Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Serological Assay
2.3. Definition of SARS-CoV-2 Infections
2.4. Statistical Analysis
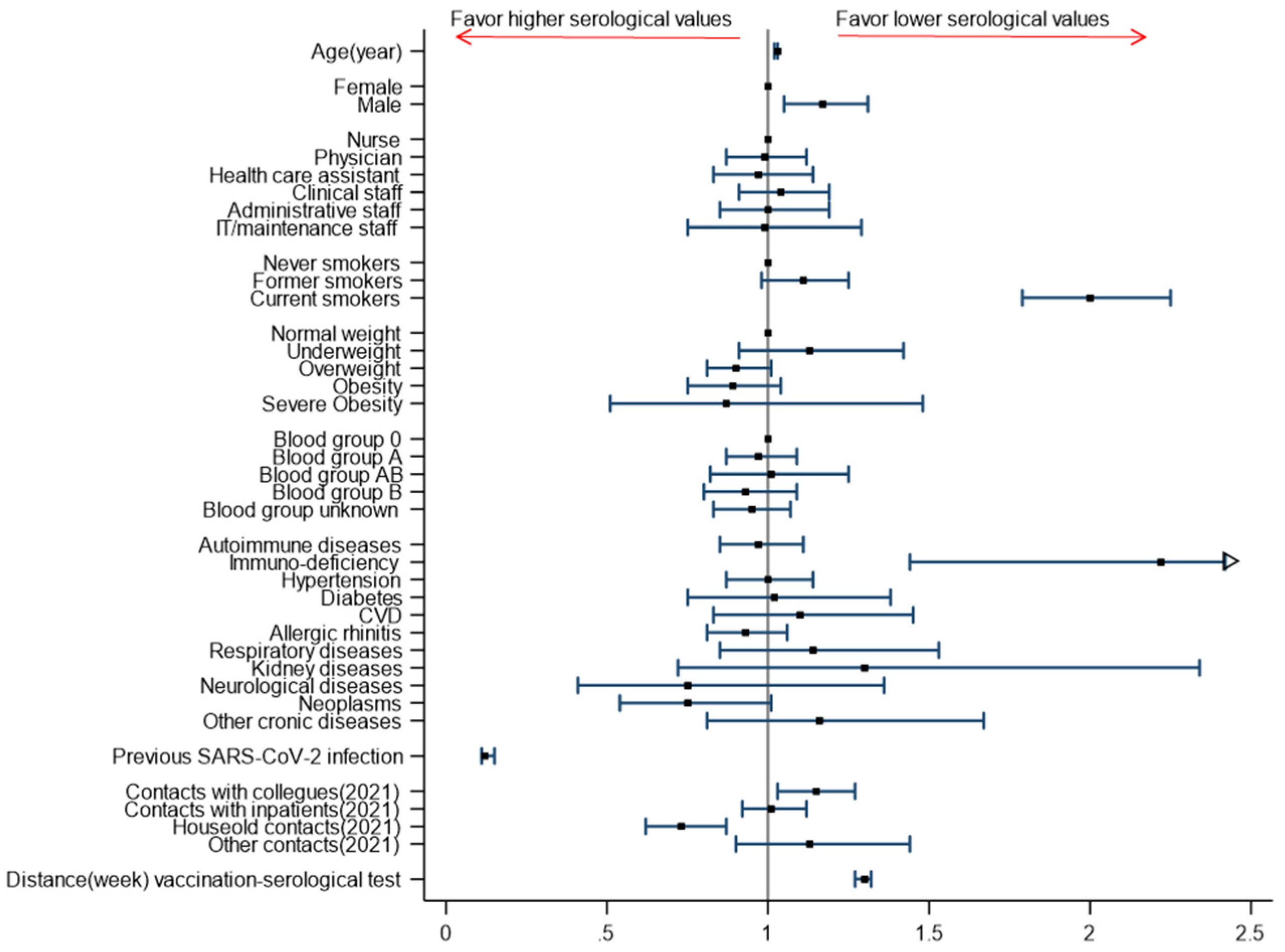
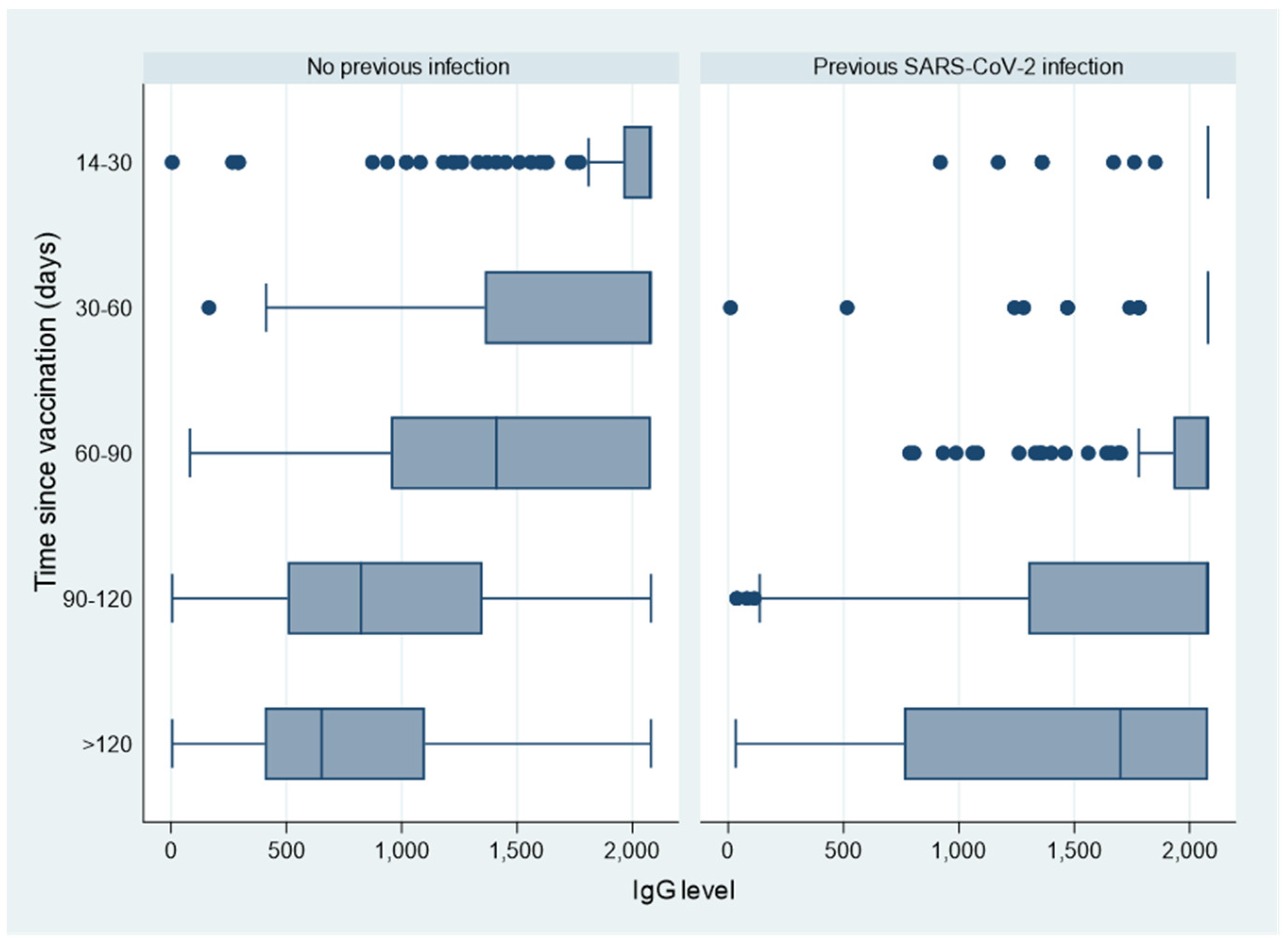
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scozzari, G.; Costa, C.; Migliore, E.; Coggiola, M.; Ciccone, G.; Savio, L.; Scarmozzino, A.; Pira, E.; Cassoni, P.; Galassi, C.; et al. Prevalence, Persistence, and Factors Associated with SARS-CoV-2 IgG Seropositivity in a Large Cohort of Healthcare Workers in a Tertiary Care University Hospital in Northern Italy. Viruses 2021, 13, 1064. [Google Scholar] [CrossRef]
- Bonelli, F.; Blocki, F.A.; Bunnell, T.; Chu, E.; De La Arriel, O.A.; Grenache, D.G.; Marzucchi, G.; Montomoli, E.; Okoye, L.; Pallavicini, L.; et al. Evaluation of the automated LIAISON® SARS-CoV-2 TrimericS IgG assay for the detection of circulating antibodies. Clin. Chem. Lab. Med. CCLM 2021, 59, 1463–1467. [Google Scholar] [CrossRef]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.-C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [Green Version]
- WHO International Standard. First WHO International Standard for anti-SARS-CoV-2 Immunoglobulin (Human). NIBSC Code: 20/136 Instructions for Use (Version 2.0, Dated 17/12/2020); NIBSC, Medicines & Healthcare Products Regulatory Agency: London, UK, 2020. [Google Scholar]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, e7–e1516. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, I.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Kontou, E.; Ranellou, K.; Zoulas, D.; Bletsa, A.; Rompola, E.; Piperaki, E.-T.; Athanasiou, N.; Ampelakiotou, K.; Pratikaki, M.; Stergiopoulou, C.; et al. Antibody Response Following a Two-Dose mRNA Vaccination Regimen, in Health Care Workers of a Tertiary Hospital in Athens, Greece. J. Pers. Med. 2021, 11, 576. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef]
- Guzmán-Martínez, O.; Guardado, K.; de Guevara, E.L.; Navarro, S.; Hernández, C.; Zenteno-Cuevas, R.; Montero, H. IgG Antibodies Generation and Side Effects Caused by Ad5-nCoV Vaccine (CanSino Biologics) and BNT162b2 Vaccine (Pfizer/BioNTech) among Mexican Population. Vaccines 2021, 9, 999. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Montero, H. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Product Information. 03/11/2021 Comirnaty—EMEA/H/C/005735—X/044; European Medicines Agency: Amsterdam, the Netherlands, 2021.
- Padoan, A.; Dall’Olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257. [Google Scholar] [CrossRef]
- Vassilaki, N.; Gargalionis, A.N.; Bletsa, A.; Papamichalopoulos, N.; Kontou, E.; Gkika, M.; Patas, K.; Theodoridis, D.; Manolis, I.; Ioannidis, A.; et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms 2021, 9, 1725. [Google Scholar] [CrossRef]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushida, K.; Hanaoka, H.; Nakada, T.-A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, e1–e1861. [Google Scholar] [CrossRef]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines 2021, 9, 1042. [Google Scholar] [CrossRef]
- Tober-Lau, P.; Schwarz, T.; Vanshylla, K.; Hillus, D.; Gruell, H.; Suttorp, N.; Landgraf, I.; Kappert, K.; Seybold, J.; Drosten, C.; et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir. Med. 2021, 9, e104–e105. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.-L.; Liu, H.; Zeng, Y.-Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [Green Version]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef] [Green Version]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogata, H.; Mori, M.; Jingushi, Y.; Matsuzaki, H.; Katahira, K.; Ishimatsu, A.; Enokizu-Ogawa, A.; Taguchi, K.; Moriwaki, A.; Yoshida, M. Impact of visceral fat on the prognosis of coronavirus disease 2019: An observational cohort study. BMC Infect. Dis. 2021, 21, 1240. [Google Scholar] [CrossRef] [PubMed]
- Picard, C.; Bobby Gaspar, H.; Al-Herz, W.; Bousfiha, A.; Casanova, J.-L.; Chatila, T.; Crow, Y.J.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J. Clin. Immunol. 2018, 38, 96–128. [Google Scholar] [CrossRef] [Green Version]
- Pulvirenti, F.; Fernandez Salinas, A.; Milito, C.; Terreri, S.; Piano Mortari, E.; Quintarelli, C.; Di Cecca, S.; Lagnese, G.; Punziano, A.; Quercio, M.; et al. B Cell Response Induced by SARS-CoV-2 Infection Is Boosted by the BNT162b2 Vaccine in Primary Antibody Deficiencies. Cells 2021, 10, 2915. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Piubelli, C.; Gobbi, F.; Martini, D.; Bertoli, G.; Ursini, T.; Moro, L.; Ronzoni, N.; Angheben, A.; Rodari, P.; et al. Antibody response induced by the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers, with or without prior SARS-CoV-2 infection: A prospective study. Clin. Microbiol. Infect. 2021, 27, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, A.; Borleri, D.; Farina, C.; Napolitano, G.; Valenti, D.; Rizzi, M.; Maggiolo, F. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 4612–4615. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Salvaggio, M.; Fusina, F.; Albani, F.; Salvaggio, M.; Beschi, R.; Ferrari, E.; Costa, A.; Agnoletti, L.; Facchi, E.; Natalini, G. Antibody Response after BNT162b2 Vaccination in Healthcare Workers Previously Exposed and Not Exposed to SARS-CoV-2. J. Clin. Med. 2021, 10, 4204. [Google Scholar] [CrossRef]
- Ferrari, D.; Di Resta, C.; Tomaiuolo, R.; Sabetta, E.; Pontillo, M.; Motta, A.; Locatelli, M. Long-term antibody persistence and exceptional vaccination response on previously SARS-CoV-2 infected subjects. Vaccine 2021, 39, 4256–4260. [Google Scholar] [CrossRef]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Appelman, B.; van der Straten, K.; Lavell, A.H.A.; Schinkel, M.; Slim, M.A.; Poniman, M.; Burger, J.A.; Oomen, M.; Tejjani, K.; Vlar, A.P.J.; et al. Time since SARS-CoV-2 infection and humoral immune response following BNT162b2 mRNA vaccination. EBioMedicine 2021, 72, 103589. [Google Scholar] [CrossRef] [PubMed]
| Participants and Prevalence of Positivity | Serological Values | ||
|---|---|---|---|
| Total—N (%) | Serological Positivity—N (%) | IgG Value (BAU/mL)—Median (IQR) | |
| OVERALL | 6687 | 6675 (99.8%) | 990 (551–1870) |
| Age (yrs) | |||
| Mean (SD) | 47.4 (11.3) | ||
| Median (IQR) | 49.5 (38.7–56.5) | ||
| Age-groups (yrs): | |||
| ≤29 | 751 (11.2%) | 751 (100.0%) | 1360 (821–2060) |
| 30–39 | 1055 (15.8%) | 1055 (100.0%) | 1090 (667–1860) |
| 40–49 | 1643 (24.6%) | 1641 (99.9%) | 912 (518–1710) |
| 50–59 | 2417 (36.1%) | 2410 (99.7%) | 910 (487–1870) |
| ≥60 | 821 (12.3%) | 818 (99.6%) | 873 (487–1770) |
| Gender | |||
| Female | 5006 (74.9%) | 4997 (99.8%) | 1030 (570–1890) |
| Male | 1681 (25.1%) | 1678 (99.8%) | 874 (511–1740) |
| Job profile | |||
| Nurse | 2315 (34.6%) | 2312 (99.9%) | 1010 (544–1950) |
| Physician | 1644 (24.6%) | 1640 (99.8%) | 975 (573–1745) |
| Health care assistant (HCA) | 748 (11.2%) | 745 (99.6%) | 1020 (511–2080) |
| Clinical staff (other than physician/nurse/HCA) | 1073 (16.0%) | 1071 (99.8%) | 960 (550–1670) |
| Administrative staff | 692 (10.3%) | 692 (100.0%) | 981 (553–1810) |
| IT/maintenance staff | 215 (3.2%) | 215 (100.0%) | 905 (496–1790) |
| Smoking habit | |||
| Never smokers | 4054 (60.6%) | 4050 (99.9%) | 1100 (624–1990) |
| Former smokers | 1152 (17.2%) | 1149 (99.7%) | 978 (555–1935) |
| Current smokers | 1481 (22.1%) | 1476 (99.7%) | 729 (399–1360) |
| Body Mass Index (BMI) | |||
| Underweight (BMI < 18.5) | 296 (4.4%) | 295 (99.7%) | 973 (493–1550) |
| Normal weight (BMI18.5–25) | 4071 (60.9%) | 4066 (99.9%) | 996 (555–1820) |
| Overweight (BMI 25–30) | 1626 (24.3%) | 1624 (99.9%) | 973 (556–1980) |
| Obesity (BMI 30–40) | 641 (9.6%) | 639 (99.7%) | 970 (528–2070) |
| Severe obesity (BMI > 40) | 53 (0.8%) | 51 (96.2%) | 1170 (533–2080) |
| AB0 Blood Group | |||
| 0 | 2375 (35.5%) | 2372 (99.9%) | 964 (545–1810) |
| A | 1939 (29.0%) | 1935 (99.8%) | 1050 (571–1930) |
| AB | 328 (4.9%) | 326 (99.4%) | 923 (500–1850) |
| B | 706 (10.6%) | 706 (100.0%) | 1000 (553–1840) |
| Unknown | 1339 (20.0%) | 1336 (99.8%) | 956 (542–1850) |
| Participants and Prevalence of Positivity | Serological Values | ||
|---|---|---|---|
| Total—N (%) | Serological Positivity—N (%) | IgG Value (BAU/mL)—Median (IQR) | |
| OVERALL | 6687 | 6675 (99.8%) | 990 (551–1870) |
| Previous SARS-CoV-2 infection * | |||
| No | 5465 (81.7%) | 5455 (99.8%) | 859 (504–1450) |
| Yes | 1222 (18.3%) | 1220 (99.8%) | 2080 (1340–2080) |
| Contacts at risk in 2021 | |||
| -with collegues | |||
| No | 4687 (70.1%) | 4678 (99.8%) | 1020 (566–1930) |
| Yes | 2000 (29.9%) | 1997 (99.9%) | 901 (519–1690) |
| -with patients | |||
| No | 3758 (56.2%) | 3749 (99.8%) | 1000 (543–1880) |
| Yes | 2929 (43.8%) | 2926 (99.9%) | 971 (559–1860) |
| -in the household | |||
| No | 6186 (92.5%) | 6174 (99.8%) | 975 (544–1850) |
| Yes | 501 (7.5%) | 501 (100.0%) | 1100 (661–2080) |
| -others | |||
| No | 6432 (96.2%) | 6420 (99.8%) | 994 (552–1870) |
| Yes | 255 (3.8%) | 255 (100.0%) | 942 (545–1570) |
| Distance (weeks) between vaccination and serological test | |||
| Mean (SD) | 14.2 (3.1) | ||
| Median (IQR) | 14.9 (13.6–16.0) | ||
| Distance categories: | |||
| 2–4 weeks | 175 (2.6%) | 174 (99.4%) | 2080 (2080–2080) |
| 4–8 weeks | 260 (3.9%) | 259 (99.6%) | 2080 (1730–2080) |
| 8–12 weeks | 422 (6.3%) | 422 (100.0%) | 1685 (1060–2080) |
| 12–16 weeks | 4214 (63.0%) | 4208 (99.9%) | 931 (553–1670) |
| >16 weeks | 1513 (22.6%) | 1509 (99.7%) | 716 (423–1300) |
| Vaccination date not known | 103 (1.5%) | 103 (100.0%) | 1480 (829–2080) |
| Participants and Prevalence of Positivity | Serological Values | ||
|---|---|---|---|
| Total—N (%) | Serological Positivity—N (%) | IgG Value (BAU/mL)—Median (IQR) | |
| OVERALL | 6687 | 6675 (99.8%) | 990 (551–1870) |
| Autoimmune diseases | |||
| No | 5812 (86.9%) | 5806 (99.9%) | 987 (552–1860) |
| Yes | 875 (13.1%) | 869 (99.3%) | 1010 (541–1920) |
| Immunodeficiency | |||
| No | 6609 (98.8%) | 6601 (99.9%) | 995 (554–1870) |
| Yes | 78 (1.2%) | 74 (94.9%) | 629 (327–1440) |
| Hypertension | |||
| No | 5660 (84.6%) | 5652 (99.9%) | 1000 (563–1860) |
| Yes | 1027 (15.4%) | 1023 (99.6%) | 896 (495–1920) |
| Diabetes | |||
| No | 6530 (97.7%) | 6519 (99.8%) | 995 (553–1860) |
| Yes | 157 (2.3%) | 156 (99.4%) | 876 (484–2080) |
| Cardiovascular diseases | |||
| No | 6499 (97.2%) | 6487 (99.8%) | 995 (555–1860) |
| Yes | 188 (2.8%) | 188 (100.0%) | 873 (439–1960) |
| Allergic rhinitis | |||
| No | 5801 (86.8%) | 5792 (99.8%) | 981 (543–1850) |
| Yes | 886 (13.2%) | 883 (99.7%) | 1040 (598–1950) |
| Respiratory diseases | |||
| No | 6513 (97.4%) | 6501 (99.8%) | 991 (553–1860) |
| Yes | 174 (2.6%) | 174 (100.0%) | 935 (481–1880) |
| Kidney diseases | |||
| No | 6647 (99.4%) | 6636 (99.8%) | 991 (552–1870) |
| Yes | 40 (0.6%) | 39 (97.5%) | 786 (378–1620) |
| Neurological diseases | |||
| Yes | 6649 (99.4%) | 6638 (99.8%) | 990 (552–1870) |
| SI | 38 (0.6%) | 37 (97.4%) | 1150 (483–1530) |
| Neoplasms | |||
| No | 6543 (97.8%) | 6532 (99.8%) | 990 (552–1860) |
| Yes | 144 (2.2%) | 143 (99.3%) | 1025 (519–1970) |
| Other chronic diseases | |||
| No | 6582 (98.4%) | 6570 (99.8%) | 989 (551–1860) |
| Yes | 105 (1.6%) | 105 (100.0%) | 1110 (585–1930) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, C.; Migliore, E.; Galassi, C.; Scozzari, G.; Ciccone, G.; Coggiola, M.; Pira, E.; Scarmozzino, A.; La Valle, G.; Cassoni, P.; et al. Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines 2022, 10, 474. https://doi.org/10.3390/vaccines10030474
Costa C, Migliore E, Galassi C, Scozzari G, Ciccone G, Coggiola M, Pira E, Scarmozzino A, La Valle G, Cassoni P, et al. Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines. 2022; 10(3):474. https://doi.org/10.3390/vaccines10030474
Chicago/Turabian StyleCosta, Cristina, Enrica Migliore, Claudia Galassi, Gitana Scozzari, Giovannino Ciccone, Maurizio Coggiola, Enrico Pira, Antonio Scarmozzino, Giovanni La Valle, Paola Cassoni, and et al. 2022. "Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers" Vaccines 10, no. 3: 474. https://doi.org/10.3390/vaccines10030474
APA StyleCosta, C., Migliore, E., Galassi, C., Scozzari, G., Ciccone, G., Coggiola, M., Pira, E., Scarmozzino, A., La Valle, G., Cassoni, P., Cavallo, R., & on behalf of the Collaborative Group. (2022). Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines, 10(3), 474. https://doi.org/10.3390/vaccines10030474






