Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Randomization
2.2. Study Vaccine Administration
2.3. Study Participants
2.4. Study Objectives and Endpoints
2.5. Statistical Methods
3. Results
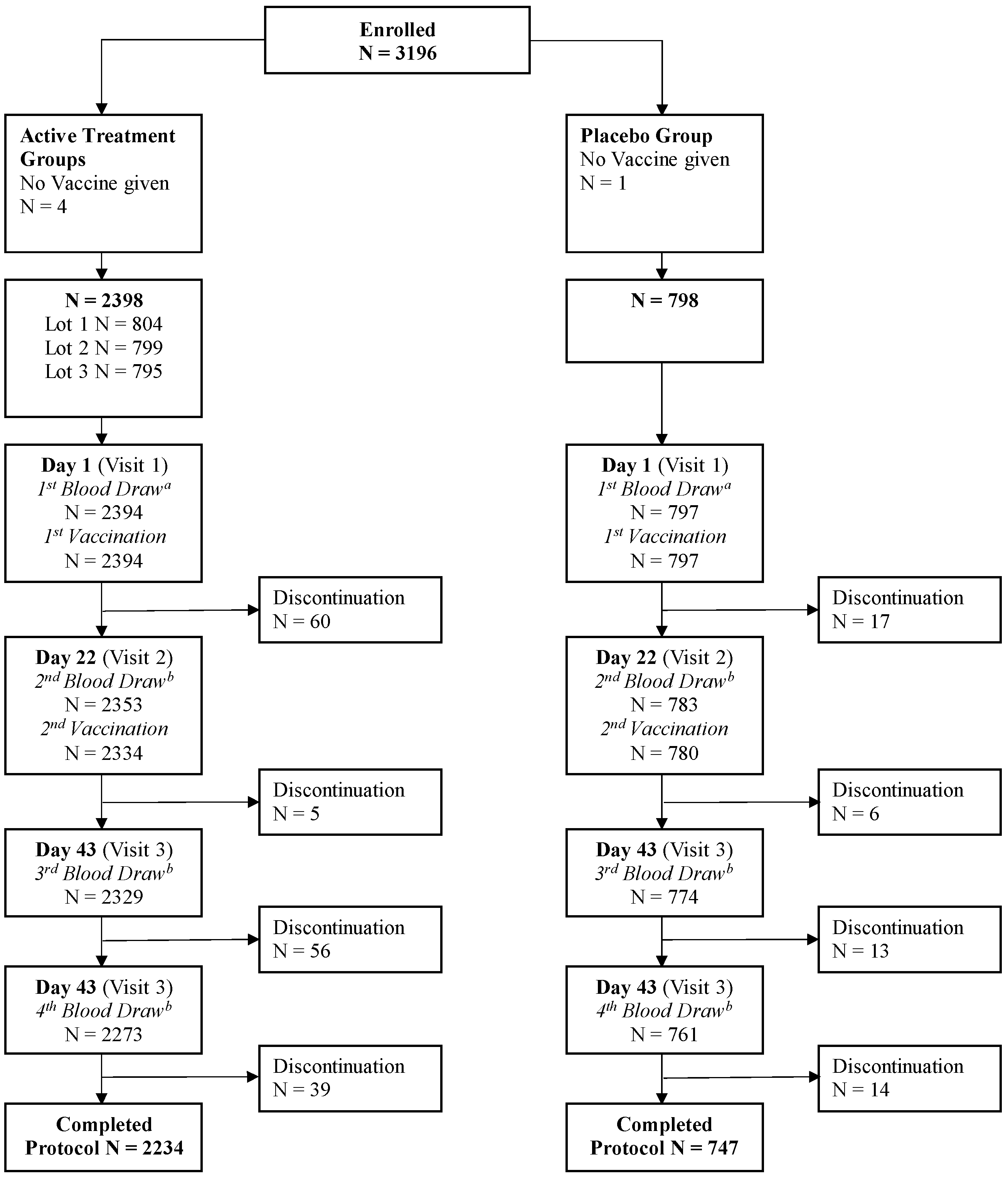
3.1. Study Population
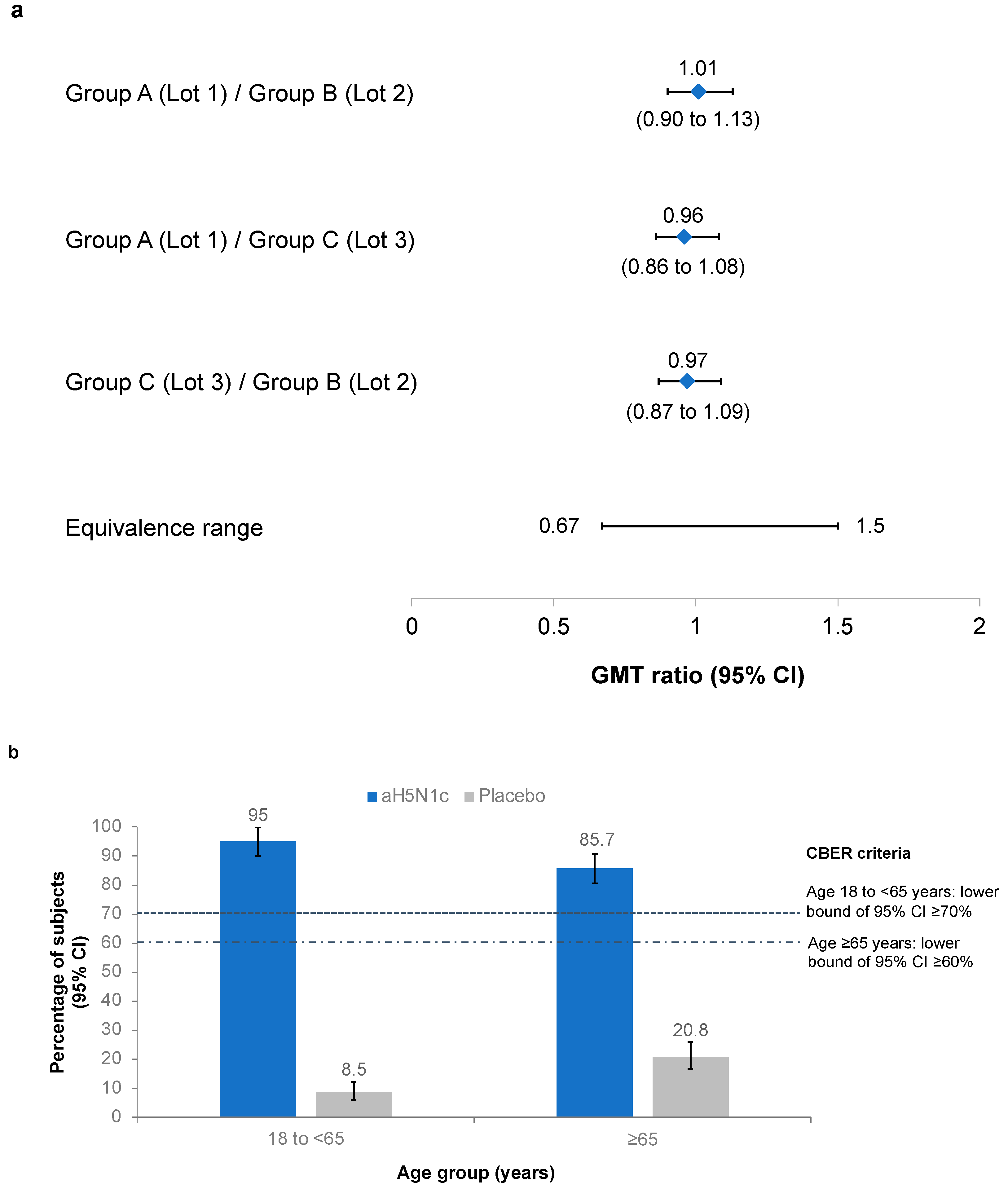
3.2. Coprimary Objectives: Lot-to-Lot Consistency and CBER Criteria
3.3. Immunogenicity
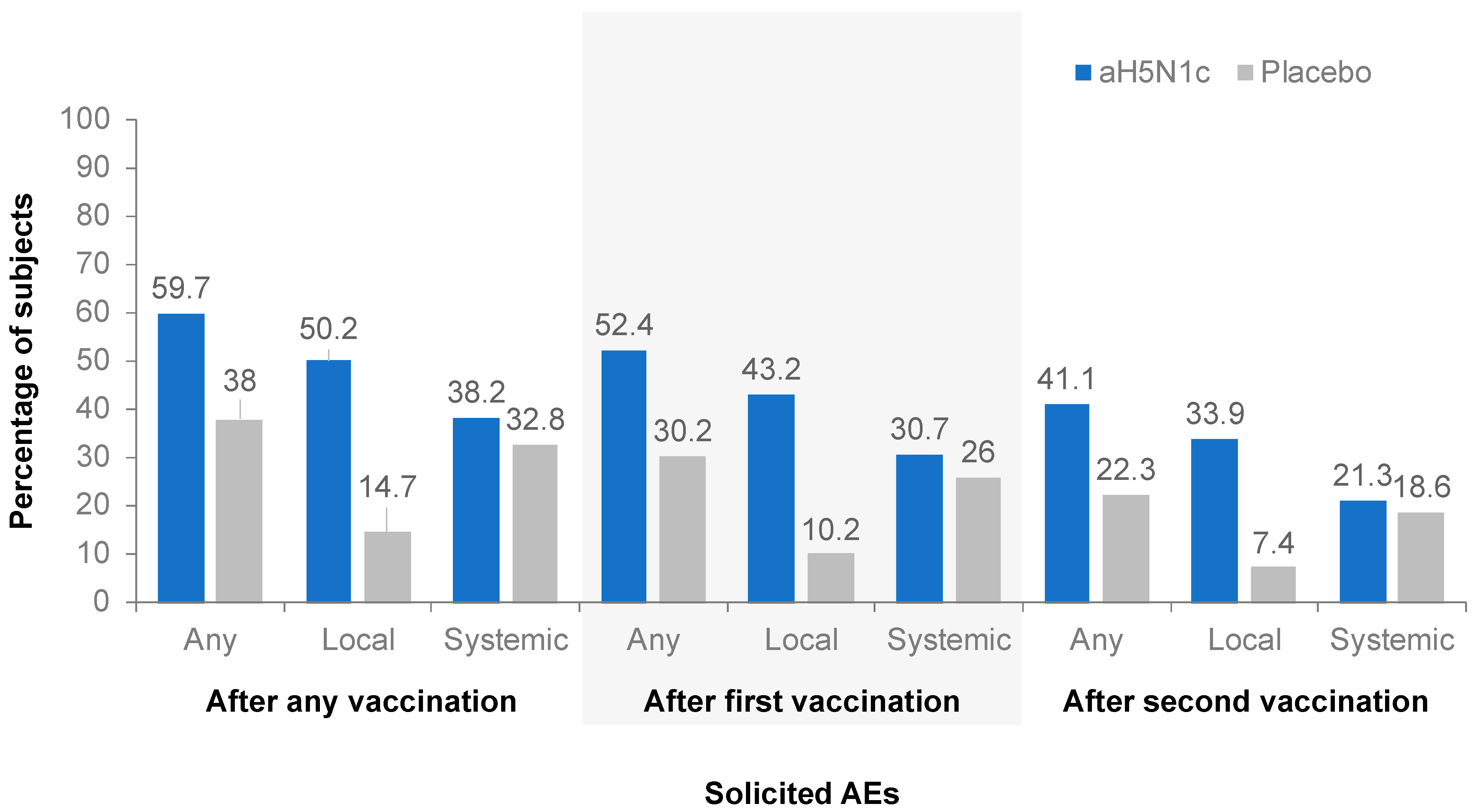
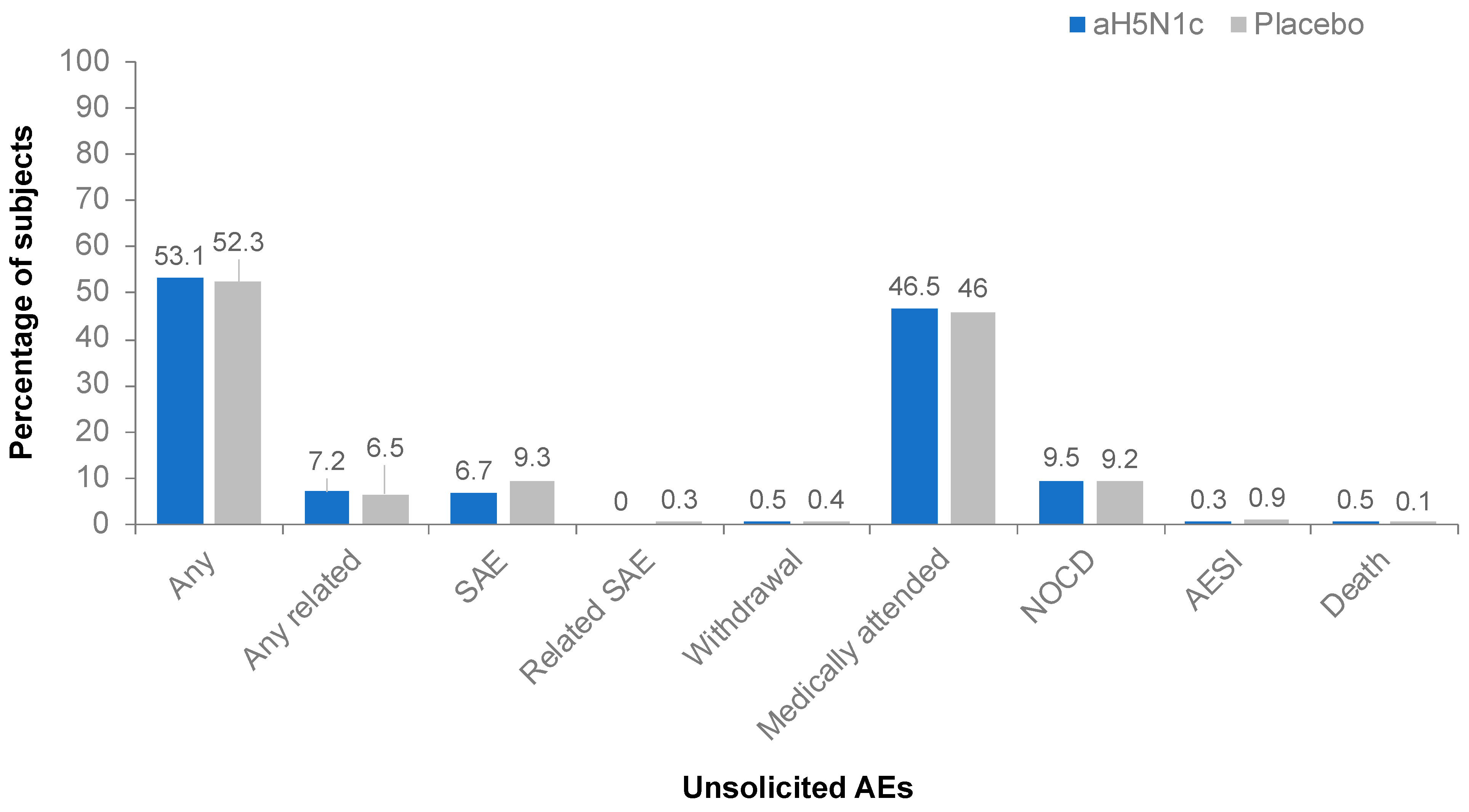
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedson, D.S. Pandemic Influenza and the Global Vaccine Supply. Clin. Infect. Dis. 2003, 36, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2009 H1N1 Pandemic (H1N1pdm09 Virus). Available online: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html (accessed on 12 April 2021).
- Webby, R.J.; Webster, R.G. Are we ready for Pandemic Influenza? In Learning from SARS: Preparing for the Next Disease Outbreak Workshop Summary; Knobler, S., Mahmoud, A., Lemon, S., Mack, A., Sivitz, L.K.O., Eds.; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar]
- World Health Organization. Human Infection with Avian Influenza A(H5) Viruses. Avian Influenza Weekly Update Number 784. Available online: https://www.who.int/docs/default-source/wpro---documents/emergency/surveillance/avian-influenza/ai-20210319.pdf (accessed on 12 April 2021).
- Homeland Security Council. National Strategy for Pandemic Influenza; Department of Homeland Security: Washington, DC, USA, 2005. [Google Scholar]
- Ferguson, N.M.; Cummings, D.A.T.; Fraser, C.; Cajka, J.; Cooley, P.C.; Burke, D.S. Strategies for mitigating an influenza pandemic. Nature 2006, 442, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.; Campbell, J.D.; Zangwill, K.M.; Rowe, T.; Wolff, M. Safety and Immunogenicity of an Inactivated Subvirion Influenza A (H5N1) Vaccine. N. Engl. J. Med. 2006, 354, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.G.; Colegate, A.E.; Podda, A.; Stephenson, I.; Wood, J.; Ypma, E.; Zambon, M.C. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: A randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001, 357, 1937–1943. [Google Scholar] [CrossRef]
- Banzhoff, A.; Pellegrini, M.; Del Giudice, G.; Fragapane, E.; Groth, N.; Podda, A. MF59®-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influ. Other Respir. Viruses 2008, 2, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Hilbert, A.K.; Bugarini, R.; Minutello, A.; Popova, O.; Toneatto, D.; Schoendorf, I.; Borkowski, A.; Rappuoli, R.; Podda, A. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine 2006, 24, 3063–3065. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines; US Department of Health and Human Services: Rockville, MD, USA, 2007. [Google Scholar]
- Committee for Medicinal Products for Human Use. Note for Guidance on Harmonisation of Requirements for Influenza Vaccines; European Agency for the Evaluation of Medicinal Products: London, UK, 1997. [Google Scholar]
- Lin, J.T.; Li, C.G.; Wang, X.; Su, N.; Liu, Y.; Qiu, Y.Z.; Yang, M.; Chen, J.T.; Fang, H.H.; Dong, X.P.; et al. Antibody persistence after 2-dose priming and booster response to a third dose of an inactivated, adjuvanted, whole-virion H5N1 vaccine. J. Infect. Dis. 2009, 199, 184–187. [Google Scholar] [CrossRef]
- Lazarus, R.; Kelly, S.; Snape, M.D.; Vandermeulen, C.; Voysey, M.; Hoppenbrouwers, K.; Hens, A.; Van Damme, P.; Pepin, S.; Leroux-Roels, I.; et al. Antibody Persistence and Booster Responses to Split-Virion H5N1 Avian Influenza Vaccine in Young and Elderly Adults. PLoS ONE 2016, 11, e0165384. [Google Scholar] [CrossRef]
- Bihari, I.; Pánczél, G.; Kovacs, J.; Beygo, J.; Fragapane, E. Assessment of Antigen-Specific and Cross-Reactive Antibody Responses to an MF59-Adjuvanted A/H5N1 Prepandemic Influenza Vaccine in Adult and Elderly Subjects. Clin. Vaccine Immunol. 2012, 19, 1943–1948. [Google Scholar] [CrossRef][Green Version]
- Versage, E.; van Twuijver, E.; Jansen, W.; Theeuwes, A.; Sawlwin, D.; Hohenboken, M. Analyses of Safety Profile and Homologous Antibody Responses to a Mammalian Cell-Based, MF59-Adjuvanted, A/H5N1, Pandemic Influenza Vaccine across Four Phase II/III Clinical Trials in Healthy Children, Adults, and Older Adults. Vaccines 2021, 9, 1468. [Google Scholar] [CrossRef]
- Vesikari, T.; Forstén, A.; Borkowski, A.; Gaitatzis, N.; Banzhoff, A.; Clemens, R. Homologous and heterologous antibody responses to a one-year booster dose of an MF59®: Adjuvanted A/H5N1 pre-pandemic influenza vaccine in pediatric subjects. Hum. Vaccines Immunother. 2012, 8, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Chanthavanich, P.; Anderson, E.; Kerdpanich, P.; Bulitta, M.; Kanesa-Thasan, N.; Hohenboken, M. Safety, Tolerability and Immunogenicity of an MF59-adjuvanted, Cell Culture-derived, A/H5N1, Subunit Influenza Virus Vaccine: Results from a Dose-finding Clinical Trial in Healthy Pediatric Subjects. Pediatr. Infect. Dis. J. 2019, 38, 757–764. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Beran, J.; Devaster, J.-M.; Esen, M.; Launay, O.; Leroux-Roels, G.; Ruiz-Palacios, G.M.; Van Essen, G.A.; Caplanusi, A.; Claeys, C.; et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: A phase 3 randomised trial. Lancet Infect. Dis. 2013, 13, 485–496. [Google Scholar] [CrossRef]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef]
- Uyemura, K.; Castle, S.C.; Makinodan, T. The frail elderly: Role of dendritic cells in the susceptibility of infection. Mech. Ageing Dev. 2002, 123, 955–962. [Google Scholar] [CrossRef]
- Seubert, A.; Monaci, E.; Pizza, M.; O’Hagan, D.T.; Wack, A. The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells. J. Immunol. 2008, 180, 5402–5412. [Google Scholar] [CrossRef]
- O’Hagan, D.; Ott, G.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; De Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar] [CrossRef]
- Khurana, S.; Chearwae, W.; Castellino, F.; Manischewitz, J.; King, L.R.; Honorkiewicz, A.; Rock, M.T.; Edwards, K.M.; Del Giudice, G.; Rappuoli, R.; et al. Vaccines with MF59 Adjuvant Expand the Antibody Repertoire to Target Protective Sites of Pandemic Avian H5N1 Influenza Virus. Sci. Transl. Med. 2010, 2, 15ra5. [Google Scholar] [CrossRef]
- Nicolay, U.; Heijnen, E.; Nacci, P.; Patriarca, P.A.; Leav, B. Immunogenicity of aIIV3, MF59-adjuvanted seasonal trivalent influenza vaccine, in older adults ≥65 years of age: Meta-analysis of cumulative clinical experience. Int. J. Infect. Dis. 2019, 85s, s1–s9. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Castilletti, C.; Tempestilli, M.; Piacentini, P.; Bordi, L.; Chiappini, R.; Agrati, C.; Squarcione, S.; Ippolito, G.; Puro, V.; et al. Cross-subtype Immunity against Avian Influenza in Persons Recently Vaccinated for Influenza. Emerg. Infect. Dis. 2008, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Banzhoff, A.; Gasparini, R.; Laghi-Pasini, F.; Staniscia, T.; Durando, P.; Montomoli, E.; Capecchi, P.L.; di Giovanni, P.; Sticchi, L.; Gentile, C.; et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE 2009, 4, e4384. [Google Scholar] [CrossRef]
- Frey, S.E.; Reyes, M.R.D.L.; Reynales, H.; Bermal, N.N.; Nicolay, U.; Narasimhan, V.; Forleo-Neto, E.; Arora, A.K. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014, 32, 5027–5034. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Reynales, H.; Poder, A.; Yu, C.Y.; Pitisuttithum, P.; Yuan, L.L.; Vermeulen, W.; Verhoeven, C.; Leav, B.; Zhang, B.; et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: A randomised, controlled, multicentre, phase 3 efficacy study. Lancet Infect. Dis. 2021, 21, 1027–1037. [Google Scholar] [CrossRef]
- World Health Organization. Report Prepared for the WHO Annual Consultation on the Composition of Influenza Vaccine for the Northern Hemisphere 2017–2018; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLOS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Perez, S.D.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef]
- Blanton, L.; Wentworth, D.E.; Alabi, N.; Azziz-Baumgartner, E.; Barnes, J.; Brammer, L.; Burns, E.; Davis, C.T.; Dugan, V.G.; Fry, A.M.; et al. Update: Influenza Activity—United States and Worldwide, May 21–September 23, 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1043–1051. [Google Scholar] [CrossRef]
- Rajaram, S.; Van Boxmeer, J.; Leav, B.; Suphaphiphat, P.; Iheanacho, I.; Kistler, K. 2556. Retrospective Evaluation of Mismatch from Egg-Based Isolation of Influenza Strains Compared with Cell-Based Isolation and the Possible Implications for Vaccine Effectiveness. Open Forum Infect. Dis. 2018, 5, S69. [Google Scholar] [CrossRef]
| aH5N1c a | ||||||
|---|---|---|---|---|---|---|
| Group A (n = 804) | Group B (n = 799) | Group C (n = 795) | All aH5N1c (n = 2398) | Placebo (n = 798) | Total (N = 3196) | |
| Mean age ± SD, years | 58.1 ± 17.67 | 57.5 ± 17.83 | 57.5 ± 18.24 | 57.7 ± 17.91 | 57.7 ± 18.29 | 57.7 ± 18.00 |
| Age group, n (%) | ||||||
| 18 to <65 years | 403 (50.1) | 399 (49.9) | 397 (49.9) | 1199 (50.0) | 398 (49.9) | 1597 (50.0) |
| ≥65 years | 401 (49.9) | 400 (50.1) | 398 (50.1) | 1199 (50.0) | 400 (50.1) | 1599 (50.0) |
| Female, n (%) | 444 (55.2) | 434 (54.3) | 447 (56.2) | 1325 (55.3) | 438 (54.9) | 1763 (55.2) |
| Race, n (%) | ||||||
| White | 668 (83.1) | 679 (85.0) | 674 (84.8) | 2021 (84.3) | 665 (83.3) | 2686 (84.0) |
| Black | 110 (13.7) | 102 (12.8) | 104 (13.1) | 316 (13.2) | 112 (14.0) | 428 (13.4) |
| Asian | 12 (1.5) | 7 (0.9) | 9 (1.1) | 28 (1.2) | 7 (0.9) | 35 (1.1) |
| Native American or Alaskan Native | 6 (0.7) | 4 (0.5) | 5 (0.6) | 15 (0.6) | 3 (0.4) | 18 (0.6) |
| Native Hawaiian or other Pacific Islander | 3 (0.4) | 2 (0.3) | 1 (0.1) | 6 (0.3) | 4 (0.5) | 10 (0.3) |
| Other | 5 (0.6) | 5 (0.6) | 2 (0.3) | 12 (0.5) | 7 (0.9) | 19 (0.6) |
| Hispanic ethnicity, n (%) | 53 (6.6) | 61 (7.6) | 64 (8.1) | 178 (7.4) | 55 (6.9) | 233 (7.3) |
| Mean weight ± SD, kg | 78.86 ± 15.0 | 80.22 ± 15.2 | 78.86 ± 15.3 | 79.31 ± 15.2 | 79.84 ± 15.3 | 79.44 ± 15.2 |
| Mean BMI ± SD, kg/m2 | 27.40 ± 4.2 | 27.86 ± 4.1 | 27.41± 4.2 | 27.56 ± 4.2 | 27.60 ± 4.2 | 27.57 ± 4.2 |
| Previous influenza vaccine in past 12 months, n (%) | ||||||
| All subjects | 430 (53.5) | 416 (52.1) | 426 (53.6) | 1272 (53.0) | 422 (52.9) | 1694 (53.0) |
| Age 18 to <65 years | 145 (36.0) | 137 (34.3) | 139 (35.0) | 421 (35.1) | 123 (30.9) | 544 (34.1) |
| Age ≥65 years | 285 (71.1) | 279 (69.8) | 287 (72.1) | 851 (71.0) | 299 (74.8) | 1150 (71.9) |
| 18 to <65 Years | ≥65 Years | Total Population | ||||
|---|---|---|---|---|---|---|
| aH5N1c | Placebo | aH5N1c | Placebo | aH5N1c | Placebo | |
| Day 1, n | 1116 | 372 | 1133 | 367 | 2249 | 739 |
| GMT (95% Cl) | 13.5 (12.8–14.2) | 13.7 (12.5- 15.0) | 20.5 (19.4–21.8) | 20.6 (18.6–22.7) | 16.6 (16.0–17.3) | 16.7 (15.6–17.9) |
| Day 22, n | 1115 | 370 | 1130 | 366 | 2245 | 736 |
| GMT (95% Cl) | 50.6 (47.6–53.8) | 11.6 (10.4–12.9) | 42.4 (40.0–45.0) | 14.5 (13.1–16.0) | 46.4 (44.5–48.4) | 13.0 (12.1–14.0) |
| GMR Day 22/Day 1 (95% Cl) | 3.81 (3.58–4.05) | 0.87 (0.79–0.97) | 2.14 (2.02–2.27) | 0.73 (0.66–0.81) | 2.86 (2.74–2.98) | 0.80 (0.74–0.86) |
| Day 43, n | 1076 | 349 | 1080 | 351 | 2156 | 700 |
| GMT (95% Cl) | 170.7 (160.5–181.6) | 11.0 (9.9–12.2) | 97.9 (92.1–104.1) | 16.7 (15.0–18.5) | 130.6 (124.8–136.6) | 13.7 (12.6–14.8) |
| GMR Day 43/Day 1 (95% Cl) | 12.70 (11.94–13.51) | 0.82 (0.73–0.91) | 4.90 (4.61–5.20) | 0.83 (0.75–0.92) | 7.96 (7.61–8.33) | 0.83 (0.77–0.90) |
| Day 183, n | 1025 | 341 | 1054 | 346 | 2079 | 687 |
| GMT (95% Cl) | 20.4 (19.3–21.6) | 6.8 (6.1–7.4) | 19.3 (18.2–20.4) | 8.6 (7.8–9.5) | 20.0 (19.2–20.8) | 7.7 (7.2–8.2) |
| GMR Day 183/Day 1 (95% Cl) | 1.53 (1.44–1.61) | 0.51 (0.46–0.56) | 0.97 (0.91–1.02) | 0.43 (0.39–0.47) | 1.22 (1.17–1.27) | 0.47 (0.44–0.50) |
| Age 18 to <65 Years | Age ≥65 Years | Total Population | ||||
|---|---|---|---|---|---|---|
| aH5N1c | Placebo | aH5N1c | Placebo | aH5N1c | Placebo | |
| Day 22, n | 1115 | 370 | 1130 | 366 | 2245 | 736 |
| Seroconversion, % (95% CI) | 40.4 (37.6–43.4) | 1.9 (0.8–3.9) | 24.2 (21.7–26.8) | 0.3 (0.0–1.5) | 32.2 (30.3–34.2) | 1.1 (0.5–2.1) |
| Day 43, n | 1076 | 349 | 1080 | 351 | 2156 | 700 |
| Seroconversion, % (95% CI) | 79.9 (77.4–82.3) | 0.3 (0.0–1.6) | 54.0 (51.0–57.0) | 1.7 (0.6–3.7) | 66.9 (64.9–68.9) | 1.0 (0.4–2.0) |
| Day 183, n | 1025 | 341 | 1054 | 346 | 2079 | 687 |
| Seroconversion, % (95% CI) | 16.2 (14.0–18.6) | 0.3 (0.0–1.6) | 8.0 (6.4–9.8) | 1.2 (0.3–2.9) | 12.0 (10.7–13.5) | 0.7 (0.2–1.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, J.; Van Twuijver, E.; Versage, E.; Hohenboken, M. Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects. Vaccines 2022, 10, 497. https://doi.org/10.3390/vaccines10040497
Peterson J, Van Twuijver E, Versage E, Hohenboken M. Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects. Vaccines. 2022; 10(4):497. https://doi.org/10.3390/vaccines10040497
Chicago/Turabian StylePeterson, James, Esther Van Twuijver, Eve Versage, and Matthew Hohenboken. 2022. "Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects" Vaccines 10, no. 4: 497. https://doi.org/10.3390/vaccines10040497
APA StylePeterson, J., Van Twuijver, E., Versage, E., & Hohenboken, M. (2022). Phase 3 Randomized, Multicenter, Placebo-Controlled Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of an Adjuvanted Cell Culture-Derived, H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects. Vaccines, 10(4), 497. https://doi.org/10.3390/vaccines10040497






