Individual Preferences for COVID-19 Vaccination under the China’s 2021 National Vaccination Policy: A Discrete Choice Experiment Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of Attributes and Levels
2.2. Experimental Design
2.3. Survey
2.4. Sample
2.5. Data Analysis
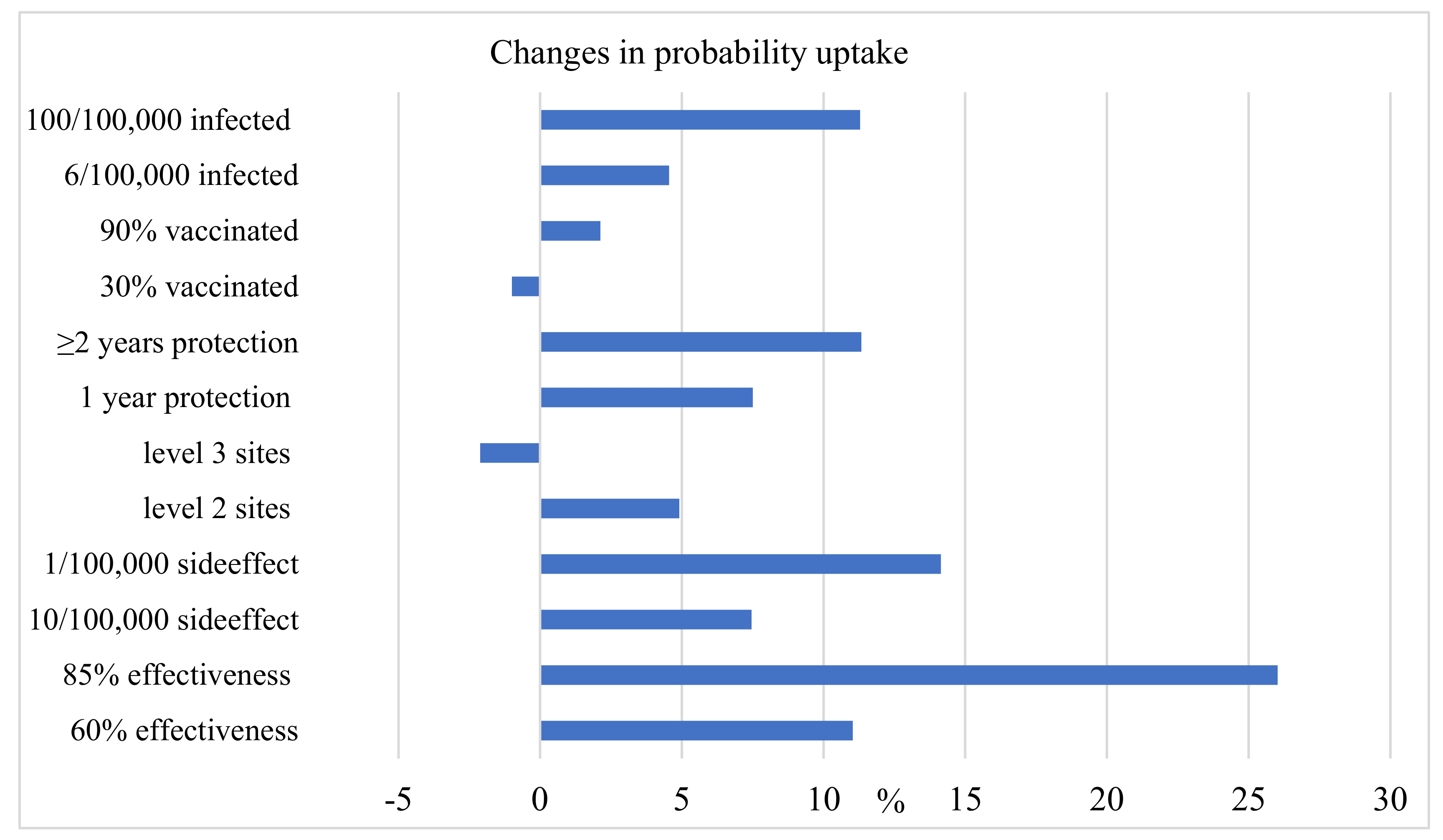
3. Results
3.1. Sample Characteristics
3.2. Estimation of Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 January 2022).
- Global Variants Report. Available online: https://covid.cdc.gov/covid-data-tracker/#global-variant-report-map (accessed on 1 January 2022).
- Covid Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 1 January 2022).
- COVID-19 Vaccine Effectiveness Research. Available online: https://www.cdc.gov/vaccines/covid-19/effectiveness-research/protocols.html (accessed on 1 January 2022).
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 85, 585–594. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- CDC Expands Eligibility for COVID-19 Booster Shots. Available online: https://www.cdc.gov/media/releases/2021/p1021-covid-booster.html (accessed on 1 January 2022).
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection against Covid-19 by BNT162b2 Booster across Age Groups. N. Engl. J. Med. 2021, 385, 2421–2430. [Google Scholar] [CrossRef]
- Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 1 January 2022).
- National and Local Health Commissions Daily Information Release. Available online: https://news.qq.com/zt2020/page/feiyan.htm#/ (accessed on 1 January 2022). (In Chinese).
- COVID-19 Daily Report, National Health Commission of the People’s Republic of China. Available online: http://www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml (accessed on 1 January 2022).
- Zheng, W.; Yan, X.; Zhao, Z.; Yang, J.; Yu, H. COVID-19 vaccination program in the mainland of China: A subnational descriptive analysis on target population size and current progress. Infect. Dis. Poverty 2021, 10, 124. [Google Scholar] [CrossRef]
- Local Governments Roll Out Booster Shots, The State Council of the PRC. Available online: http://english.www.gov.cn/news/topnews/202110/11/content_WS61638a3ec6d0df57f98e1769.html (accessed on 1 January 2022).
- Yang, F.; Li, X.; Su, X.; Xiao, T.; Wang, Y.; Hu, P.; Li, H.; Guan, J.; Tian, H.; Wang, P.; et al. A study on willingness and influencing factors to receive COVID-19 vaccination among Qingdao residents. Hum. Vaccine Immunother. 2021, 17, 408–413. [Google Scholar] [CrossRef]
- Bai, W.; Cai, H.; Liu, S.; Liu, H.; Qi, H.; Chen, X.; Liu, R.; Cheung, T.; Su, Z.; Ng, C.H.; et al. Attitudes toward COVID-19 vaccines in Chinese college students. Int. J. Biol. Sci. 2021, 17, 1469–1475. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C. Acceptance of a COVID-19 Vaccine Before it is Available in China During the Pandemic. Int. J. Public Health 2021, 66, 1604092. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Lai, X.; Ma, X.; Cao, L.; Lei, H.; Wang, J.; Zhang, H.; Jing, R.; Feng, H.; Guo, J.; et al. The Acceptance of COVID-19 Vaccination Under Different Methods of Investigation: Based on Online and On-Site Surveys in China. Front. Public Health 2021, 9, 760388. [Google Scholar] [CrossRef] [PubMed]
- Mo, P.K.H.; Luo, S.; Wang, S.; Zhao, J.; Zhang, G.; Li, L.; Li, L.; Xie, L.; Lau, J.T. Intention to Receive the COVID-19 Vaccination in China: Application of the Diffusion of Innovations Theory and the Moderating Role of Openness to Experience. Vaccines 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Chen, J.; Wen, Z.; Feng, F.; Zou, H.; Fu, C.; Chen, L.; Shu, Y.; Sun, C. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum. Vaccine Immunother. 2021, 17, 2279–2288. [Google Scholar] [CrossRef]
- Gan, L.; Chen, Y.; Hu, P.; Wu, D.; Zhu, Y.; Tan, J.; Li, Y.; Zhang, D. Willingness to Receive SARS-CoV-2 Vaccination and Associated Factors among Chinese Adults: A Cross Sectional Survey. Int. J. Environ. Res. Public Health 2021, 18, 1993. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.D.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef]
- Han, K.; Francis, M.R.; Zhang, R. Confidence, Acceptance and Willingness to Pay for the COVID-19 Vaccine among Migrants in Shanghai, China: A Cross-Sectional Study. Vaccines 2021, 9, 443. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; Rauh, L.; Rabin, K.; Ratzan, S.; Gostin, L.O.; Larson, H.J.; El-Mohandes, A. Hesitant or Not? The Association of Age, Gender, and Education with Potential Acceptance of a COVID-19 Vaccine: A Country-level Analysis. J. Health Commun. 2020, 25, 799–807. [Google Scholar] [CrossRef]
- Kerekes, S.; Ji, M.; Shih, S.-F.; Chang, H.-Y.; Harapan, H.; Rajamoorthy, Y.; Singh, A.; Kanwar, S.; Wagner, A.L. Differential Effect of Vaccine Effectiveness and Safety on COVID-19 Vaccine Acceptance across Socioeconomic Groups in an International Sample. Vaccines 2021, 9, 1010. [Google Scholar] [CrossRef]
- China Grants Conditional Approval to First COVID Vaccine, National Medical Products Administration. Available online: http://english.nmpa.gov.cn/2020-12/31/c_579192.htm (accessed on 21 February 2022).
- Shakeel, C.S.; Mujeeb, A.A.; Mirza, M.S.; Chaudhry, B.; Khan, S.J. Global COVID-19 Vaccine Acceptance: A Systematic Review of Associated Social and Behavioural Factors. Vaccines 2022, 10, 110. [Google Scholar] [CrossRef]
- Walker, A.N.; Zhang, T.; Peng, X.Q.; Ge, J.J.; Gu, H.; You, H. Vaccine Acceptance and Its Influencing Factors: An Online Cross-Sectional Study among International College Students Studying in China. Vaccines 2021, 9, 585. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Q.; Guan, H.; Fan, K.; Bi, X.; Huang, Y.; Liang, L.; Khoso, A.R.; Xu, X.; Ma, Y.; et al. Who is more likely to hesitate to accept COVID-19 vaccine: A cross-sectional survey in China. Expert Rev. Vaccines 2022, 21, 397–406. [Google Scholar] [CrossRef]
- Wang, Q.; Xiu, S.; Zhao, S.; Wang, J.; Han, Y.; Dong, S.; Huang, J.; Cui, T.; Yang, L.; Shi, N.; et al. Vaccine Hesitancy: COVID-19 and Influenza Vaccine Willingness among Parents in Wuxi, China—A Cross-Sectional Study. Vaccines 2021, 9, 342. [Google Scholar] [CrossRef]
- Liu, X.-J.; Mesch, G.S. The Adoption of Preventive Behaviors during the COVID-19 Pandemic in China and Israel. Int. J. Environ. Res. Public Health 2020, 17, 7170. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Zhao, Q.; Alias, H.; Danaee, M.; Wong, L.P. Understanding COVID-19 vaccine demand and hesitancy: A nationwide online survey in China. PLoS Negl. Trop. Dis. 2020, 14, e0008961. [Google Scholar] [CrossRef]
- Kwok, K.O.; Li, K.K.; Wei, W.I.; Tang, A.; Wong, S.Y.S.; Lee, S.S. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey. Int. J. Nurs. Stud. 2021, 114, 103854. [Google Scholar] [CrossRef]
- Zhang, K.C.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.Q.; Zhou, X.; Wang, Z. Parental Acceptability of COVID-19 Vaccination for Children Under the Age of 18 Years: Cross-Sectional Online Survey. JMIR Pediatr. Parent. 2020, 3, e24827. [Google Scholar] [CrossRef]
- Suo, L.; Ma, R.; Wang, Z.Z.; Tang, T.; Wang, H.L.; Liu, F.; Tang, J.F.; Peng, X.H.; Guo, X.; Lu, L.; et al. Perception of the COVID-19 Epidemic and Acceptance of Vaccination Among Healthcare Workers Prior to Vaccine Licensure—Beijing Municipality, China, May–July 2020. China CDC Wkly. 2021, 3, 569–575. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Wong, E.L.Y.; Huang, J.; Cheung, A.W.L.; Law, K.; Chong, M.K.C.; Ng, R.W.Y.; Lai, C.K.C.; Boon, S.S.; Lau, J.T.F.; et al. Acceptance of the COVID-19 vaccine based on the health belief model: A population-based survey in Hong Kong. Vaccine 2021, 39, 1148–1156. [Google Scholar] [CrossRef]
- Meng, Z.; Shan, S.; Zhang, R. China’s COVID-19 Vaccination Strategy and Its Impact on the Global Pandemic. Risk Manag. Health Policy 2021, 14, 4649–4655. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, Y.; Zhang, H.; Jing, R.; Lai, X.; Feng, H.; Knoll, M.D.; Fang, H. Willingness to pay and financing preferences for COVID-19 vaccination in China. Vaccine 2021, 39, 1968–1976. [Google Scholar] [CrossRef]
- Dong, D.; Xu, R.H.; Wong, E.L.; Hung, C.T.; Feng, D.; Feng, Z.; Yeoh, E.K.; Wong, S.Y. Public preference for COVID-19 vaccines in China: A discrete choice experiment. Health Expect. 2020, 23, 1543–1578. [Google Scholar] [CrossRef] [PubMed]
- Leng, A.; Maitland, E.; Wang, S.; Nicholas, S.; Liu, R.; Wang, J. Individual preferences for COVID-19 vaccination in China. Vaccine 2021, 39, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Beijing Municipal Commission of Development and Reform. Kexing New Crown Vaccine Has Become the Focus of the Chinese Brand Day for Beijing Pavilion. Available online: http://fgw.beijing.gov.cn/gzdt/fgzs/mtbdx/bzwlxw/202105/t20210511_2386120.htm (accessed on 13 February 2022). (In Chinese)
- The Annual Production Capacity of Sinopharm’s New Crown Vaccine Will Reach 3 Billion Doses This Year. Available online: http://finance.people.com.cn/n1/2021/0602/c1004-32120760.html (accessed on 21 February 2022). (In Chinese).
- The Joint Prevention and Control Mechanism of the State Council News Conference 9 January 2021. Available online: http://www.gov.cn/xinwen/gwylflkjz144/index.htm (accessed on 22 February 2022). (In Chinese)
- Liu, R.; Zhang, Y.; Nicholas, S.; Leng, A.; Maitland, E.; Wang, J. COVID-19 Vaccination Willingness among Chinese Adults under the Free Vaccination Policy. Vaccines 2021, 9, 292. [Google Scholar] [CrossRef]
- Gao, H.; Guo, D.; Wu, J.; Zhao, Q.; Li, L. Changes of the Public Attitudes of China to Domestic COVID-19 Vaccination After the Vaccines Were Approved: A Semantic Network and Sentiment Analysis Based on Sina Weibo Texts. Front. Public Health 2021, 9, 723015. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, Z.; Tao, X.; Huang, L.; Zhu, E.; Yu, L.; Zhang, M. COVID-19 vaccine hesitancy among Chinese residents under the free vaccination policy. Rev. Assoc. Med. Bras. 2021, 67, 1317–1321. [Google Scholar] [CrossRef]
- Huang, Y.; Su, X.; Xiao, W.; Wang, H.; Si, M.; Wang, W.; Gu, X.; Ma, L.; Li, L.; Zhang, S.; et al. COVID-19 vaccine hesitancy among different population groups in China: A national multicenter online survey. BMC Infect. Dis. 2022, 22, 153. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Han, X.; Shi, A.; Cheng, Z.; Mou, H. Addressing People’s Vaccine Hesitancy Will Be Helpful for COVID-19 Vaccination in China. Asia Pac. J. Public Health 2021, 33, 603–604. [Google Scholar] [CrossRef]
- Wang, C.; Han, B.; Zhao, T.; Liu, H.; Liu, B.; Chen, L.; Xie, M.; Liu, J.; Zheng, H.; Zhang, S.; et al. Vaccination willingness, vaccine hesitancy, and estimated coverage at the first round of COVID-19 vaccination in China: A national cross-sectional study. Vaccine 2021, 39, 2833–2842. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Silver Tarimo, C.; Wang, M.; Gu, J.; Wei, W.; Ma, M.; Zhao, L.; Mu, Z.; Miao, Y. COVID-19 Vaccine Hesitancy Among Chinese Population: A Large-Scale National Study. Front. Immunol. 2021, 12, 781161. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Nicholas, S.; Maitland, E.; Leng, A.; Liu, R. The Intention to Receive the COVID-19 Vaccine in China: Insights from Protection Motivation Theory. Vaccines 2021, 9, 445. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Liu, L.; Sun, J.; Yan, W.; Yuan, K.; Zheng, Y.B.; Lu, Z.A.; Liu, L.; Ni, S.Y.; Su, S.; et al. Public Willingness and Determinants of COVID-19 Vaccination at the Initial Stage of Mass Vaccination in China. Vaccines 2021, 9, 1172. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Gao, J.; Liu, X.; Mao, Y.; Wang, R.; Zheng, P.; Xiao, Q.; Jia, Y.; Fu, H.; et al. Health Belief Model Perspective on the Control of COVID-19 Vaccine Hesitancy and the Promotion of Vaccination in China: Web-Based Cross-sectional Study. J. Med. Internet Res. 2021, 23, e29329. [Google Scholar] [CrossRef]
- Liu, T.; He, Z.; Huang, J.; Yan, N.; Chen, Q.; Huang, F.; Zhang, Y.; Akinwunmi, O.M.; Akinwunmi, B.O.; Zhang, C.J.P.; et al. A Comparison of Vaccine Hesitancy of COVID-19 Vaccination in China and the United States. Vaccines 2021, 9, 649. [Google Scholar] [CrossRef]
- Huang, W.; Shao, X.; Wagner, A.; Chen, Y.; Guan, B.; Boulton, M.L.; Li, B.; Hu, L.; Lu, Y. COVID-19 vaccine coverage, concerns, and preferences among Chinese ICU clinicians: A nationwide online survey. Expert Rev. Vaccines 2021, 20, 1361–1367. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Wang, M.; Ma, Z. Attribute nonattendance in COVID-19 vaccine choice: A discrete choice experiment based on Chinese public preference. Health Expect. 2022, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Wang, Y.; Yan, H.H.; Gao, J.L. Public preference and vaccination willingness for COVID-19 vaccine in China. Fudan Univ. J. Med. Sci. 2021, 48, 578–585, 617. (In Chinese) [Google Scholar]
- Report for the Seventh National Census of Shandong Province, Shandong Province Bureau of Statistics. Available online: http://tjj.shandong.gov.cn/art/2021/5/21/art_6293_10287511.html (accessed on 18 February 2022).
- 2020 Shandong Cities GDP Release, Souhu. Available online: https://www.sohu.com/a/448734313_120927771 (accessed on 21 February 2022). (In Chinese).
- China’s New Qingdao Jiaodong International Airport Starts Operations, Airport Technology. Available online: https://www.airport-technology.com/news/qingdao-jiaodong-international-airport/ (accessed on 21 February 2022).
- Jiang, H. Research on Qingdao Port Logistics Development under the Background of “Belt and Road”. IOP Conf. Ser. Earth Environ. Sci. 2019, 330, 052017. [Google Scholar] [CrossRef]
- China Vaccinating Key Groups against COVID-19, Xinhua. Available online: http://www.xinhuanet.com/english/2021-01/04/c_139641409.htm (accessed on 21 February 2021). (In Chinese).
- The Vaccination Rate of New Crown Vaccine among People Aged 60–69 in Qingdao Has Exceeded 90%, and the City Has Received a Total of 24.1663 Million Doses. Available online: https://view.inews.qq.com/a/20220130A09HHN00 (accessed on 21 February 2022). (In Chinese).
- COVID-19 Outbreak Shandong Real-Time Tracking, Sina. Available online: https://news.sina.cn/project/fy2020/yq_province.shtml?province=shandong (accessed on 22 February 2022). (In Chinese).
- Kelly, H.; Carville, K.; Grant, K.; Jacoby, P.; Tran, T.; Barr, I. Estimation of influenza vaccine effectiveness from routine surveillance data. PLoS ONE 2009, 4, e5079. [Google Scholar] [CrossRef]
- The Vaccination Protection Rate of HIN1 Vaccine Exceeds 85%. Persistence Is Still under Observation. Available online: https://www.ixueshu.com/document/756eb25879eaa0d6318947a18e7f9386.html (accessed on 21 November 2009).
- How Well Does the Flu Vaccine Work, CDD. Available online: https://www.cdc.gov/flu/about/qa/vaccineeffect.htm (accessed on 12 May 2017).
- More than 150 Cases of HINI Vaccination Have Been Reported Abnormal Reaction in More than 300,000 People Nationwide. Available online: chinagate.cn/infocus/2009-10/10/content_18678045.htm (accessed on 10 October 2009). (In Chinese).
- Orme, B. Getting Started with Conjoint Analysis: Strategies for Product Design and Pricing Research, 2nd ed.; Research Publishers LLC: Los Angeles, CA, USA, 2010. [Google Scholar]
- Hole, A.R.; Kolstad, J.R. Mixed logit estimation of willingness to pay distributions: A comparison of models in preference and WTP space using data from a health-related choice experiment. Empir. Econ. 2012, 42, 445–469. [Google Scholar] [CrossRef]
- Greene, W.H.; Hensher, D.A. A latent class model for discrete choice analysis: Contrasts with mixed logit. Transp. Res. Part B Methodol. 2003, 37, 681–698. [Google Scholar] [CrossRef]
- Hauber, A.B.; González, J.M.; Groothuis-Oudshoorn, C.G.; Prior, T.; Marshall, D.A.; Cunningham, C.; IJzerman, M.J.; Bridges, J.F. Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR conjoint analysis good research practices task force. Value Health 2016, 19, 300–315. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, E.S.; Holtgrave, D.R.; Dorabawila, V.; Conroy, M.; Greene, D.; Lutterloh, E.; Backenson, B.; Hoefer, D.; Morne, J.; Bauer, U.; et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status—New York, 3 May–25 July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.Y.; Wang, W.B.; Gao, R.D.; Zhou, A.M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Attributes | Levels | Descriptions |
|---|---|---|
| Vaccine effectiveness | 40% | Protects 40% of vaccinated |
| 60% | Protects 60% of vaccinated | |
| 85% | Protects 85% of vaccinated | |
| Self-assessed vaccine-related side effects | 50/100,000 | 50 out of 100,000 risk of severe side effects |
| 10/100,000 | 10 out of 100,000 risk of severe side effects | |
| 1/100,000 | 1 out of 100,000 risk of severe side effects | |
| Vaccination sites | Level 1 | village clinic or community health station |
| Level 2 | township or community health centre | |
| Level 3 | county hospital and above | |
| Duration of vaccine protection | six months | Six months of vaccine protection |
| one year | One year of vaccine protection | |
| More than two years | More than two years | |
| Acquaintances vaccinated | 30% | 30% of your family, friends and acquaintances already vaccinated |
| 60% | 60% of your family, friends and acquaintances already vaccinated | |
| 90% | 90% of your family, friends and acquaintances already vaccinated | |
| Risk perception (probability including yourself and acquaintances being infected with COVID-19) | 100/100,000 | 100 out of 100,000 contracting COVID-19 |
| 6/100,000 | 6 out of 100,000 contracting COVID-19 | |
| 1/100,000 | 1 out of 100,000 contracting COVID-19 |
| Q1 | Vaccine A | Vaccine B |
|---|---|---|
| Vaccine effectiveness | 40% | 60% |
| Vaccine-related side effects | 10/100,000 | 50/100,000 |
| Vaccination sites | Level 1 | Level 2 |
| Duration of vaccine protection | one year | six months |
| Acquaintances vaccinated | 30% | 60% |
| The probability of infection with COVID-19 | 100/100,000 | 6/100,000 |
| Which vaccine do you prefer? |
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Male | 453 | 53.357 |
| Female | 396 | 46.643 |
| Age | ||
| Age 18–30 | 168 | 19.788 |
| Age 31–50 | 416 | 48.999 |
| Age 51+ | 265 | 31.213 |
| Marital status | ||
| married | 660 | 77.739 |
| unmarried/widowed/divorced | 189 | 22.261 |
| Residence | ||
| urban area | 301 | 35.453 |
| rural area | 548 | 64.547 |
| Years of education | ||
| low education (≤12 years) | 481 | 56.655 |
| medium education (13— ≤16years) | 263 | 30.978 |
| high education (>16 years) | 105 | 12.367 |
| Occupation | ||
| farmer | 257 | 30.271 |
| government/public institution staff | 180 | 21.201 |
| company employees (including migrant workers, individual businesses, etc.) | 290 | 34.158 |
| Other (including retired, student) | 122 | 14.370 |
| Household yearly income | ||
| low income level | 114 | 13.428 |
| medium income level | 637 | 75.029 |
| high income level | 98 | 11.543 |
| Elderly at home | 107 | 12.603 |
| Children at home | 200 | 23.557 |
| Attribute | ß | SE | p Values | 95% CI |
|---|---|---|---|---|
| Vaccine effectiveness (reference = 40%) | ||||
| 60% | 0.423 | 0.036 | 0.000 | 0.351, 0.494 |
| 85% | 0.806 | 0.041 | 0.000 | 0.727, 0.886 |
| Vaccine-related side effects (reference = 50/100,000) | ||||
| 10/100,000 | 0.251 | 0.035 | 0.000 | 0.182, 0.320 |
| 1/100,000 | 0.432 | 0.037 | 0.000 | 0.358, 0.507 |
| Vaccination sites (reference = Level 1) | ||||
| level 2 | 0.141 | 0.037 | 0.000 | 0.067, 0.214 |
| level 3 | −0.067 | 0.036 | 0.063 | −0.138, 0.004 |
| Duration of vaccine protection (reference = six months) | ||||
| one year | 0.245 | 0.037 | 0.000 | 0.173, 0.316 |
| more than two years | 0.350 | 0.036 | 0.000 | 0.279, 0.421 |
| Acquaintances vaccinated (reference = 30%) | ||||
| 60% | 0.031 | 0.037 | 0.409 | −0.042, 0.103 |
| 90% | 0.093 | 0.037 | 0.011 | 0.021, 0.165 |
| The probability of respondents/acquaintances infected (reference = 1/100,000) | ||||
| 6/100,000 | 0.221 | 0.037 | 0.000 | 0.148, 0.293 |
| 100/100,000 | 0.346 | 0.036 | 0.000 | 0.274, 0.417 |
| Model fit | ||||
| Observations = 13,584 | ||||
| Respondents = 849 | ||||
| Prob > chi2 = 0.000 | ||||
| Pseudo R2 = 0.0857 | ||||
| LR chi2(13) = 4304.62 | ||||
| AIC = 8633.24 | ||||
| BIC = 8723.44 | ||||
| Variables | ß | SD | p Values | 95% CI |
|---|---|---|---|---|
| Vaccine effectiveness (reference = 40%) | ||||
| 60% | 0.543 | 0.005 | 0.001 | 0.465, 0.621 |
| 85% | 1.537 | 1.164 | 0.001 | 1.418, 1.656 |
| Vaccine-related side effects (reference = 50/100,000) | ||||
| 10/100,000 | 0.658 | 0.535 | 0.001 | 0.581, 0.736 |
| 1/100,000 | 1.402 | 1.326 | 0.001 | 1.285, 1.519 |
| Vaccination sites (reference = level 1) | ||||
| level 2 | 0.055 | 0.885 | 0.187 | −0.027, 0.137 |
| level 3 | −0.361 | 0.637 | 0.001 | −0.445, −0.276 |
| one year | −0.048 | −0.268 | 0.197 | −0.122, 0.025 |
| more than two years | 0.096 | 0.408 | 0.013 | 0.020, 0.171 |
| Acquaintances vaccinated (reference = 30%) | ||||
| 60% | 0.226 | 0.014 | 0.001 | 0.149, 0.304 |
| 90% | 0.440 | 0.058 | 0.001 | 0.361, 0.519 |
| 6/100,000 | 0.252 | −0.392 | 0.001 | 0.175, 0.329 |
| 100/100,000 | 0.374 | 0.744 | 0.001 | 0.290, 0.459 |
| Attribute | Class 1 | Class 2 | Class 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ß | SE | p Value | ß | SE | p Value | ß | SE | p Value | |
| Vaccine effectiveness (reference = 40%) | |||||||||
| 60% | 3.007 | 0.333 | 0.000 | −0.052 | 0.080 | 0.502 | 0.902 | 0.114 | 0.000 |
| 85% | 6.344 | 0.771 | 0.000 | 0.060 | 0.086 | 0.488 | 1.351 | 0.149 | 0.000 |
| Vaccine-related side effects (reference = 50/100,000) | |||||||||
| 10/100,000 | −0.027 | 0.390 | 0.946 | 0.633 | 0.070 | 0.000 | 0.261 | 0.089 | 0.000 |
| 1/100,000 | 0.093 | 0.349 | 0.791 | 1.247 | 0.095 | 0.000 | −0.442 | 0.119 | 0.000 |
| Vaccination sites (reference = first level) | |||||||||
| second level | 3.317 | 0.859 | 0.000 | 0.276 | 0.072 | 0.000 | −0.388 | 0.112 | 0.000 |
| third level | −2.244 | 0.278 | 0.000 | −0.181 | 0.079 | 0.021 | 0.684 | 0.113 | 0.000 |
| Duration of vaccine protection (reference = six months) | |||||||||
| one year | 1.732 | 0.264 | 0.000 | 0.082 | 0.073 | 0.259 | 0.098 | 0.105 | 0.350 |
| more than two years | 0.475 | 0.326 | 0.145 | 0.197 | 0.072 | 0.005 | 0.991 | 0.116 | 0.000 |
| Acquaintances vaccinated (reference = 30%) | |||||||||
| 60% | −1.259 | 0.366 | 0.001 | 0.081 | 0.067 | 0.228 | 0.044 | 0.103 | 0.679 |
| 90% | −2.354 | 0.348 | 0.000 | 0.474 | 0.075 | 0.000 | −0.112 | 0.107 | 0.293 |
| The probability of individuals/acquaintances infected (reference = 1/100,000) | |||||||||
| 6/100,000 | 0.667 | 0.476 | 0.117 | −0.536 | 0.077 | 0.000 | 0.10 | 0.111 | 0.363 |
| 100/100,000 | 2.588 | 0.554 | 0.000 | −1.133 | 0.088 | 0.000 | −0.081 | 0.093 | 0.386 |
| Class probability model | |||||||||
| age | 0.041 | 0.159 | 0.795 | −0.281 | 0.131 | 0.032 | - | - | - |
| education | 0.213 | 0.181 | 0.239 | 0.128 | 0.144 | 0.374 | - | - | - |
| urban/rural residence | 0.477 | 0.238 | 0.045 | 0.528 | 0.186 | 0.005 | - | - | - |
| average monthly household income | −0.185 | 0.209 | 0.374 | −0.431 | 0.170 | 0.011 | - | - | - |
| elderly at home | −0.131 | 0.297 | 0.658 | −0.746 | 0.316 | 0.018 | - | - | - |
| children at home | −0.110 | 0.266 | 0.678 | −0.030 | 0.232 | 0.870 | - | - | - |
| constant | −1.126 | 0.772 | 0.144 | 0.894 | 0.525 | 0.089 | - | - | - |
| Class probability | |||||||||
| Average | 0.220 | 0.468 | 0.312 | ||||||
| Model fit | |||||||||
| Observations = 13584 | |||||||||
| Respondents = 849 | |||||||||
| AIC = 7740.432 | |||||||||
| BIC = 7977.635 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Nicholas, S.; Maitland, E.; Leng, A. Individual Preferences for COVID-19 Vaccination under the China’s 2021 National Vaccination Policy: A Discrete Choice Experiment Study. Vaccines 2022, 10, 543. https://doi.org/10.3390/vaccines10040543
Wang S, Nicholas S, Maitland E, Leng A. Individual Preferences for COVID-19 Vaccination under the China’s 2021 National Vaccination Policy: A Discrete Choice Experiment Study. Vaccines. 2022; 10(4):543. https://doi.org/10.3390/vaccines10040543
Chicago/Turabian StyleWang, Siyuan, Stephen Nicholas, Elizabeth Maitland, and Anli Leng. 2022. "Individual Preferences for COVID-19 Vaccination under the China’s 2021 National Vaccination Policy: A Discrete Choice Experiment Study" Vaccines 10, no. 4: 543. https://doi.org/10.3390/vaccines10040543
APA StyleWang, S., Nicholas, S., Maitland, E., & Leng, A. (2022). Individual Preferences for COVID-19 Vaccination under the China’s 2021 National Vaccination Policy: A Discrete Choice Experiment Study. Vaccines, 10(4), 543. https://doi.org/10.3390/vaccines10040543







