [CrossRef]
- Jameson, S.C.; Masopust, D. Understanding Subset Diversity in T Cell Memory. Immunity 2018, 48, 214–226. [Google Scholar] [CrossRef]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Özcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef]
- Zhang, H.; Weyand, C.M.; Goronzy, J.J. Hallmarks of the aging T-cell system. FEBS J. 2021, 288, 7123–7142. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Mechanisms underlying T cell ageing. Nat. Rev. Immunol. 2019, 19, 573–583. [Google Scholar] [CrossRef]
- Hu, B.; Li, G.; Ye, Z.; Gustafson, C.E.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Transcription factor networks in aged naïve CD4 T cells bias lineage differentiation. Aging Cell 2019, 18, e12957. [Google Scholar] [CrossRef]
- Jeng, M.Y.; Hull, P.A.; Fei, M.; Kwon, H.-S.; Tsou, C.-L.; Kasler, H.; Ng, C.-P.; Gordon, D.E.; Johnson, J.; Krogan, N.; et al. Metabolic reprogramming of human CD8+ memory T cells through loss of SIRT1. J. Exp. Med. 2018, 215, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Weng, N.-P.; Akbar, A.N.; Goronzy, J. CD28(-) T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Fulop, T. From “truly naïve” to “exhausted senescent” T cells: When markers predict functionality. Cytom. A 2014, 85, 25–35. [Google Scholar] [CrossRef]
- Fülöp, T.; Larbi, A.; Pawelec, G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef]
- Brunner, S.; Herndler-Brandstetter, D.; Weinberger, B.; Grubeck-Loebenstein, B. Persistent viral infections and immune aging. Ageing Res. Rev. 2011, 10, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Mackay, L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Nikolich-Zugich, J.; Rudd, B.D. Immune memory and aging: An infinite or finite resource? Curr. Opin. Immunol. 2010, 22, 535–540. [Google Scholar] [CrossRef]
- Pawelec, G.; Bronikowski, A.; Cunnane, S.C.; Ferrucci, L.; Franceschi, C.; Fülöp, T.; Gaudreau, P.; Gladyshev, V.N.; Gonos, E.S.; Gorbunova, V.; et al. The conundrum of human immune system “senescence”. Mech. Ageing Dev. 2020, 192, 111357. [Google Scholar] [CrossRef]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and human longevity in the omics era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccin. Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Garcia, D.; Blomberg, B.B. B Cell Immunosenescence. Annu. Rev. Cell Dev. Biol. 2020, 36, 551–574. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B.; Garcia, D.; Keilich, S.R.; Haynes, L. Age-related factors that affect B cell responses to vaccination in mice and humans. Immunol. Rev. 2020, 296, 142–154. [Google Scholar] [CrossRef]
- Cancro, M.P.; Hao, Y.; Scholz, J.L.; Riley, R.L.; Frasca, D.; Dunn-Walters, D.; Blomberg, B.B. B cells and aging: Molecules and mechanisms. Trends Immunol. 2009, 30, 313–318. [Google Scholar] [CrossRef]
- Frasca, D. Senescent B cells in aging and age-related diseases: Their role in the regulation of antibody responses. Exp. Gerontol. 2018, 107, 55–58. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun. Ageing 2020, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Pritz, T.; Lair, J.; Ban, M.; Keller, M.; Weinberger, B.; Krismer, M.; Grubeck-Loebenstein, B. Plasma cell numbers decrease in bone marrow of old patients. Eur. J. Immunol. 2015, 45, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.L.; Wu, Y.-C.; Barnett, Y.; Duggan, O.; Vaughan, R.; Kondeatis, E.; Nilsson, B.-O.; Wikby, A.; Kipling, D.; Dunn-Walters, D.K. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009, 8, 18–25. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigeneticclock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jadhav, R.R.; Gustafson, C.E.; Le Saux, S.; Ye, Z.; Li, X.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Distinct Age-Related Epigenetic Signatures in CD4 and CD8 T Cells. Front. Immunol. 2020, 11, 585168. [Google Scholar] [CrossRef]
- Ucar, D.; Márquez, E.J.; Chung, C.-H.; Marches, R.; Rossi, R.; Uyar, A.; Wu, T.-C.; George, J.; Stitzel, M.L.; Palucka, A.K.; et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 2017, 214, 3123–3144. [Google Scholar] [CrossRef]
- Moskowitz, D.M.; Zhang, D.W.; Hu, B.; Le Saux, S.; Yanes, R.E.; Ye, Z.; Buenrostro, J.D.; Weyand, C.M.; Greenleaf, W.J.; Goronzy, J.J. Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol. 2017, 2, eaag0192. [Google Scholar] [CrossRef]
- Akbar, A.N.; Beverley, P.C.; Salmon, M. Will telomere erosion lead to a loss of T-cell memory? Nat. Rev. Immunol. 2004, 4, 737–743. [Google Scholar] [CrossRef]
- Libertini, G.; Shubernetskaya, O.; Corbi, G.; Ferrara, N. Is Evidence Supporting the Subtelomere-Telomere Theory of Aging? Biochemistry (Mosc) 2021, 86, 1526–1539. [Google Scholar] [CrossRef]
- Bektas, A.; Zhang, Y.; Lehmann, E.; Wood, W.H.; Becker, K.G.; Madara, K.; Ferrucci, L.; Sen, R. Age-associated changes in basal NF-κB function in human CD4+ T lymphocytes via dysregulation of PI3 kinase. Aging 2014, 6, 957–974. [Google Scholar] [CrossRef]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e6. [Google Scholar] [CrossRef] [PubMed]
- Geltink, R.I.K.; Kyle, R.L.; Pearce, E.L. Unraveling the Complex Interplay between T Cell Metabolism and Function. Annu. Rev. Immunol. 2018, 36, 461–488. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yu, M.; Lee, W.-W.; Tsang, M.; Krishnan, E.; Weyand, C.M.; Goronzy, J.J. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat. Med. 2012, 18, 1518–1524. [Google Scholar] [CrossRef]
- Le Page, A.; Fortin, C.; Garneau, H.; Allard, N.; Tsvetkova, K.; Tan, C.T.Y.; Larbi, A.; Dupuis, G.; Fülöp, T. Downregulation of inhibitory SRC homology 2 domain-containing phosphatase-1 (SHP-1) leads to recovery of T cell responses in elderly. Cell Commun. Signal. 2014, 12, 2. [Google Scholar] [CrossRef]
- Fulop, T.; Le Page, A.; Fortin, C.; Witkowski, J.M.; Dupuis, G.; Larbi, A. Cellular signaling in the aging immune system. Curr. Opin. Immunol. 2014, 29, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Larbi, A.; Dupuis, G.; Khalil, A.; Douziech, N.; Fortin, C.; Fülöp, T., Jr. Differential role of lipid rafts in the functions of CD4+ and CD8+ human T lymphocytes with aging. Cell Signal. 2006, 18, 1017–1030. [Google Scholar] [CrossRef]
- Smigielska-Czepiel, K.; Berg, A.V.D.; Jellema, P.; Slezak-Prochazka, I.; Maat, H.; Bos, H.V.D.; Van Der Lei, R.J.; Kluiver, J.; Brouwer, E.; Boots, A.M.H.; et al. Dual role of miR-21 in CD4+ T-cells: Activation-induced miR-21 supports survival of memory T-cells and regulates CCR7 expression in naive T-cells. PLoS ONE 2013, 8, e76217. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hu, B.; Jadhav, R.R.; Jin, J.; Zhang, H.; Cavanagh, M.M.; Akondy, R.S.; Ahmed, R.; Weyand, C.M.; Goronzy, J.J. Activation of miR-21-Regulated Pathways in Immune Aging Selects against Signatures Characteristic of Memory T Cells. Cell Rep. 2018, 25, 2148–2162.e5. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The integration of inflammaging in age-related diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Fourati, S.; Cristescu, R.; Loboda, A.; Talla, A.; Filali, A.; Railkar, R.; Schaeffer, A.K.; Favre, D.; Gagnon, D.; Peretz, Y.; et al. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat. Commun. 2016, 7, 10369. [Google Scholar] [CrossRef] [PubMed]
- Vukmanovic-Stejic, M.; Chambers, E.S.; Suárez-Fariñas, M.; Sandhu, D.; Fuentes-Duculan, J.; Patel, N.; Agius, E.; Lacy, K.E.; Turner, C.; Larbi, A.; et al. Enhancement of cutaneous immunity during aging by blocking p38 mitogen-activated protein (MAP) kinase-induced inflammation. J. Allergy Clin. Immunol. 2018, 142, 844–856. [Google Scholar] [CrossRef]
- Pereira, B.; Xu, X.N.; Akbar, A.N. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Front. Immunol. 2020, 11, 583019. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, Y.; Cheng, Y.; Glanville, J.; Zhang, D.; Lee, J.-Y.; Olshen, R.A.; Weyand, C.M.; Boyd, S.D.; Goronzy, J.J. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. USA 2014, 111, 13139–13144. [Google Scholar] [CrossRef] [PubMed]
- Robins, H.S.; Campregher, P.V.; Srivastava, S.K.; Wacher, A.; Turtle, C.J.; Kahsai, O.; Riddell, S.R.; Warren, E.; Carlson, C.S. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood 2009, 114, 4099–4107. [Google Scholar] [CrossRef] [PubMed]
- de Greef, P.C.; Oakes, T.; Gerritsen, B.; Ismail, M.; Heather, J.; Hermsen, R.; Chain, B.; de Boer, R.J. The naive T-cell receptor repertoire has an extremely broad distribution of clone sizes. Elife 2020, 9, e49900. [Google Scholar] [CrossRef]
- Drabkin, M.J.; Meyer, J.I.; Kanth, N.; Lobel, S.; Fogel, J.; Grossman, J.; Krumenacker, J.H. Age-stratified Patterns of Thymic Involution on Multidetector CT. J. Thorac. Imaging 2018, 33, 409–416. [Google Scholar] [CrossRef]
- Mold, J.E.; Réu, P.; Olin, A.; Bernard, S.; Michaëlsson, J.; Rane, S.; Yates, A.; Khosravi, A.; Salehpour, M.; Possnert, G.; et al. Cell generation dynamics underlying naive T-cell homeostasis in adult humans. PLoS Biol. 2019, 17, e3000383. [Google Scholar] [CrossRef]
- Nicoli, F.; Clave, E.; Wanke, K.; von Braun, A.; Bondet, V.; Alanio, C.; Douay, C.; Baque, M.; Lependu, C.; Marconi, P.; et al. Primary immune responses are negatively impacted by persistent herpesvirus infections in older people: Results from an observational study on healthy subjects and a vaccination trial on subjects aged more than 70 years old. EBioMedicine 2022, 76, 103852. [Google Scholar] [CrossRef]
- Britanova, O.V.; Putintseva, E.V.; Shugay, M.; Merzlyak, E.M.; Turchaninova, M.A.; Staroverov, D.B.; Bolotin, D.A.; Lukyanov, S.; Bogdanova, E.A.; Mamedov, I.Z.; et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J. Immunol. 2014, 192, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Horn, V.; Semmler, M.; Schweppe, C. Older People in Germany During the COVID-19 Pandemic: The Least, the More, and the Most Affected. J. Popul. Ageing 2021. [Google Scholar] [CrossRef]
- Maltese, G.; Corsonello, A.; Di Rosa, M.; Soraci, L.; Vitale, C.; Corica, F.; Lattanzio, F. Frailty and COVID-19: A Systematic Scoping Review. J. Clin. Med. 2020, 9, 2106. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Mellaerts, B.; Vandewinckele, H.; Lybeert, P.; Frans, E.; Ombelet, S.; Lemahieu, W.; Symons, R.; Ho, E.; Frans, J.; et al. Frailty and Mortality in Hospitalized Older Adults With COVID-19: Retrospective Observational Study. J. Am. Med. Dir. Assoc. 2020, 21, 928–932.e1. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Dupuis, G.; Baehl, S.; Le Page, A.; Bourgade, K.; Frost, E.; Witkowski, J.M.; Pawelec, G.; Larbi, A.; Cunnane, S. From inflamm-aging to immune-paralysis: A slippery slope during aging for immune-adaptation. Biogerontology 2016, 17, 147–157. [Google Scholar] [CrossRef]
- Bindu, S.; Dandapat, S.; Manikandan, R.; Dinesh, M.; Subbaiyan, A.; Mani, P.; Dhawan, M.; Tiwari, R.; Bilal, M.; Bin Emran, T.; et al. Prophylactic and therapeutic insights into trained immunity: A renewed concept of innate immune memory. Hum. Vaccin. Immunother. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ietto, G.; Mortara, L.; Gasperina, D.D.; Iovino, D.; Azzi, L.; Baj, A.; Ageno, W.; Genoni, A.P.; Acquati, F.; Gallazzi, M.; et al. Immune-Mediated Mechanisms in Patients Testing Positive for SARS-CoV-2: Protocol for a Multianalysis Study. JMIR Res. Protoc. 2022, 11, e29892. [Google Scholar] [CrossRef]
- Edelman, G.M.; Gally, J.A. Degeneracy and complexity in biological systems. Proc. Natl. Acad. Sci. 2001, 98, 13763–13768. [Google Scholar] [CrossRef]
- Riley, T.P.; Hellman, L.; Gee, M.H.; Mendoza, J.L.; Alonso, J.A.; Foley, K.C.; Nishimura, M.I.; Kooi, C.W.V.; Garcia, K.C.; Baker, B.M. T cell receptor cross-reactivity expanded by dramatic peptide-MHC adaptability. Nat. Chem. Biol. 2018, 14, 934–942. [Google Scholar] [CrossRef]
- Petrova, G.; Ferrante, A.; Gorski, J. Cross-reactivity of T cells and its role in the immune system. Crit. Rev. Immunol. 2012, 32, 349–372. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Kriete, A. Robustness and aging—A systems-level perspective. Biosystems 2013, 112, 37–48. [Google Scholar] [CrossRef]
- Johnson, P.L.; Yates, A.J.; Goronzy, J.J.; Antia, R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc. Natl. Acad. Sci. USA 2012, 109, 21432–21437. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wong, G.; Hwang, Y.Y.; Larbi, A. The untwining of immunosenescence and aging. Semin, Immunopathol. 2020, 42, 559–572. [Google Scholar] [CrossRef]
- Verschoor, C.P.; Andrew, M.K.; Loeb, M.; Pawelec, G.; Haynes, L.; Kuchel, G.A.; McElhaney, J.E. Antibody and Cell-Mediated Immune Responses Are Correlates of Protection against Influenza Infection in Vaccinated Older Adults. Vaccines 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Pedrero, M.Á.; Osterhaus, A.D.M.E.; Becker, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Aging and Options to Halt Declining Immunity to Virus Infections. Front. Immunol. 2021, 12, 681449. [Google Scholar] [CrossRef]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir Rev. 2021, 30, 200258. [Google Scholar] [CrossRef]
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Di Pietrantonj, C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018, 2, CD001269. [Google Scholar] [CrossRef]
- Available online: https://www.influenza.org.nz/ (accessed on 11 February 2022).
- Nguyen, T.H.O.; Sant, S.; Bird, N.L.; Grant, E.J.; Clemens, E.B.; Koutsakos, M.; Valkenburg, S.A.; Gras, S.; Lappas, M.; Jaworowski, A.; et al. Perturbed CD8(+) T cell immunity across universal influenza epitopes in the elderly. J. Leukoc. Biol. 2018, 103, 321–339. [Google Scholar] [CrossRef]
- Park, H.-J.; Shin, M.S.; Kim, M.; Bilsborrow, J.B.; Mohanty, S.; Montgomery, R.; Shaw, A.C.; You, S.; Kang, I. Transcriptomic analysis of human IL-7 receptor alpha (low) and (high) effector memory CD8(+) T cells reveals an age-associated signature linked to influenza vaccine response in older adults. Aging Cell 2019, 18, e12960. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef]
- McElhaney, J.E.; Verschoor, C.P.; Andrew, M.K.; Haynes, L.; Kuchel, G.A.; Pawelec, G. The immune response to influenza in older humans: Beyond immune senescence. Immun. Ageing 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Zheng, N.-Y.; Huang, M.; Cabanov, A.; Rojas, K.T.; Kaur, K.; Andrews, S.F.; Palm, A.-K.; Chen, Y.-Q.; Li, Y.; et al. Influenza Virus Vaccination Elicits Poorly Adapted B Cell Responses in Elderly Individuals. Cell Host Microbe 2019, 25, 357–366.e6. [Google Scholar] [CrossRef] [PubMed]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Lee, J.K.; Lam, G.K.; Shin, T.; Samson, S.I.; Greenberg, D.P.; Chit, A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: An updated systematic review and meta-analysis. Vaccine 2021, 39 (Suppl. 1), A24–A35. [Google Scholar] [CrossRef]
- Tsai, T.F. Fluad-MF59-Adjuvanted Influenza Vaccine in Older Adults. Infect. Chemother. 2013, 45, 159–174. [Google Scholar] [CrossRef]
- Domnich, A.; Arata, L.; Amicizia, D.; Puig-Barberà, J.; Gasparini, R.; Panatto, D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine 2017, 35, 513–520. [Google Scholar] [CrossRef]
- Beran, J.; Reynales, H.; Poder, A.; Yu, C.Y.; Pitisuttithum, P.; Yuan, L.L.; Vermeulen, W.; Verhoeven, C.; Leav, B.; Zhang, B.; et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: A randomised, controlled, multicentre, phase 3 efficacy study. Lancet Infect. Dis. 2021, 21, 1027–1037. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.; Goldenthal, K.L.; Muse, D.; Callahan, J.; Cox, M.M. PSC12 Study Team. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N. Engl. J. Med. 2017, 376, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Chi, R.C.; Rock, M.T.; Neuzil, K.M. Immunogenicity and safety of intradermal influenza vaccination in healthy older adults. Clin. Infect. Dis. 2010, 50, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Merani, S.; Kuchel, G.; Kleppinger, A.; McElhaney, J.E. Influenza vaccine-mediated protection in older adults: Impact of influenza infection, cytomegalovirus serostatus and vaccine dosage. Exp. Gerontol. 2018, 107, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.W.Y.; Cowling, B.J.; Gao, H.Z.; Thompson, M.G. Comparative Immunogenicity of Enhanced Seasonal Influenza Vaccines in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 2019, 219, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Cowling, B.J.; Perera, R.A.P.M.; A Valkenburg, S.; Leung, N.H.L.; Iuliano, A.D.; Tam, Y.H.; Wong, J.H.F.; Fang, V.J.; Li, A.P.Y.; So, H.C.; et al. Comparative Immunogenicity of Several Enhanced Influenza Vaccine Options for Older Adults: A Randomized, Controlled Trial. Clin. Infect. Dis. 2020, 71, 1704–1714. [Google Scholar] [CrossRef]
- Weinberger, B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 2018, 41, 34–41. [Google Scholar] [CrossRef]
- Falkenhorst, G.; Remschmidt, C.; Harder, T.; Hummers-Pradier, E.; Wichmann, O.; Bogdan, C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169368. [Google Scholar] [CrossRef]
- Diao, W.-Q.; Shen, N.; Yu, P.-X.; Liu, B.-B.; He, B. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: A systematic review and meta-analysis of randomized trials. Vaccine 2016, 34, 1496–1503. [Google Scholar] [CrossRef]
- Moberley, S.; Holden, J.; Tatham, D.P.; Andrews, R.M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst. Rev. 2013, 2013, CD000422. [Google Scholar] [CrossRef]
- Wu, Y.-C.B.; Kipling, D.; Dunn-Walters, D.K. Age-Related Changes in Human Peripheral Blood IGH Repertoire Following Vaccination. Front. Immunol. 2012, 3, 193. [Google Scholar] [CrossRef]
- Berild, J.D.; Winje, B.A.; Vestrheim, D.F.; Slotved, H.-C.; Valentiner-Branth, P.; Roth, A.; Storsäter, J. A Systematic Review of Studies Published between 2016 and 2019 on the Effectiveness and Efficacy of Pneumococcal Vaccination on Pneumonia and Invasive Pneumococcal Disease in an Elderly Population. Pathogens 2020, 9, 259. [Google Scholar] [CrossRef]
- Gessner, B.D.; Jiang, Q.; Van Werkhoven, C.H.; Sings, H.L.; Webber, C.; Scott, D.; Gruber, W.C.; Grobbee, D.E.; Bonten, M.J.; Jodar, L. A post-hoc analysis of serotype-specific vaccine efficacy of 13-valent pneumococcal conjugate vaccine against clinical community acquired pneumonia from a randomized clinical trial in the Netherlands. Vaccine 2019, 37, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Dion, S.B.; Major, M.; Grajales, A.G.; Nepal, R.M.; Cane, A.; Gessner, B.; Vojicic, J.; Suaya, J.A. Invasive pneumococcal disease in Canada 2010–2017: The role of current and next-generation higher-valent pneumococcal conjugate vaccines. Vaccine 2021, 39, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.M.; Jiang, Q.; Isturiz, R.E.; Sings, H.L.; Swerdlow, D.L.; Gessner, B.D.; Carrico, R.M.; Peyrani, P.; Wiemken, T.L.; Mattingly, W.A.; et al. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Hospitalization for Community-Acquired Pneumonia in Older US Adults: A Test-Negative Design. Clin. Infect. Dis. 2018, 67, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; Van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Bruxvoort, K.J.; Fischer, H.; Hong, V.X.; Grant, L.R.; Jódar, L.; Cané, A.; Gessner, B.D.; Tartof, S.Y. Effectiveness of 13-valent pneumococcal conjugate vaccine against medically-attended lower respiratory tract infection and pneumonia among older adults. Clin. Infect. Dis. 2021, ciab1051. [Google Scholar] [CrossRef]
- Cannon, K.; Elder, C.; Young, M.; Scott, D.A.; Scully, I.L.; Baugher, G.; Peng, Y.; Jansen, K.U.; Gruber, W.C.; Watson, W. A trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in populations of adults ≥65 years of age with different prior pneumococcal vaccination. Vaccine 2021, 39, 7494–7502. [Google Scholar] [CrossRef]
- Essink, B.; Sabharwal, C.; Cannon, K.; Frenck, R.; Lal, H.; Xu, X.; Sundaraiyer, V.; Peng, Y.; Moyer, L.; Pride, M.W.; et al. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults 18 Years and Older. Clin. Infect. Dis. 2021, ciab990. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Warren, J.L.; Regev-Yochay, G.; Givon-Lavi, N.; Dagan, R.; Weinberger, D.M. Serotype Patterns of Pneumococcal Disease in Adults Are Correlated with Carriage Patterns in Older Children. Clin. Infect. Dis. 2021, 72, e768–e775. [Google Scholar] [CrossRef]
- Blom, K.; Yin, L.; Arnheim-Dahlström, L. Effectiveness of the herpes zoster vaccine Zostavax® in Stockholm County, Sweden. Vaccine 2019, 37, 4401–4406. [Google Scholar] [CrossRef]
- Levin, M.J.; Weinberg, A. Immune responses to zoster vaccines. Hum. Vaccin. Immunother. 2019, 15, 772–777. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.F.; Volpe, S.; Catteau, G.; Chlibek, R.; David, M.P.; Richardus, J.H.; Lal, H.; Oostvogels, L.; Pauksens, K.; Ravault, S.; et al. Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum. Vaccin. Immunother. 2018, 14, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Popmihajlov, Z.; E Schmader, K.; Johnson, M.J.; Caldas, Y.; Salazar, A.T.; Canniff, J.; McCarson, B.J.; Martin, J.; Pang, L.; et al. Persistence of Varicella-Zoster Virus Cell-Mediated Immunity after the Administration of a Second Dose of Live Herpes Zoster Vaccine. J. Infect. Dis. 2019, 219, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Heineman, T.C.; Cunningham, A.; Levin, M. Understanding the immunology of Shingrix, a recombinant glycoprotein E adjuvanted herpes zoster vaccine. Curr. Opin. Immunol. 2019, 59, 42–48. [Google Scholar] [CrossRef]
- Sullivan, N.L.; Eberhardt, C.S.; Wieland, A.; A Vora, K.; Pulendran, B.; Ahmed, R. Understanding the immunology of the Zostavax shingles vaccine. Curr. Opin. Immunol. 2019, 59, 25–30. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Sasso, B.L.; Giglio, R.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.; Ciaccio, A.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Grupel, D.; Gazit, S.; Schreiber, L.; Nadler, V.; Wolf, T.; Lazar, R.; Supino-Rosin, L.; Perez, G.; Peretz, A.; Ben Tov, A.; et al. Kinetics of SARS-CoV-2 anti-S IgG after BNT162b2 vaccination. Vaccine 2021, 39, 5337–5340. [Google Scholar] [CrossRef]
- Mwimanzi, F.M.; Lapointe, H.R.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Datwani, S.; Omondi, F.H.; Burns, L.; et al. Older Adults Mount Less Durable Humoral Responses to a Two-dose COVID-19 mRNA Vaccine Regimen, but Strong Initial Responses to a Third Dose. medRxiv 2022. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2021, 22, 483–495. [Google Scholar] [CrossRef]
- Mudd, P.A.; Remy, K.E. Prolonged adaptive immune activation in COVID-19: Implications for maintenance of long-term immunity? J. Clin. Investig. 2021, 131, e143928. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.K.; Jasny, E.; Yoon, H.; Horscroft, N.; Schanen, B.; Geter, T.; Fotin-Mleczek, M.; Petsch, B.; Wittman, V. Adjuvant effects of a sequence-engineered mRNA vaccine: Translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 2017, 15, 1. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Lacroix, C.; Exposito, J.-Y.; Verrier, B. Tailoring mRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends Mol. Med. 2020, 26, 311–323. [Google Scholar] [CrossRef]
- Zierer, J.; Menni, C.; Kastenmüller, G.; Spector, T.D. Integration of ‘omics’ data in aging research: From biomarkers to systems biology. Aging Cell 2015, 14, 933–944. [Google Scholar] [CrossRef]
- Rodrigues, S.; Desroches, M.; Krupa, M.; Cortes, J.M.; Sejnowski, T.J.; Ali, A.B. Time-coded neurotransmitter release at excitatory and inhibitory synapses. Proc. Natl. Acad. Sci. 2016, 113, E1108–E1115. [Google Scholar] [CrossRef]
- Fülöp, T.; Desroches, M.; A Cohen, A.; Santos, F.A.N.; Rodrigues, S. Why we should use topological data analysis in ageing: Towards defining the “topological shape of ageing”. Mech. Ageing Dev. 2020, 192, 111390. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Activation of immunosuppressive network in the aging process. Ageing Res. Rev. 2020, 57, 100998. [Google Scholar] [CrossRef]
- Churov, A.V.; Mamashov, K.Y.; Novitskaia, A.V. Homeostasis and the functional roles of CD4(+) Treg cells in aging. Immunol. Lett. 2020, 226, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J. Mol. Med. (Berl) 2021, 99, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Picard, E.; Bueno, V.; Verschoor, C.P.; Ostrand-Rosenberg, S. MDSCs, ageing and inflammageing. Cell Immunol. 2021, 362, 104297. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Hirokawa, K.; Cohen, A.A.; Witkowski, J.M. Immunosenescence is both functional/adaptive and dysfunctional/maladaptive. Semin. Immunopathol. 2020, 42, 521–536. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Successful and Maladaptive T Cell Aging. Immunity 2017, 46, 364–378. [Google Scholar] [CrossRef]
- Hussien, H.; Nastasa, A.; Apetrii, M.; Nistor, I.; Petrovic, M.; Covic, A. Different aspects of frailty and COVID-19: Points to consider in the current pandemic and future ones. BMC Geriatr. 2021, 21, 389. [Google Scholar] [CrossRef]
- Cohen, A.A. Complex systems dynamics in aging: New evidence, continuing questions. Biogerontology 2016, 17, 205–220. [Google Scholar] [CrossRef]
- Mangel, M.J. Complex adaptive systems, aging and longevity. Theor. Biol. 2001, 213, 559–571. [Google Scholar] [CrossRef]
- Holden, L.M. Complex adaptive systems: Concept analysis. J. Adv. Nurs. 2005, 52, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Nakaya, H.I.; Kazmin, D.A.; Oh, J.Z.; Pulendran, B. Systems biological approaches to measure and understand vaccine immunity in humans. Semin. Immunol. 2013, 25, 209–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pulendran, B.; Li, S.; Nakaya, H. Systems vaccinology. Immunity 2010, 33, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Sherman, A.C.; Rouphael, N.G.; Pulendran, B. Systems Biological Analysis of Immune Response to Influenza Vaccination. Cold Spring Harb. Perspect. Med. 2021, 11, a038596. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Tomic, A.; Pollard, A.; Davis, M. Systems Immunology: Revealing Influenza Immunological Imprint. Viruses 2021, 13, 948. [Google Scholar] [CrossRef]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J.; Netea, M.G. Overcoming immune dysfunction in the elderly: Trained immunity as a novel approach. Int. Immunol. 2020, 32, 741–753. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Van De Merwe, R.C.; Louie, R.; Bracken, O.V.; Devine, O.; Goldstein, D.R.; Uddin, M.; Akbar, A.N.; Gilroy, D.W. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 2020, 21, 615–625. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.P.; Kritchevsky, S.B.; Espeland, M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Lanna, A.; O Gomes, D.C.; Muller-Durovic, B.; McDonnell, T.; Escors, D.; Gilroy, D.W.; Lee, J.H.; Karin, M.; Akbar, A.N. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat. Immunol. 2017, 18, 354–363. [Google Scholar] [CrossRef] [PubMed]
| Vaccines | Younger Individuals | Older Individuals |
|---|---|---|
| Influenza | ||
| Standard dose | +/− | - |
| High dose | + | ++ |
| Herpes Zoster | ||
| Zostavax | NIL | + |
| Shingrix | NIL | ++ |
| SARS-CoV-2 (after 3rd dose) | + | + |
| Pneumococcus | ||
| Polysaccharide | +/− | - |
| Conjugated | + | + |
| Yellow fever | + | + |
| Hepatitis B virus | + | + |
| Japanese encephalitis virus | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fulop, T.; Larbi, A.; Pawelec, G.; Cohen, A.A.; Provost, G.; Khalil, A.; Lacombe, G.; Rodrigues, S.; Desroches, M.; Hirokawa, K.; et al. Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change. Vaccines 2022, 10, 607. https://doi.org/10.3390/vaccines10040607
Fulop T, Larbi A, Pawelec G, Cohen AA, Provost G, Khalil A, Lacombe G, Rodrigues S, Desroches M, Hirokawa K, et al. Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change. Vaccines. 2022; 10(4):607. https://doi.org/10.3390/vaccines10040607
Chicago/Turabian StyleFulop, Tamas, Anis Larbi, Graham Pawelec, Alan A. Cohen, Guillaume Provost, Abedelouahed Khalil, Guy Lacombe, Serafim Rodrigues, Mathieu Desroches, Katsuiku Hirokawa, and et al. 2022. "Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change" Vaccines 10, no. 4: 607. https://doi.org/10.3390/vaccines10040607
APA StyleFulop, T., Larbi, A., Pawelec, G., Cohen, A. A., Provost, G., Khalil, A., Lacombe, G., Rodrigues, S., Desroches, M., Hirokawa, K., Franceschi, C., & Witkowski, J. M. (2022). Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change. Vaccines, 10(4), 607. https://doi.org/10.3390/vaccines10040607









