Frequent Onsets of Cellulitis in Lower Limbs with Lymphedema Following COVID-19 mRNA Vaccination
Abstract
:1. Introduction
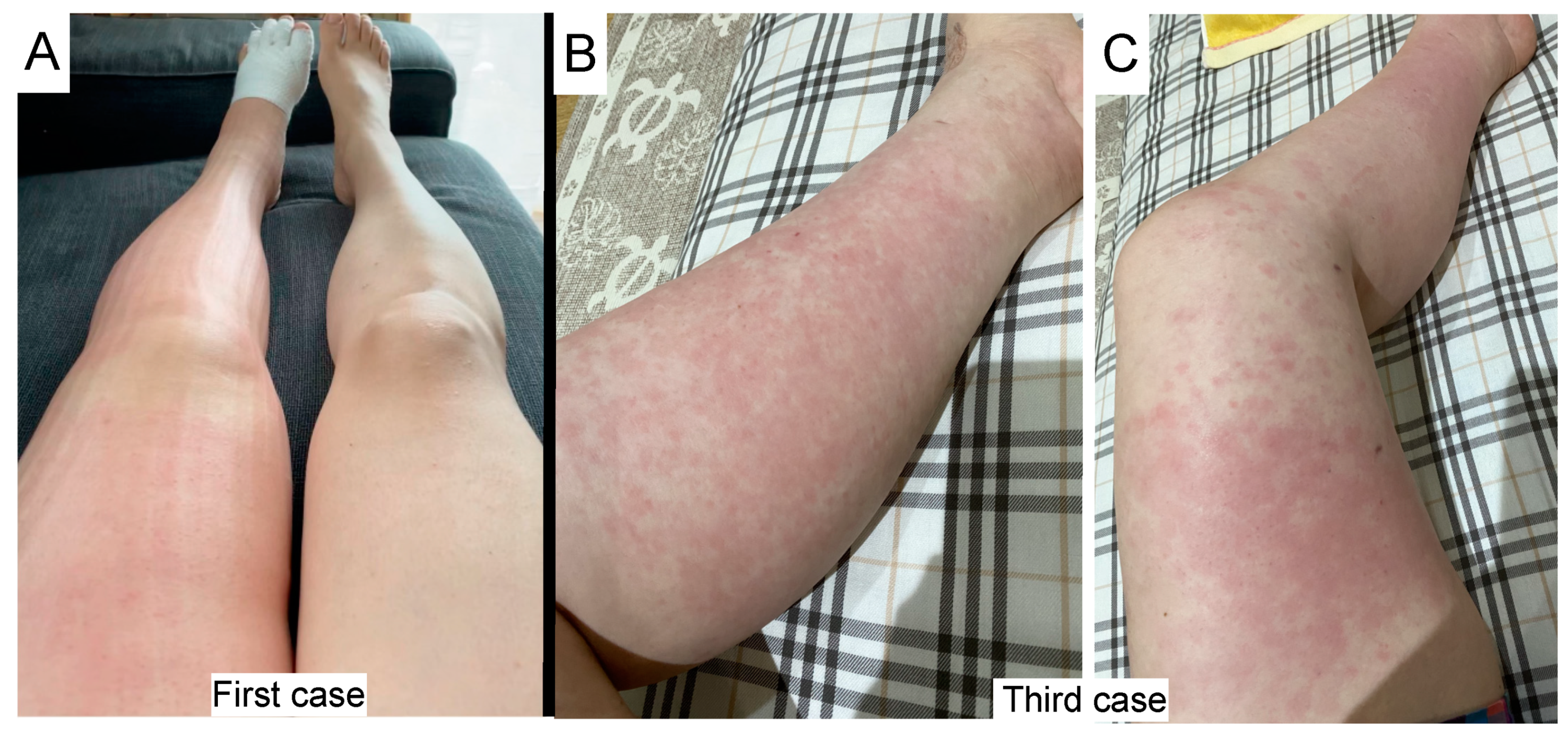
2. Cases
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Arora, H.; Lewis, S.; Gunasekaran, K.; Muruganandam, M.; Nagaraju, S.; Padmanabhan, P. COVID-19 vaccine-induced cellulitis and myositis. Clevel. Clin. J. Med. 2021, 88, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fathy, R.; McMahon, D.E.; Freeman, E.E. COVID-19 Vaccines and the Skin: The Landscape of Cutaneous Vaccine Reactions Worldwide. Dermatol. Clin. 2021, 39, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Gajula, V.; Madathala, R.R.; Chennaiahgari, N.; Malayala, S.V. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: A randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J. Med. Virol. 2021, 93, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Munavalli, G.G.; Guthridge, R.; Knutsen-Larson, S.; Brodsky, A.; Matthew, E.; Landau, M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: A challenging clinical conundrum in diagnosis and treatment. Arch. Dermatol. Res. 2022, 314, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Mendez, U.; Gilbert, R.J.; Keim, A.P.; Goldman, J. Increased hyaluronan expression at distinct time points in acute lymphedema. Lymphat. Res. Biol. 2012, 10, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukita, Y.; Okazaki, T.; Ebihara, S.; Komatsu, R.; Nihei, M.; Kobayashi, M.; Hirano, T.; Sugiura, H.; Tamada, T.; Tanaka, N.; et al. Beneficial effects of sunitinib on tumor microenvironment and immunotherapy targeting death receptor5. Oncoimmunology 2019, 8, e1543526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebedev, L.; Sapojnikov, M.; Wechsler, A.; Varadi-Levi, R.; Zamir, D.; Tobar, A.; Levin-Iaina, N.; Fytlovich, S.; Yagil, Y. Minimal Change Disease Following the Pfizer-BioNTech COVID-19 Vaccine. Am. J. Kidney Dis. 2021, 78, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Okazaki, T.; Ebihara, S.; Kobayashi, M.; Niu, K.; Gui, P.; Tamai, T.; Nukiwa, T.; Yamaya, M.; Kikuchi, T.; et al. Chronic inflammation, lymphangiogenesis, and effect of an anti-VEGFR therapy in a mouse model and in human patients with aspiration pneumonia. J. Pathol. 2015, 235, 632–645. [Google Scholar] [CrossRef] [PubMed]
| Age | Vaccination Type | History of Cellulitis Before the Vaccination | Repetition of Cellulitis | Onset after First or Second Vaccination | |
|---|---|---|---|---|---|
| Case 1 | 52 | BNT162b2 mRNA | No | No | First vaccination |
| Case 2 | 52 | mRNA-1273 | Yes | Yes | First vaccination |
| Case 3 | 49 | BNT162b2 mRNA | Yes | No | Second vaccination |
| Case 4 | 45 | mRNA-1273 | No | Yes | First vaccination |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okazaki, T.; Matashiro, M.; Kodama, G.; Tshubota, T.; Furusawa, Y.; Izumi, S.-I. Frequent Onsets of Cellulitis in Lower Limbs with Lymphedema Following COVID-19 mRNA Vaccination. Vaccines 2022, 10, 517. https://doi.org/10.3390/vaccines10040517
Okazaki T, Matashiro M, Kodama G, Tshubota T, Furusawa Y, Izumi S-I. Frequent Onsets of Cellulitis in Lower Limbs with Lymphedema Following COVID-19 mRNA Vaccination. Vaccines. 2022; 10(4):517. https://doi.org/10.3390/vaccines10040517
Chicago/Turabian StyleOkazaki, Tatsuma, Momoko Matashiro, Gaku Kodama, Takeshi Tshubota, Yoshihito Furusawa, and Shin-Ichi Izumi. 2022. "Frequent Onsets of Cellulitis in Lower Limbs with Lymphedema Following COVID-19 mRNA Vaccination" Vaccines 10, no. 4: 517. https://doi.org/10.3390/vaccines10040517
APA StyleOkazaki, T., Matashiro, M., Kodama, G., Tshubota, T., Furusawa, Y., & Izumi, S.-I. (2022). Frequent Onsets of Cellulitis in Lower Limbs with Lymphedema Following COVID-19 mRNA Vaccination. Vaccines, 10(4), 517. https://doi.org/10.3390/vaccines10040517






