Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
- –
- Cohort 1: HCP uninfected after COVID-19 vaccination named “COVID-19 Negative”;
- –
- Cohort 2: HCP infected after COVID-19 vaccination named “COVID-19 Positive”.
2.2. Data and Sample Collection
2.3. Data Source
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 July 2021).
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 1, 585354. [Google Scholar] [CrossRef] [PubMed]
- Awadasseid, A.; Wu, Y.; Tanaka, Y.; Zhang, W. Current advances in the development of SARS-CoV-2 vaccines. Int. J. Biol. Sci. 2021, 17, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- Dror, A.A.; Eisenbach, N.; Taiber, S.; Morozov, N.G.; Mizrachi, M.; Zigron, A.; Srouji, S.; Sela, E. Vaccine hesitancy: The next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020, 35, 775–779. [Google Scholar] [CrossRef]
- European Medicine Agency. EMA Receives Application for Conditional Marketing Authorisation of COVID-19 mRNA Vaccine BNT162b2. Available online: https://www.ema.europa.eu/en/news/ema-receives-application-conditional-marketing-authorisation-covid-19-mrna-vaccine-bnt162b2 (accessed on 1 July 2021).
- Italian Medicine Agency (AIFA). BioNTech/Pfizer Vaccine Authorised Answers to Frequently Asked Questions on AIFA’s Website. Available online: https://www.aifa.gov.it/en/-/autorizzato-il-vaccino-biontech-pfizer (accessed on 1 July 2021).
- European Medicine Agency. EMA Recommends COVID-19 Vaccine AstraZeneca for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu (accessed on 1 July 2021).
- European Medicine Agency. EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu. (accessed on 1 July 2021).
- Italian Medicine Agency. COVID-19: AIFA Authorizes Moderna Vaccine. Available online: https://www.aifa.gov.it/en/-/covid-19-aifa-autorizza-vaccino-moderna (accessed on 1 July 2021).
- European Medicine Agency. COVID-19 Vaccine Janssen. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-janssen (accessed on 1 July 2021).
- Italian Medicine Agency. AIFA Approves Janssen Vaccine. Available online: https://www.aifa.gov.it/en/-/aifa-approva-il-vaccino-janssen (accessed on 1 July 2021).
- Amit, S.; Beni, S.A.; Biber, A.; Grinberg, A.; Leshem, E.; Regev-Yochay, G. Postvaccination COVID-19 among Healthcare Workers, Israel. Emerg. Infect. Dis. 2021, 27, 1220–1222. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahman, K.M.; Sun, Y.; Qureshi, M.O.; Abdi, I.; Chughtai, A.A.; Seale, H. Current knowledge of COVID-19 and infection prevention and control strategies in healthcare settings: A global analysis. Infect. Control Hosp. Epidemiol. 2020, 41, 1196–1206. [Google Scholar] [CrossRef]
- Varia, M.; Wilson, S.; Sarwal, S.; McGeer, A.; Gournis, E.; Galanis, E.; Henry, B. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ 2003, 169, 285–292. [Google Scholar]
- Oh, M.D.; Park, W.B.; Park, S.W.; Choe, P.G.; Bang, J.H.; Song, K.H.; Kim, E.S.; Kim, H.B.; Kim, N.J. Middle East respiratory syndrome: What we learned from the 2015 outbreak in the Republic of Korea. Korean J. Intern. Med. 2018, 33, 233–246. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Rhee, C.; Kanjilal, S.; Baker, M.; Klompas, M. Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectivity: When Is It Safe to Discontinue Isolation? Clin. Infect. Dis. 2021, 72, 1467–1474. [Google Scholar] [CrossRef]
- Perrella, A.; Brita, M.; Coletta, F.; Cotena, S.; De Marco, G.; Longobardi, A.; Sala, C.; Sannino, D.; Tomasello, A.; Perrella, M.; et al. SARS-CoV-2 in Urine May Predict a Severe Evolution of COVID-19. J. Clin. Med. 2021, 10, 4061. [Google Scholar] [CrossRef]
- Perrella, A.; Orlando, V.; Trama, U.; Bernardi, F.F.; Menditto, E.; Coscioni, E. Pre-Exposure Prophylaxis with Hydroxychloroquine Does Not Prevent COVID-19 nor Virus Related Venous Thromboembolism. Viruses 2021, 13, 2052. [Google Scholar] [CrossRef]
- Orlando, V.; Coscioni, E.; Guarino, I.; Mucherino, S.; Perrella, A.; Trama, U.; Limongelli, G.; Menditto, E. Drug-utilisation profiles and COVID-19. Sci. Rep. 2021, 11, 8913. [Google Scholar] [CrossRef]
- Orlando, V.; Rea, F.; Savaré, L.; Guarino, I.; Mucherino, S.; Perrella, A.; Trama, U.; Coscioni, E.; Menditto, E.; Corrao, G. Development and validation of a clinical risk score to predict the risk of SARS-CoV-2 infection from administrative data: A population-based cohort study from Italy. PLoS ONE 2021, 16, e0237202. [Google Scholar] [CrossRef]
- Bliek-Bueno, K.; Mucherino, S.; Poblador-Plou, B.; González-Rubio, F.; Aza-Pascual-Salcedo, M.; Orlando, V.; Clerencia-Sierra, M.; Ioakeim-Skoufa, I.; Coscioni, E.; Carmona-Pírez, J.; et al. Baseline Drug Treatments as Indicators of Increased Risk of COVID-19 Mortality in Spain and Italy. Int. J. Environ. Res. Public Health 2021, 18, 11786. [Google Scholar] [CrossRef]
- Scala, D.; Menditto, E.; Caruso, G.; Monetti, V.M.; Orlando, V.; Guerriero, F.; Buonomo, G.; Caruso, D.; D’Avino, M. Are you more concerned about or relieved by medicines? An explorative randomized study of the impact of telephone counseling by pharmacists on patients’ beliefs regarding medicines and blood pressure control. Patient Educ Couns 2018, 101, 679–686. [Google Scholar] [PubMed]
- Perrella, A.; Carannante, N.; Berretta, M.; Rinaldi, M.; Maturo, N.; Rinaldi, L. Novel Coronavirus 2019 (SARS-CoV2): A global emergency that needs new approaches? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2162–2164. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, D.; Gonzalez-Parra, G.; Villanueva, R.J. Analysis of Key Factors of a SARS-CoV-2 Vaccination Program: A Mathematical Modeling Approach. Epidemiologia 2021, 2, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Tu, X.Y.; Liu, M.; Liang, Z.W.; Chen, J.N.; Li, J.J.; Jiang, L.G.; Xing, F.Q.; Jiang, Y. Efficacy and safety of COVID-19 vaccines: A systematic review. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Feaster, M.; Goh, Y.Y. High proportion of asymptomatic SARS-CoV-2 infections in 9 long-term care facilities, Pasadena, California, USA, April 2020. Emerg. Infect. Dis. 2020, 26, 2416–2419. [Google Scholar] [CrossRef]
- Akinbami, L.J.; Vuong, N.; Petersen, L.R.; Sami, S.; Patel., A.; Lukacs, S.L.; Mackey, L.; Grohskopf, L.A.; Shehu, A.; Atas, J. SARS-CoV-2 seroprevalence among healthcare, first response, and public safety personnel, Detroit metropolitan area, Michigan, USA, May–June 2020. Emerg. Infect. Dis. 2020, 26, 2863–2871. [Google Scholar] [CrossRef]
- Calcagno, A.; Ghisetti, V.; Emanuele, T.; Trunfio, M.; Faraoni, S.; Boglione, L.; Burdino, E.; Audagnotto, S.; Lipani, F.; Nigra, M.; et al. Risk for SARS-CoV-2 infection in healthcare workers, Turin, Italy. Emerg. Infect. Dis. 2021, 27, 303–305. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNAbased COVID-19 vaccine candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Figueiredo, J.C.; Eyk, J.E.V.; Braun, J.G.; Cheng, S.; Sobhani, K. Prior COVID-19 Infection and Antibody Response to Single Versus Double Dose mRNA SARS-CoV-2 Vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
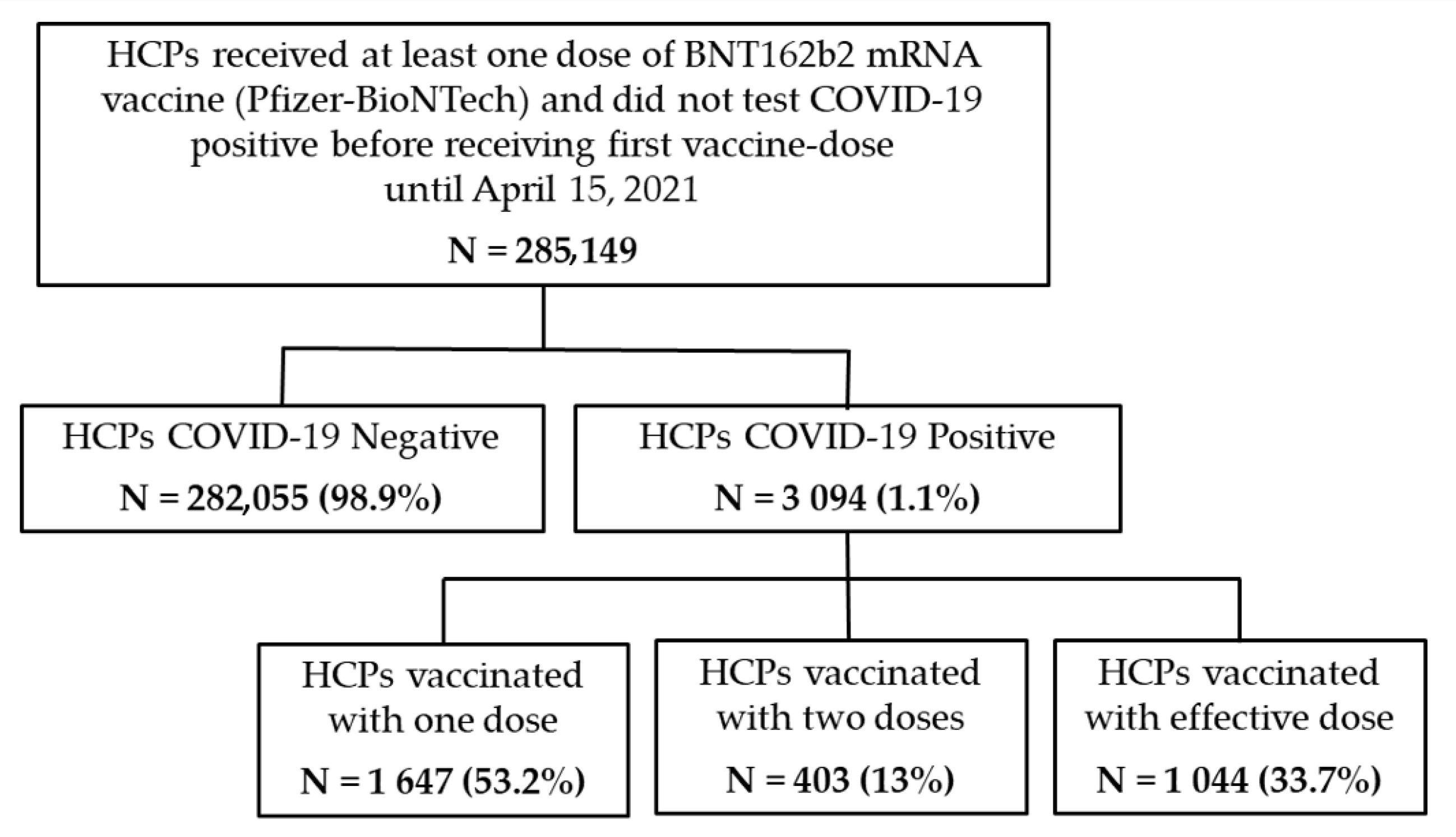
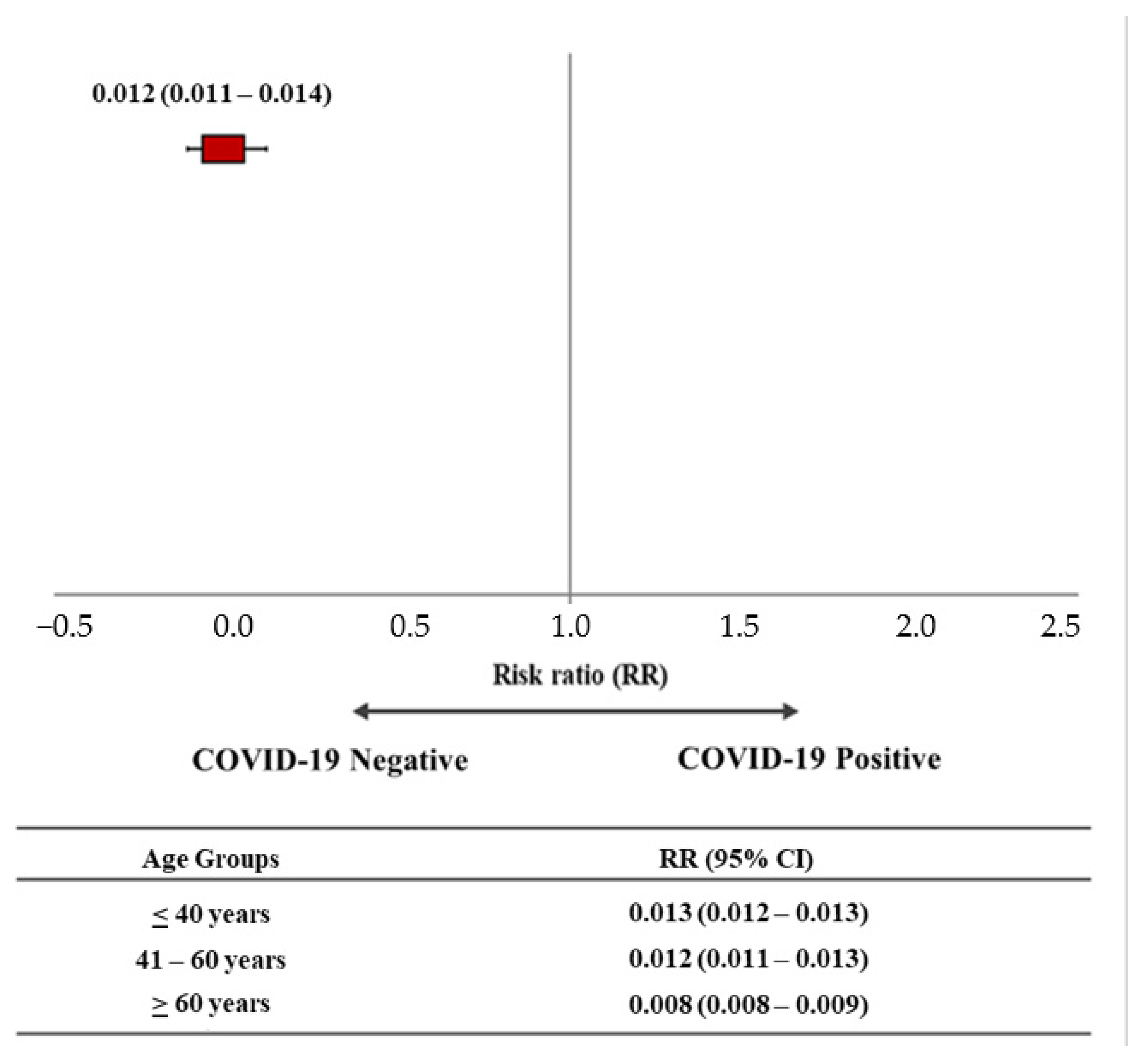
| COVID-19 Negative | COVID-19 Positive | Total | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total | 282,055 (98.9) | 3094 (1.1) | 285,149 |
| Sex | |||
| Male | 140,137 (98.9) | 1497 (1.1) | 141,634 (49.7) |
| Female | 141,918 (98.8) | 1597 (1.2) | 143,515 (50.3) |
| Mean Age (±SD) | 52 (±14) | 55 (±94) | 52 (±17) |
| Age Groups | |||
| ≤40 years | 66,121 (98.7) | 899 (1.3) | 67,020 (2.4) |
| 41–60 years | 114,413 (98.7) | 1372 (1.3) | 115,785 (40.6) |
| ≥60 years | 101,521 (99.2) | 823 (0.8) | 102,344 (35.9) |
| Symptoms and Duration of COVID-19 | Vaccination Status N (%) | Overall N (%) | ||
|---|---|---|---|---|
| 1st Vaccine Dose | 2nd Vaccine Dose | Effective Dose * | ||
| 1647 (53.2) | 403 (13.0) | 1044 (33.7) | 3094 | |
| Disease Symptoms (%) ° | ||||
| Asymptomatic | 1368 (83.1) | 354 (87.8) | 744 (71.3) | 2466 (79.7) |
| Paucysinintomatic | 77 (4.7) | 9 (2.2) | 12 (1.1) | 98 (3.2) |
| Mild | 60 (3.6) | 8 (2.0) | 17 (1.6) | 85 (2.7) |
| Severe | 2 (0.1) | - | - | 2 (0.1) |
| Critical | 1 (0.1) | - | - | 1 (0.03) |
| Days from 1st positive test to 1st negative test (Mean; ±SD) | 13 (±9) | 11 (±8) | 10 (±9) | 12 (±8) |
| Vaccination Status | OR | 95% CI | p-Value ° |
|---|---|---|---|
| Effective dose * | Reference | Reference | Reference |
| 1st vaccine dose | 2.303 | 1.535–3.455 | 0.001 |
| 2nd vaccine dose | 1.133 | 0.324–1.763 | 0.654 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrella, A.; Mucherino, S.; Guarino, I.; Nerilli, M.; Maraolo, A.E.; Capoluongo, N.; Coscioni, E.; Trama, U.; Menditto, E.; Orlando, V. Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study. Vaccines 2022, 10, 511. https://doi.org/10.3390/vaccines10040511
Perrella A, Mucherino S, Guarino I, Nerilli M, Maraolo AE, Capoluongo N, Coscioni E, Trama U, Menditto E, Orlando V. Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study. Vaccines. 2022; 10(4):511. https://doi.org/10.3390/vaccines10040511
Chicago/Turabian StylePerrella, Alessandro, Sara Mucherino, Ilaria Guarino, Mariagiovanna Nerilli, Alberto Enrico Maraolo, Nicolina Capoluongo, Enrico Coscioni, Ugo Trama, Enrica Menditto, and Valentina Orlando. 2022. "Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study" Vaccines 10, no. 4: 511. https://doi.org/10.3390/vaccines10040511
APA StylePerrella, A., Mucherino, S., Guarino, I., Nerilli, M., Maraolo, A. E., Capoluongo, N., Coscioni, E., Trama, U., Menditto, E., & Orlando, V. (2022). Postvaccination SARS-CoV-2 Infections among Healthcare Professionals: A Real World Evidence Study. Vaccines, 10(4), 511. https://doi.org/10.3390/vaccines10040511







