Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases
Abstract
:1. Introduction
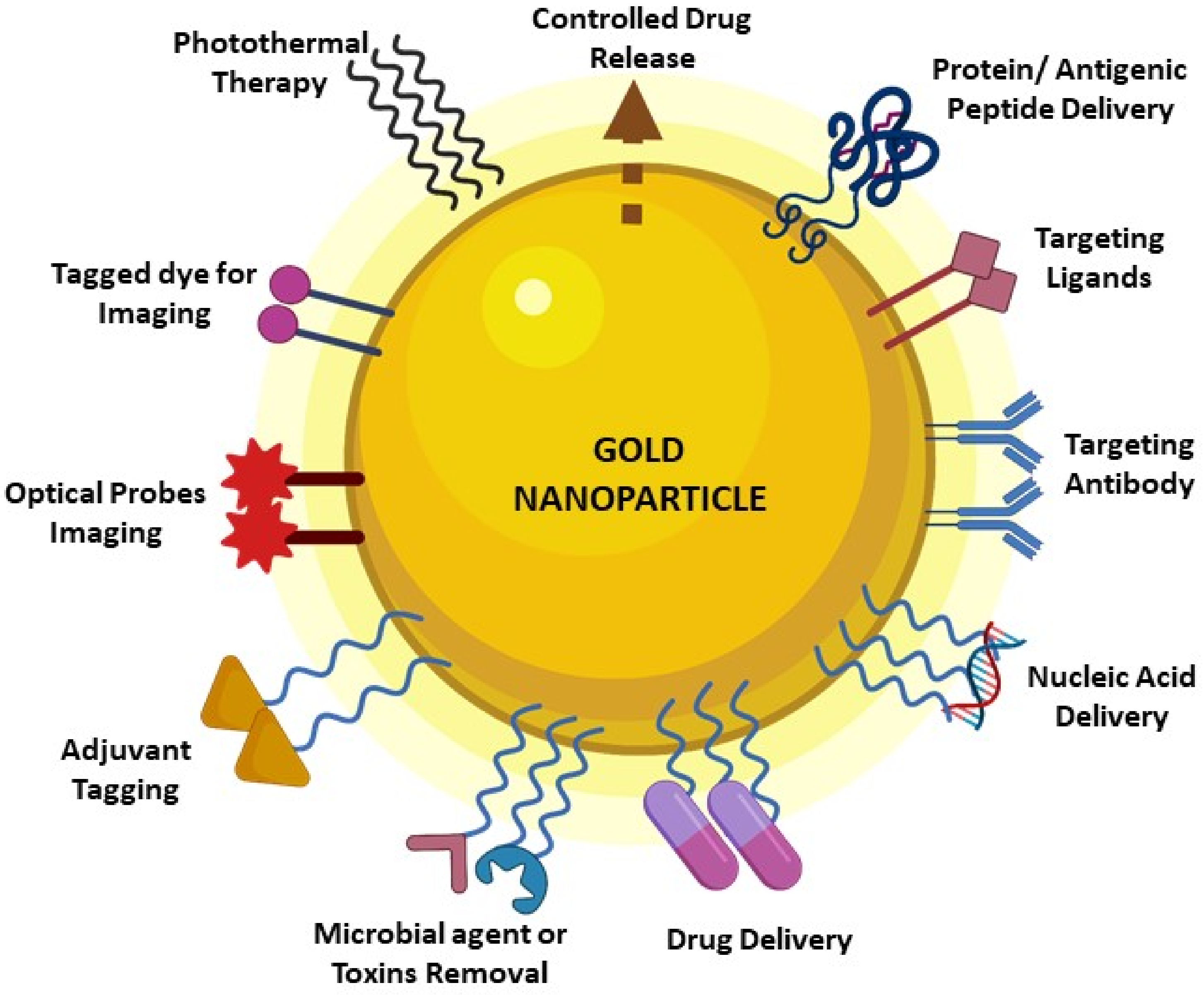
2. GNP Characteristics and Features Make It Indispensable in Vaccine Development Research
3. Shape and Size of GNP Influence Its Impact on the Immune System
4. Effect of GNPs on Dendritic Cells, Macrophages, and Natural Killer Cells
4.1. Dendritic Cells
4.2. Macrophages
4.3. Natural Killer Cells
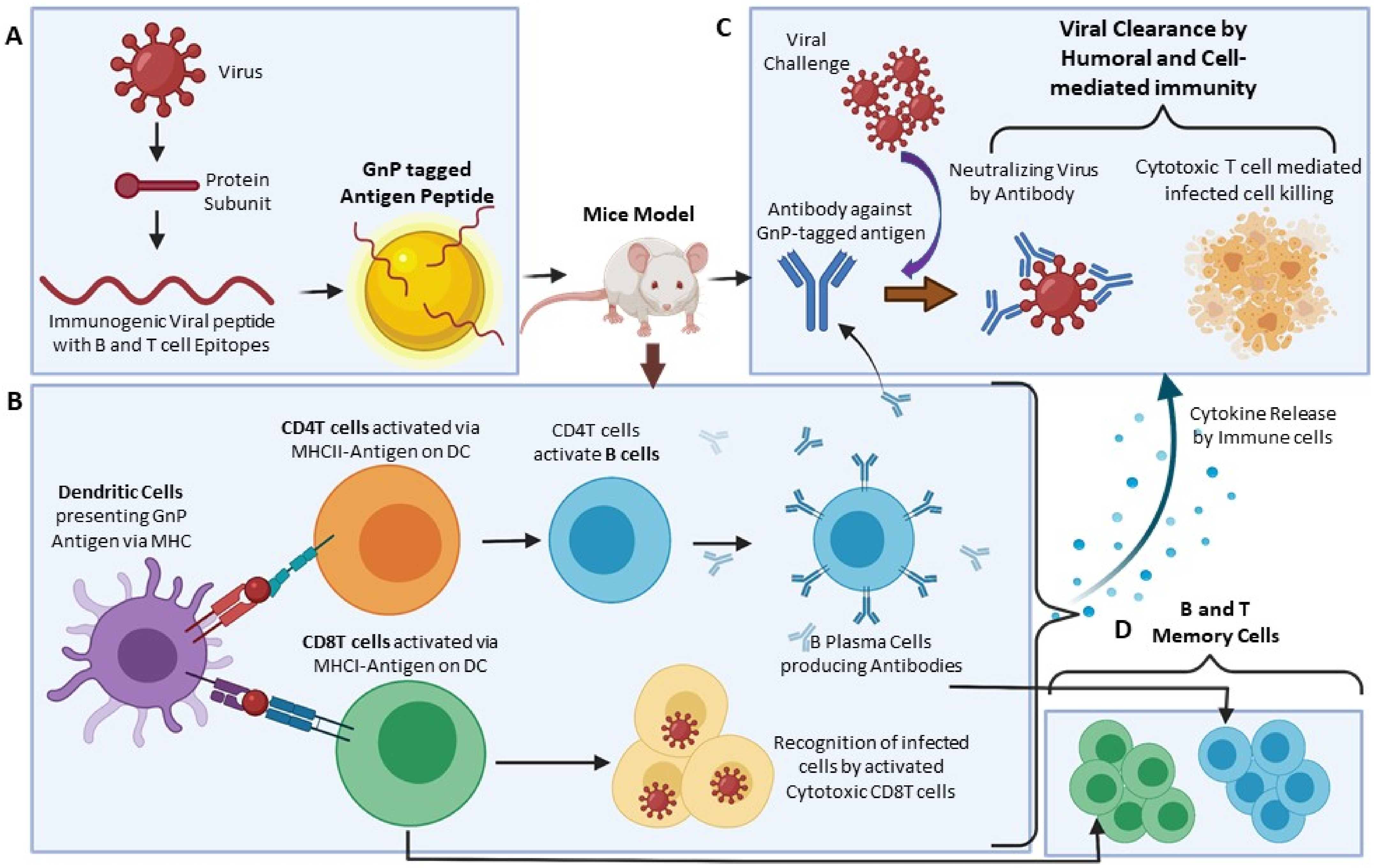
5. Use of GNP in Antiviral Immunization
5.1. HIV
5.2. Hepatitis B
5.3. Hepatitis C
5.4. Dengue
5.5. Influenza
6. Use of GNP in Antibacterial Immunization
7. Use of GNP in Anti-Parasitic Immunization
8. Limitations of the GNP
9. Discussion and Future Perspectives
- Biocompatible
- Easy synthesis process
- Size- and shape-dependent varied immune response
- Colloidal stability
- Optical properties
- Efficiency in molecule loading on the surface
- Surface functionalization flexibility and multi functionalization property
- Can be designed for targeted delivery and controlled release of drugs
- Photothermal conversion potential
- Inherent adjuvant potential
- Usage in imaging techniques
- High binding affinity with wide range of molecules
- Higher surface area to volume ratio
- Large surface energy and charge
- Non-biodegradable
- Non-porous
- Limited penetration depth
- Altered biodistribution profile upon surface modification
- Surface functionalization-mediated toxicity and pharmacokinetics issues
- Limited knowledge of impact on multiple cell types
- Clearance by macrophage phagocytosis system and renal pathway
- Accumulation in cellular organelles such as mitochondria, lysosomes, etc., hampering normal cellular metabolism and ROS production
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Comber, J.D.; Bamezai, A. Gold nanoparticles (GnPs): A new frontier in vaccine delivery. J. Nanomedine Biother. Discov. 2015, 5, 4. [Google Scholar]
- Salazar-González, J.A.; González-Ortega, O.; Rosales-Mendoza, S. Gold nanoparticles and vaccine development. Expert Rev. Vaccines 2015, 14, 1197–1211. [Google Scholar] [PubMed]
- Fadeel, B. Hide and seek: Nanomaterial interactions with the immune system. Front. Immunol. 2019, 10, 133. [Google Scholar]
- Frey, M.; Bobbala, S.; Karabin, N.; Scott, E. Influences of nanocarrier morphology on therapeutic immunomodulation. Nano-Med. 2018, 13, 1795–1811. [Google Scholar]
- Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Immunological basis for enhanced immunity of nanoparticle vaccines. Expert Rev. Vac.-Cines 2019, 18, 269–280. [Google Scholar]
- Fai, T.K.; Kumar, P.V. Revolution in the Synthesis, Physio-chemical and Biological Characterization of Gold Nanoplatform. Curr. Pharm. Des. 2021, 27, 2482–2504. [Google Scholar] [CrossRef] [PubMed]
- Personick, M.; Langille, M.R.; Zhang, J.; Mirkin, C.A. Shape Control of Gold Nanoparticles by Silver Underpotential Deposition. Nano Lett. 2011, 11, 3394–3398. [Google Scholar] [PubMed]
- Rescignano, N.; Kenny, J.M. Stimuli-Responsive Core-Shell Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Rosemary, M.J.; MacLaren, I.; Pradeep, T. Investigations of the Antibacterial Properties of Ciprofloxacin@SiO2. Langmuir 2006, 22, 10125–10129. [Google Scholar] [CrossRef]
- Shittu, K.O.; Bankole, M.T.; Abdulkareem, A.S.; Abubakre, O.K.; Ubaka, A.U. Application of gold nanoparticles for improved drug efficiency. Adv. Nat. Sci. Nano Sci. Nanotechnol. 2017, 8, 035014. [Google Scholar] [CrossRef]
- Roshmi, T.; Soumya, K.R.; Jyothis, M.; Radhakrishnan, E.K. Effect of biofabricated gold nanoparticle-based antibiotic conjugates on minimum inhibitory concentration of bacterial isolates of clinical origin. Gold Bull. 2015, 48, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Pauer, A.C.; Gonzales, A.A.; Fenniri, H. Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int. J. Nano Med. 2019, 14, 7281–7289. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef] [Green Version]
- Gajendiran, M.; Yousuf, S.M.J.; Elangovan, V.; Balasubramanian, S. Gold nanoparticle conjugated PLGA–PEG–SA–PEG–PLGA multiblock copolymer nanoparticles: Synthesis, characterization, in vivo release of rifampicin. J. Mater. Chem. B 2013, 2, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.; Waghwani, H.K.; Connor, M.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel Synthesis of Kanamycin Conjugated Gold Nanoparticles with Potent Antibacterial Activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Quach, Q.H.; Ang, S.K.; Chu, J.-H.J.; Kah, J.C.Y. Size-dependent neutralizing activity of gold nanoparticle-based subunit vaccine against dengue virus. Acta Biomater. 2018, 78, 224–235. [Google Scholar]
- Dykman, L.A.; Staroverov, S.; Mezhennyj, P.; Fomin, A.S.; Kozlov, S.; Volkov, A.; Laskavy, V.N.; Shchyogolev, S.Y. Use of a synthetic foot-and-mouth disease virus peptide conjugated to gold nanoparticles for enhancing immunological response. Gold Bull. 2015, 48, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Hung, Y.-C.; Lin, W.-H.; Huang, G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology 2010, 21, 195101. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.-M.J.; Zhang, L. Modulating Antibacterial Immunity via Bacterial Membrane-Coated Nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Villamil, J.I.; Tapia, D.; Torres, A.G. Development of a Gold Nanoparticle Vaccine against Enterohemorrhagic Esch-erichia coli O157:H7. mBio 2019, 10, e01869-19. [Google Scholar] [CrossRef] [Green Version]
- Gregory, A.; Williamson, E.; Prior, J.; Butcher, W.; Thompson, I.; Shaw, A.; Titball, R. Conjugation of Y. pestis F1-antigen to gold nanoparticles improves immunogenicity. Vaccine 2012, 30, 6777–6782. [Google Scholar] [CrossRef] [Green Version]
- Barhate, G.A.; Gaikwad, S.M.; Jadhav, S.S.; Pokharkar, V.B. Structure function attributes of gold nanoparticle vaccine asso-ciation: Effect of particle size and association temperature. Int. J. Pharm. 2014, 471, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Dakterzada, F.; Mobarez, A.M.; Roudkenar, M.H.; Mohsenifar, A. Induction of humoral immune response against Pseudomonas aeruginosa flagellin(1-161) using gold nanoparticles as an adjuvant. Vaccine 2016, 34, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.R.-D.; Marradi, M.; González, R.C.; Cabanes, E.F.; Penadés, S.; Petrovsky, N.; Alvarez-Dominguez, C. A gold gly-co-nanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine 2015, 33, 1465–1473. [Google Scholar] [CrossRef] [Green Version]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [PubMed]
- Gregory, A.; Judy, B.M.; Qazi, O.; Blumentritt, C.A.; Brown, K.A.; Shaw, A.; Torres, A.G.; Titball, R.W. A gold nanoparti-cle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomed. Nanotechnol. Biol. Med. 2014, 11, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.G.; Gregory, A.; Hatcher, C.L.; Vinet-Oliphant, H.; Morici, L.A.; Titball, R.W.; Roy, C.J. Protection of non-human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine 2014, 33, 686–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manea, F.; Bindoli, C.; Fallarini, S.; Lombardi, G.; Polito, L.; Lay, L.; Bonomi, R.; Mancin, F.; Scrimin, P. Multivalent, Sac-cha-ride-Functionalized Gold Nanoparticles as Fully Synthetic Analogs of Type A Neisseria meningitidis Antigens. Adv. Mater. 2008, 20, 4348–4352. [Google Scholar]
- Khlebtsov, N.; Bogatyrev, V.; Dykman, L.; Khlebtsov, B.; Staroverov, S.; Shirokov, A.; Matora, L.; Khanadeev, V.; Pylaev, T.; Tsyganova, N.; et al. Analytical and Theranostic Applications of Gold Nanoparticles and Multifunctional Nanocomposites. Theranostics 2013, 3, 167–180. [Google Scholar]
- Staroverov, S.A.; Dykman, L.A. Use of gold nanoparticles for the preparation of antibodies to tuberculin, the immunoassay of mycobacteria, and animal vaccination. Nanotechnol. Russ. 2013, 8, 816–822. [Google Scholar]
- Parween, S.; Gupta, P.K.; Chauhan, V.S. Induction of humoral immune response against PfMSP-119 and PvMSP-119 using gold nanoparticles along with alum. Vaccine 2011, 29, 2451–2460. [Google Scholar] [CrossRef]
- Kumar, R.; Ray, P.C.; Datta, D.; Bansal, G.P.; Angov, E.; Kumar, N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine 2015, 33, 5064–5071. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Choi, J.-W. Application of Plasmonic Gold Nanoparticle for Drug Delivery System. Curr. Drug Targets 2018, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Luboshits, G.; Firer, M.A. Synthesis of Gold Nanoparticle: Peptide-Drug Conjugates for Targeted Drug Delivery. Methods Mol. Biol. 2019, 2059, 145–154. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C. Applications of Gold Nanoparticles in Nanomedicine: Recent Advances in Vaccines. Molecules 2017, 22, 857. [Google Scholar]
- Fan, J.; Cheng, Y.; Sun, M. Functionalized Gold Nanoparticles: Synthesis, Properties and Biomedical Applications. Chem. Rec. 2020, 20, 1474–1504. [Google Scholar] [CrossRef]
- Cao-Milán, R.; Liz-Marzán, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef]
- Kohout, C.; Santi, C.; Polito, L. Anisotropic Gold Nanoparticles in Biomedical Applications. Int. J. Mol. Sci. 2018, 19, 3385. [Google Scholar] [CrossRef] [Green Version]
- Benne, N.; van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slütter, B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control. Release 2016, 234, 124–134. [Google Scholar] [CrossRef]
- Carnovale, C.; Bryant, G.; Shukla, R.; Bansal, V. Identifying Trends in Gold Nanoparticle Toxicity and Uptake: Size, Shape, Capping Ligand, and Biological Corona. ACS Omega 2019, 4, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Moon, J.J. Particulate delivery systems for vaccination against bioterrorism agents and emerging infectious pathogens. Wiley Interdiscip Rev. Nanomed. Nanobiotechnol. 2017, 9, e1403. [Google Scholar]
- Neto, L.M.M.; Kipnis, A.; Junqueira-Kipnis, A.P. Role of metallic nanoparticles in vaccinology: Implications for infectious disease vaccine development. Front. Immunol. 2017, 8, 239. [Google Scholar]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [PubMed] [Green Version]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar]
- Lopes, T.S.; Alves, G.G.; Pereira, M.R.; Granjeiro, J.M.; Leite, P.E.C. Advances and potential application of gold nanoparticles in nanomedicine. J. Cell. Biochem. 2019, 120, 16370–16378. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Ding, Y. Gold nanoparticle-conjugated nanomedicine: Design, construction, and structure–efficacy re-lationship studies. J. Mater. Chem. B 2020, 8, 4813–4830. [Google Scholar] [CrossRef]
- Ahmad, S.; Zamry, A.A.; Tan, H.-T.T.; Wong, K.K.; Lim, J.; Mohamud, R. Targeting dendritic cells through gold nanoparticles: A review on the cellular uptake and subsequent immunological properties. Mol. Immunol. 2017, 91, 123–133. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef]
- Stead, S.O.; Kireta, S.; McInnes, S.J.P.; Kette, F.D.; Sivanathan, K.N.; Kim, J.; Cueto-Diaz, E.J.; Cunin, F.; Durand, J.-O.; Drogemuller, C.J.; et al. Murine and Non-Human Primate Dendritic Cell Targeting Nanoparticles for in Vivo Generation of Regulatory T-Cells. ACS Nano 2018, 12, 6637–6647. [Google Scholar]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced antitumor immunity by targeting dendritic cells with tumor cell lysate-loaded chitosan nanoparticles vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar]
- Yang, M.; Ding, J.; Zhang, Y.; Chang, F.; Wang, J.; Gao, Z.; Zhuang, X.; Chen, X. Activated macrophage-targeted dex-tran-methotrexate/folate conjugate prevents deterioration of collagen-induced arthritis in mice. J. Mater. Chem. B 2016, 4, 2102–2113. [Google Scholar] [PubMed]
- Heo, R.; Park, J.-S.; Jang, H.J.; Kim, S.-H.; Shin, J.M.; Suh, Y.D.; Jeong, J.H.; Jo, D.-G.; Park, J.H. Hyaluronan nanoparticles bearing γ-secretase inhibitor: In vivo therapeutic effects on rheumatoid arthritis. J. Control. Release 2014, 192, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ding, J.; Feng, X.; Chang, F.; Wang, Y.; Gao, Z.; Zhuang, X.; Chen, X. Scavenger Receptor-Mediated Targeted Treatment of Collagen-Induced Arthritis by Dextran Sulfate-Methotrexate Prodrug. Theranostics 2017, 7, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Versiani, A.F.; Andrade, L.M.; Martins, E.M.; Scalzo, S.; Geraldo, J.M.; Chaves, C.R.; Ferreira, D.C.; Ladeira, M.; Guatimosim, S.; Ladeira, L.O.; et al. Gold nanoparticles and their applications in biomedicine. Futur. Virol. 2016, 11, 293–309. [Google Scholar]
- Faa, G.; Gerosa, C.; Fanni, D.; Lachowicz, J.; Nurchi, V. Gold-Old Drug with New Potentials. Curr. Med. Chem. 2018, 25, 75–84. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Ju, E.G.; Ren, H.; Ren, J.S. Gold nanocluster-based vaccines for dual-delivery of antigens and im-munostimulatory oligonucleotides. Nanoscale 2015, 7, 12419–12426. [Google Scholar]
- Wang, Y.; Wang, Y.; Kang, N.; Liu, Y.; Shan, W.; Bi, S.; Ren, L.; Zhuang, G. Construction and Immunological Evaluation of CpG-Au@HBc Virus-Like Nanoparticles as a Potential Vaccine. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Sekimukai, H.; Iwata-Yoshikawa, N.; Fukushi, S.; Tani, H.; Kataoka, M.; Suzuki, T.; Hasegawa, H.; Niikura, K.; Arai, K.; Nagata, N. Gold nanoparticle-adjuvanted S protein induces a strong antigen-specific IgG response against severe acute res-piratory syndrome-related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiol. Immunol. 2019, 64, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [PubMed]
- Van Haute, D.; Berlin, J.M. Challenges in realizing selectivity for nanoparticle biodistribution and clearance: Lessons from gold nanoparticles. Ther. Deliv. 2017, 8, 763–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, A.T.; Cornejo, Y.R.; Van Haute, D.; Berlin, J.M. A Systematic comparison of in vitro cell uptake and in vivo biodistribution for three classes of gold nanoparticles with saturated PEG coatings. PLoS ONE 2020, 15, e0234916. [Google Scholar] [CrossRef]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [PubMed]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Bertoli, F.; Garry, D.; Monopoli, M.P.; Salvati, A.; Dawson, K.A. The Intracellular Destiny of the Protein Corona: A Study on its Cellular Internalization and Evolution. ACS Nano 2016, 10, 10471–10479. [Google Scholar] [CrossRef]
- Cai, F.; Li, S.; Huang, H.; Iqbal, J.; Wang, C.; Jiang, X. Green synthesis of gold nanoparticles for immune response regulation: Mechanisms, applications, and perspectives. J. Biomed. Mater. Res. Part A 2021, 110, 424–442. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Kawaguchi, A.; Hasegawa, H.; Kajino, K.; Ninomiya, T.; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.R.; Dixon, D.; Coulter, J.A. Gold nanoparticle surface functionalization: A necessary requirement in the development of novel nanotherapeutics. Nanomedicine 2015, 10, 1315–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farfán-Castro, S.; García-Soto, M.J.; Comas-García, M.; Arévalo-Villalobos, J.I.; Palestino, G.; González-Ortega, O.; Rosales-Mendoza, S. Synthesis and immunogenicity assessment of a gold nanoparticle conjugate for the delivery of a peptide from SARS-CoV-2. Nanomedicine 2021, 34, 102372. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Balfourier, A.; Nicolás-Boluda, A.; Carn, F.; Gazeau, F. Endocytosis-driven gold nanoparticle fractal rear-rangement in cells and its influence on photothermal conversion. Nanoscale 2020, 12, 21832–21849. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Matos, C.A.; Millington, O.R.; Wark, A.W.; Zagnoni, M. Real-time assessment of nanoparticle-mediated antigen de-livery and cell response. Lab Chip 2016, 16, 3374–3381. [Google Scholar] [CrossRef] [Green Version]
- Bahamonde, J.; Brenseke, B.; Chan, M.; Kent, R.D.; Vikesland, P.J.; Prater, M.R. Gold Nanoparticle Toxicity in Mice and Rats: Species Differences. Toxicol. Pathol. 2018, 46, 431–443. [Google Scholar] [CrossRef]
- Deville, S.; Baré, B.; Piella, J.; Tirez, K.; Hoet, P.; Monopoli, M.P.; Dawson, K.A.; Puntes, V.; Nelissen, I. Interaction of gold nanoparticles and nickel(II) sulfate affects dendritic cell maturation. Nanotoxicology 2016, 10, 1395–1403. [Google Scholar] [CrossRef]
- El-Sayed, N.; Korotchenko, E.; Scheiblhofer, S.; Weiss, R.; Schneider, M. Functionalized multifunctional nanovaccine for targeting dendritic cells and modulation of immune response. Int. J. Pharm. 2020, 593, 120123. [Google Scholar] [CrossRef]
- Staroverov, S.A.; Volkov, A.A.; Mezhennyj, P.; Domnitsky, I.Y.; Fomin, A.S.; Kozlov, S.V.; Dykman, L.A.; Guliy, O.I. Pro-spects for the use of spherical gold nanoparticles in immunization. Appl. Microbiol. Biotechnol. 2018, 103, 437–447. [Google Scholar]
- Fytianos, K.; Rodriguez-Lorenzo, L.; Clift, M.J.; Blank, F.; Vanhecke, D.; von Garnier, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Uptake efficiency of surface modified gold nanoparticles does not correlate with functional changes and cytokine secretion in human dendritic cells in vitro. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 633–644. [Google Scholar]
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J. Nanobiotechnology 2020, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Van Der Mei, H.C.; Dijk, M.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; Ren, Y.; Chen, H.; Busscher, H.J. Po-larization of Macrophages, Cellular Adhesion, and Spreading on Bacterially Contaminated Gold Nanoparticle-Coatings in Vitro. ACS Biomater. Sci. Eng. 2020, 6, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Tyner, K.; Bancos, S.; Stevens, D. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. Int. J. Nanomed. 2014, 10, 183–206. [Google Scholar] [CrossRef] [Green Version]
- Kingston, M.; Pfau, J.C.; Gilmer, J.; Brey, R. Selective inhibitory effects of 50-nm gold nanoparticles on mouse macrophage and spleen cells. J. Immunotoxicol. 2015, 13, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Jiao, P.; Otto, M.; Geng, Q.; Li, C.; Li, F.; Butch, E.R.; Snyder, S.E.; Zhou, H.; Yan, B. Enhancing both CT imaging and natural killer cell-mediated cancer cell killing by a GD2-targeting nanoconstruct. J. Mater. Chem. B 2015, 4, 513–520. [Google Scholar]
- Qu, Y.; Li, Y.; Liao, S.; Sun, J.; Li, M.; Wang, D.; Xia, C.; Luo, Q.; Hu, J.; Luo, K.; et al. Linear and Core-Crosslinked Glyco-polymer-Gadolinium Conjugates: Preparation and Their Behaviors as Nanoscale Magnetic Resonance Imaging Contrast Agents. J. Biomed. Nanotechnol. 2019, 15, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects of Gold Nanoparticles Synthesized from Hypoxis hemerocallidea Aqueous Extract and Hypoxoside on Macrophage and Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef] [Green Version]
- Abia, I.; Peng, T.Y.; Mains, S.; Pohl, N. Design and synthesis of thiol-terminated oligosaccharides for attachment on gold nanoparticles: Toward the development of an HIV vaccine. Abstr. Pap. Am. Chem. Soc. 2013, 246, 1155. [Google Scholar]
- Chiodo, F.; Enriquez-Navas, P.M.; Angulo, J.; Marradi, M.; Penades, S. Assembling different antennas of the gp120 high mannose-type glycans on gold nanoparticles provides superior binding to the anti-HIV antibody 2G12 than the individual antennas. Carbohydr. Res. 2015, 405, 102–109. [Google Scholar]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y.; et al. Surface-Engineered Gold Nanorods: Promising DNA Vaccine Adjuvant for HIV-1 Treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [PubMed]
- Di Gianvincenzo, P.; Calvo, J.; Perez, S.; Álvarez, A.; Bedoya, L.M.; Alcamí, J.; Penadés, S. Negatively charged glyco-nanoparticles modulate and stabilize the secondary structures of a gp120 V3 loop peptide: Toward fully synthetic HIV vaccine candidates. Bioconjug Chem. 2015, 26, 755–765. [Google Scholar]
- Negahdari, B.; Darvishi, M.; Saeedi, A.A. Gold nanoparticles and hepatitis B virus. Artif. Cells Nanomed. Biotechnol. 2019, 47, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Widera, G.; Rabussay, D. Enhancement of the effectiveness of electroporation-augmented cutaneous DNA vaccination by a particulate adjuvant. Bioelectrochemistry 2004, 63, 369–373. [Google Scholar] [PubMed]
- Yavuz, E.; Bagriacik, E.U. Gold-based nano-adjuvants. In Proceedings of the IEEE 7th International Conference on Nanomaterials: Applications and Properties, Odesa, Ukraine, 10–15 September 2017. [Google Scholar]
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.; Chun, H.S.; Jo, C.-H.; Choi, B.G.; Huh, Y.S.; et al. Het-eroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Zhao, L.; Protzer, U.; Van De Klundert, M.A.A. Applicability of Metal Nanoparticles in the Detection and Monitoring of Hepatitis B Virus Infection. Viruses 2017, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Draz, M.S.; Wang, Y.J.; Chen, F.F.; Xu, Y.H.; Shafiee, H. Electrically Oscillating Plasmonic Nanoparticles for Enhanced DNA Vaccination against Hepatitis C Virus. Adv. Funct. Mater. 2017, 27, 1604139. [Google Scholar]
- Li, Y.; Jin, Q.; Ding, P.; Zhou, W.; Chai, Y.; Li, X.; Wang, Y.; Zhang, G.-P. Gold nanoparticles enhance immune responses in mice against recombinant classical swine fever virus E2 protein. Biotechnol. Lett. 2020, 42, 1169–1180. [Google Scholar] [CrossRef]
- Paul, A.; Shi, Y.; Acharya, D.; Douglas, J.R.; Cooley, A.; Anderson, J.F.; Huang, F.; Bai, F. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J. Gen. Virol. 2014, 95, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Hurst, B.L.; Shakya, A.K.; Uddin, J.; Ingrole, R.S.; Hernandez-Sanabria, M.; Arya, R.; Bimler, L.; Paust, S.; Tarbet, B.; et al. Consensus M2e peptide conjugated to gold nanoparticles confers protection against H1N1, H3N2 and H5N1 influenza A viruses. Antivir. Res. 2017, 141, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Tao, W.; Gill, H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33, 2307–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, W.; Ziemer, K.S.; Gill, H.S. Gold nanoparticle–M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine 2014, 9, 237–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bimler, L.; Song, A.Y.; Le, D.T.; Schafer, A.M.; Paust, S. GnP-M2e + sCpG vaccination of juvenile mice generates lifelong protective immunity to influenza a virus infection. Immun. Ageing 2019, 16, 23. [Google Scholar] [PubMed] [Green Version]
- Mezhenny, P.V.; Staroverov, S.A.; Volkov, A.A.; Kozlov, S.V.; Laskavy, V.N.; Dykman, L.A.; Isayeva, A.Y. Con-struction of conjugates of colloidal selenium and colloidal gold with the protein of influenza virus and the study of their im-munogenic properties. Bull Saratov State Agrar. Univ. 2013, 2, 29–32. [Google Scholar]
- Wang, C.; Zhu, W.; Wang, B.-Z. Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of re-combinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int. J. Nanomed. 2017, 12, 4747–4762. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.-Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1349–1360. [Google Scholar] [CrossRef]
- Chen, H.-W.; Huang, C.-Y.; Lin, S.-Y.; Fang, Z.-S.; Hsu, C.-H.; Lin, J.-C.; Chen, Y.-I.; Yao, B.-Y.; Hu, C.-M.J. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials 2016, 106, 111–118. [Google Scholar]
- Staroverov, S.A.; Vidyasheva, I.V.; Gabalov, K.P.; Vasilenko, O.A.; Laskavyi, V.N.; Dykman, L.A. Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull. Exp. Biol. Med. 2011, 151, 436–439. [Google Scholar]
- Stone, J.; Thornburg, N.J.; Blum, D.L.; Kuhn, S.J.; Wright, D.W.; Crowe, J.E. Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology 2013, 24, 295102. [Google Scholar]
- Bawage, S.; Tiwari, P.M.; Singh, A.; Dixit, S.; Pillai, S.R.; Dennis, V.A.; Singh, S.R. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2299–2310. [Google Scholar] [CrossRef] [Green Version]
- DeRussy, B.M.; Aylward, M.A.; Fan, Z.; Ray, P.C.; Tandon, R. Inhibition of cytomegalovirus infection and photo-thermolysis of infected cells using bioconjugated gold nanoparticles. Sci. Rep. 2014, 4, 5550. [Google Scholar] [PubMed]
- Ding, P.; Zhang, T.; Li, Y.; Teng, M.; Sun, Y.; Liu, X.; Chai, S.; Zhou, E.; Jin, Q.; Zhang, G. Nanoparticle orientationally displayed antigen epitopes improve neutralizing antibody level in a model of porcine circovirus type 2. Int. J. Nano Med. 2017, 12, 5239–5254. [Google Scholar]
- Dykman, L.A.; Volokh, O.A.; Kuznetsova, E.M.; Nikiforov, A.K. Immunogenicity of Conjugates of Protective Antigen Complexes of Tularemia Microbe with Gold Nanoparticles. Nanotechnol. Russ. 2018, 13, 384–392. [Google Scholar]
- Staroverov, S.A.; Ermilov, D.N.; Shcherbakov, A.A.; Semenov, S.V.; Shchegolev, S.I.; Dykman, L.A. Generation of antibodies to Yersinia pseudotuberculosis antigens using the colloid gold particles as an adjuvant. Zh Mikrobiol. Epidemiol. Immunobiol. 2003, 3, 54–57. [Google Scholar]
- Chowdhury, R.; Ilyas, H.; Ghosh, A.; Ali, H.; Ghorai, A.; Midya, A.; Jana, N.R.; Das, S.; Bhunia, A. Multivalent gold nanoparticle–peptide conjugates for targeting intracellular bacterial infections. Nanoscale 2017, 9, 14074–14093. [Google Scholar] [CrossRef]
- Fallarini, S.; Paoletti, T.; Battaglini, C.O.; Ronchi, P.; Lay, L.; Bonomi, R.; Jha, S.; Mancin, F.; Scrimin, P.; Lombardi, G. Factors affecting T cell responses induced by fully synthetic glyco-gold-nanoparticles. Nanoscale 2012, 5, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Gonzalez, R.; Terán-Navarro, H.; Frande-Cabanes, E.; Ferrández-Fernández, E.; Freire, J.; Penadés, S.; Marradi, M.; García, I.; Gomez-Román, J.; Yañez-Díaz, S.; et al. Pregnancy Vaccination with Gold Glyco-Nanoparticles Car-rying Listeria monocytogenes Peptides Protects against Listeriosis and Brain- and Cutaneous-Associated Morbidities. Nanomaterials 2016, 6, 151. [Google Scholar]
- Vetro, M.; Safari, D.; Fallarini, S.; Salsabila, K.; Lahmann, M.; Penadés, S.; Lay, L.; Marradi, M.; Compostella, F. Preparation and immunogenicity of gold glyco-nanoparticles as antipneumococcal vaccine model. Nanomedicine 2017, 12, 13–23. [Google Scholar]
- Barhate, G.; Gautam, M.; Gairola, S.; Jadhav, S.; Pokharkar, V. Quillaja saponaria extract as mucosal adjuvant with chitosan functionalized gold nanoparticles for mucosal vaccine delivery: Stability and immunoefficiency studies. Int. J. Pharm. 2013, 441, 636–642. [Google Scholar]
- Barhate, G.; Gautam, M.; Gairola, S.; Jadhav, S.; Pokharkar, V. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: Charac-terization, immunogenicity, and stability assessment. J. Pharm. Sci. 2014, 103, 3448–3456. [Google Scholar]
- Liu, J.; Wang, J.; Li, Z.; Meng, H.; Zhang, L.; Wang, H.; Li, J.; Qu, L. A lateral flow assay for the determination of human tetanus antibody in whole blood by using gold nanoparticle labeled tetanus antigen. Mikrochim. Acta 2018, 185, 110. [Google Scholar] [CrossRef] [PubMed]
- Assis, N.R.; Caires, A.; Figueiredo, B.C.; Morais, S.B.; Mambelli, F.S.; Marinho, F.; Ladeira, L.O.; Oliveira, S.C. The use of gold nanorods as a new vaccine platform against schistosomiasis. J. Control. Release 2018, 275, 40–52. [Google Scholar] [PubMed]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar]
- Homberger, M.; Simon, U. On the application potential of gold nanoparticles in nanoelectronics and biomedicine. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1405–1453. [Google Scholar]
- Glazer, E.S.; Zhu, C.; Hamir, A.N.; Borne, A.; Thompson, C.S.; Curley, S.A. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology 2011, 5, 459–468. [Google Scholar]
- Li, J.J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y.L. Autophagy and oxidative stress associated with gold nano-particles. Biomaterials 2010, 31, 5996–6003. [Google Scholar] [PubMed]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar]
- Wang, L.; Liu, Y.; Li, W.; Jiang, X.; Ji, Y.; Wu, X.; Xu, L.; Qiu, Y.; Zhao, K.; Wei, T.; et al. Selective Targeting of Gold Nanorods at the Mitochondria of Cancer Cells: Implications for Cancer Therapy. Nano Lett. 2011, 11, 772–780. [Google Scholar]
- Chang, M.-Y.; Shiau, A.-L.; Chen, Y.-H.; Chang, C.-J.; Chen, H.H.-W.; Wu, C.-L. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008, 99, 1479–1484. [Google Scholar]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Weintraub, K. Biomedicine: The new gold standard. Nature 2013, 495, S14–S16. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [PubMed] [Green Version]
- Rosa, S.; Connolly, C.; Schettino, G.; Butterworth, K.T.; Prise, K.M. Biological mechanisms of gold nanoparticle radio-sensitization. Cancer Nanotechnol. 2017, 8, 2. [Google Scholar] [PubMed] [Green Version]
- Havaki, S.; Kotsinas, A.; Chronopoulos, E.; Kletsas, D.; Georgakilas, A.; Gorgoulis, V.G. The role of oxidative DNA damage in radiation induced bystander effect. Cancer Lett. 2015, 356, 43–51. [Google Scholar] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small 2009, 5, 2067–2076. [Google Scholar]
- Katas, H.; Moden, N.Z.; Lim, C.S.; Celesistinus, T.; Chan, J.Y.; Ganasan PSuleman Ismail Abdalla, S. Biosynthesis and potential applications of silver and gold nanoparticles and their chitosan-based nanocomposites in nanomedicine. J. Nanotechnol. 2018, 2018, 4290705. [Google Scholar]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An overview of the synthesis of gold nanoparticles using radiation technologies. Nanomaterials 2018, 8, 939. [Google Scholar]
- Chen, H.; Dorrigan, A.; Saad, S.; Hare, D.J.; Cortie, M.B.; Valenzuela, S.M. In vivo study of spherical gold nanoparticles: Inflammatory effects and distribution in mice. PLoS ONE 2013, 8, e58208. [Google Scholar]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar]
| SN | Antigen Conjugated with AuNP | GNP/Adjuvant | Immunization Mechanism | Immune Response | Ref. |
|---|---|---|---|---|---|
| 1 | Surface antigens spike glycoprotein of avian coronavirus | Virus-like particles (VLP) by incubating the antigen with 100 nm AuNPs | Dose: Single, 10 μg Mode: Intramuscularly Animals: BALB/C mice and specific pathogen-free chickens |
| [109] |
| 2 | Surface antigens gastroenteritis virus | Conjugated with 15 nm AuNPs | Guinea pigs twice subcutaneously with 125 μg, mice once intraperitoneally with 70 μg, and rabbits three times subcutaneously with 220 μg |
| [84,110] |
| 3 | Glycoprotein antigen of respiratory syncytial virus | Nanorods | Human cell treatment in vitro | Human dendritic cells induced an immune activation (proliferation and expansion) of primary T cells. | [111] |
| 4 | Glycoprotein isolated from fixed rabies virus, strain Moscow 3253 | Conjugated with 15 nm AuNPs | Animal: Mice Mode: Intraperitoneally Dose: 25 μg in four booster doses, 50 μg was used | Develop highly specific neutralizing antibodies against the virus. | [112] |
| 5 | Surface glycoprotein (gB) of human cytomegalovirus (CMV, a herpes virus) | Conjugated with AuNP | In vitro |
| [113] |
| 6 | West Nile fever virus | Multiple sizes and shapes of AuNPs used: 20 and 40 nm nanospheres, 40 × 20 nm nanorods, and 40 × 40 × 40 nm nanocubes | Animal: Mice Mode: Intraperitoneally Dose: 100 μg No. of doses: 2 |
| [72] |
| 7 | Capsid (Cap) protein from pathogenic porcine circovirus | Conjugated with 23 nm GNPs | In vitro and mice immunized twice subcutaneously |
| [114] |
| SN | Antigen Conjugated with AuNP | GNP/Adjuvant | Immunization Mechanism | Immune Response | Ref. |
|---|---|---|---|---|---|
| 1 | Listeriolysin O peptide (LLO91-99) from Listeria monocytogenes | Conjugated with AuNP | A single intravenous or intraperitoneal immunization of mice |
| [95,119] |
| 2 | A synthetic tetrasaccharide epitope, similar to the capsular polysaccharide of Streptococcus pneumoniae type14 | Conjugated with 2 nm AuNP + T helper peptide | Animal: Mice Dose: 3 μg Mode: Intradermal No. of doses: 1 |
| [25,120] |
| 3 | Bacterial vesicles of the outer membrane of Escherichia coli | Conjugated with 30 nm AuNPs | Injected in mice three times subcutaneously |
| [19] |
| 4 | Tetanus toxoid Clostridium tetani | Conjugated with 25 nm AuNPs + plant adjuvants (saponins) from Quillaja saponaria (79) and Asparagus racemosus (80) | Subcutaneous injection, or transmucosal delivery | Oral administration highly enhanced mucosal immune response in the presence of plant adjuvants. | [121,122,123] |
| 5 | Burkholderia mallei recombinant protein: Hc fragment of tetanus toxin, hemolysin (produced by both B. mallei and B. pseudomallei), and flagellin (produced by B. pseudomallei) | 15 nm AuNP functionalized with purified LPS from a nonvirulent B. thailandensis strain | BALB/C mice, intranasal, 3 different dose concentrations |
| [26] |
| 6 | 7.5 μg of tuberculin (mixture of the surface antigens of various types of mycobacteria) | Conjugated with 15 nm AuNPs | Rabbits, four times intramuscularly | High antibody production against multiple types of mycobacteria. | [29,30] |
| 7 | Specific immunogenic antigens LomW and EscC from enterohemorrhagic strain E. coli O157: H7 | Conjugated with AuNP | Mice, three times subcutaneously, 2-week intervals |
| [20] |
| SN | Antigen | AuNP/Adjuvant | Immunization Mechanism | Immune Response | Ref. |
|---|---|---|---|---|---|
| 1 | Recombinant protein from rSm2 Schistosoma mansoni | Gold nanorods conjugated | Mice immunization intraperitoneally with 2 μg dose |
| [124] |
| 2 | Surface protein Pfs25 from the P. falciparum | Attached to various AuNPs, including nanospheres, nanostars, nanocages, and nanoprisms | Mice were immunized with the resulting conjugates. Dose: 10 μg, three times, intramuscularly |
| [32] |
| 3 | C-terminal 19 kDa fragment of merozoite surface protein 1 from the malaria pathogen Plasmodium falciparum | 17 nm AuNP conjugated + adjuvant Alhydrogel® | Mice were immunized three times subcutaneously at a dose of 25 μg |
| [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, A.; Azharuddin, M.; Al-Otaibi, N.; Hinkula, J. Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines 2022, 10, 505. https://doi.org/10.3390/vaccines10040505
Sengupta A, Azharuddin M, Al-Otaibi N, Hinkula J. Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines. 2022; 10(4):505. https://doi.org/10.3390/vaccines10040505
Chicago/Turabian StyleSengupta, Anirban, Mohammad Azharuddin, Noha Al-Otaibi, and Jorma Hinkula. 2022. "Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases" Vaccines 10, no. 4: 505. https://doi.org/10.3390/vaccines10040505
APA StyleSengupta, A., Azharuddin, M., Al-Otaibi, N., & Hinkula, J. (2022). Efficacy and Immune Response Elicited by Gold Nanoparticle- Based Nanovaccines against Infectious Diseases. Vaccines, 10(4), 505. https://doi.org/10.3390/vaccines10040505






