An Outbreak of Equine Herpesvirus-4 in an Ecological Donkey Milk Farm in Romania
Abstract
1. Introduction
2. Outbreak Description
2.1. Background and Premises
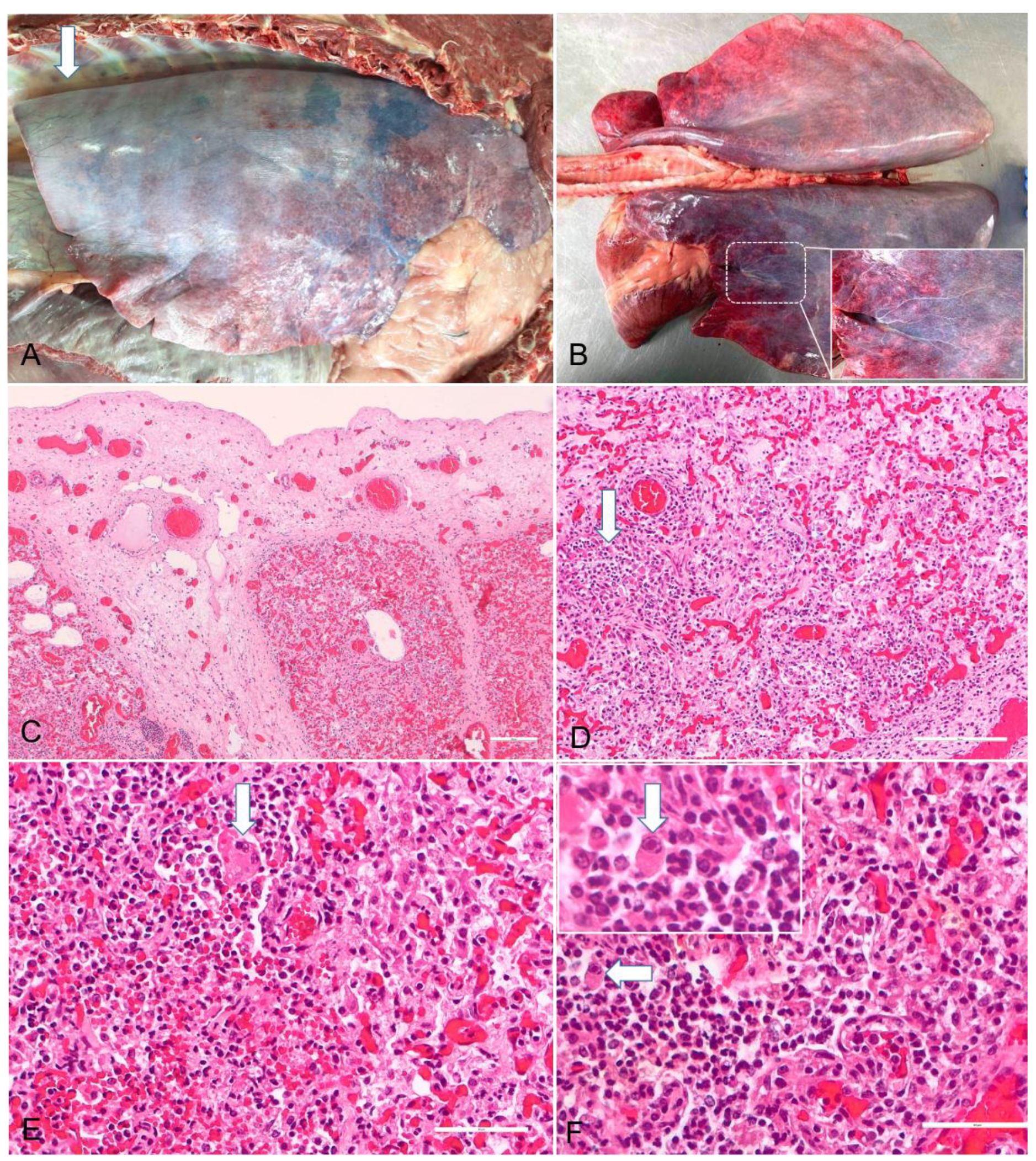
2.2. Diagnosis
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, J.R.; Heldens, J. Equine herpesviruses 1 (EHV-1) and 4 (EHV-4)—Epidemiology, disease and immunoprophylaxis: A brief review. Vet. J. 2005, 170, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Azab, W.; Osterrieder, N. Equine herpesviruses type 1 (EHV-1) and 4 (EHV-4)-masters of co-evolution and a constant threat to equids and beyond. Vet. Microbiol. 2013, 167, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.; Sattler, C.; Adler, H. Herpesviruses and Their Host Cells: A Successful Liaison. Trends Microbiol. 2017, 25, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Leutenegger, C.M.; Wilson, W.D.; Watson, J.L.; Ferraro, G.L.; Madigan, J.E. Equine herpesvirus-4 kinetics in peripheral blood leukocytes and nasopharyngeal secretions in foals using quantitative real-time TaqMan PCR. J. Vet. Diagn. Investig. 2005, 17, 578–581. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Dubovnik, E.J. Herpesvirales in Fenner’s Veterinary Virology, 5th ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 9; pp. 189–216. ISBN 9780128009468. [Google Scholar] [CrossRef]
- Mekonnen, A.; Eshetu, A.; Gizaw, D. Equine herpesvirus 1 and/or 4 in working equids: Seroprevalence and risk factors in North Shewa Zone, Ethiopia. Ethiop. Vet. J. 2017, 21, 28–39. [Google Scholar] [CrossRef][Green Version]
- Yildirim, Y.; Yilmaz, V.; Kirmizigul, A.H. Equine herpes virus type 1 (EHV-1) and 4 (EHV-4) infections in horses and donkeys in northeastern Turkey. Iran. J. Vet. Res. 2015, 16, 341–344. [Google Scholar]
- Badenhorst, M.; Page, P.; Ganswindt, A.; Laver, P.; Guthrie, A.; Schulman, M. Detection of equine herpesvirus-4 and physiological stress patterns in young Thoroughbreds consigned to a South African auction sale. BMC Vet. Res. 2015, 11, 126. [Google Scholar] [CrossRef][Green Version]
- Carlson, J.K.; Traub-Dargatz, J.L.; Lunn, D.P.; Morley, P.S.; Kohler, A.; Kasper, K.; Landolt, G.A.; Barnett, C.; Lunn, K.F. Equine viral respiratory pathogen surveillance at horse shows and sales. J. Equine Vet. Sci. 2013, 33, 229–237. [Google Scholar] [CrossRef]
- Gilkerson, J.R.; Teague, N.; Whalley, J.M.; Love, D.N. A prospective cohort study of upper respiratory tract disease in one- and two-year-old racehorses. Serological evaluation of the role of equine herpesviruses 1 and 4 (EHV-1 and EHV-4) in respiratory disease. Aust. Equine Vet. 1999, 17, 76–81. [Google Scholar]
- Pusterla, N.; Kass, P.H.; Mapes, S.; Johnson, C.; Barnett, D.C.; Vaala, W.; Gutierrez, C.; Mcdaniel, R.; Whitehead, B.; Manning, J. Surveillance programme for important equine infectious respiratory pathogens in the USA. Vet. Rec. 2011, 169, 12. [Google Scholar] [CrossRef]
- Temesgen, T.; Getachew, Y.; Negussie, H. Molecular Identification of Equine Herpesvirus 1, 2, and 5 in Equids with Signs of Respiratory Disease in Central Ethiopia. Vet. Med. 2021, 12, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Bain, F.; James, K.; Mapes, S.; Kenelty, K.; Barnett, D.C.; Gaughan, E.; Craig, B.; Chappell, D.E.; Vaala, W. Frequency of molecular detection of equine herpesvirus-4 in nasal secretions of 3028 horses with upper airway infection. Vet. Rec. 2017, 180, 593. [Google Scholar] [CrossRef] [PubMed]
- Ploszay, G.; Rola, J.; Larska, M.; Zmudzinski, J.F. First report on equine herpesvirus type 4 isolation in Poland--evaluation of diagnostic tools. Pol. J. Vet. Sci. 2013, 16, 493–500. [Google Scholar] [CrossRef][Green Version]
- Diallo, I.; Hewitson, G.; Wright, L.; Kelly, M.; Rodwell, B.; Corney, B. Multiplex real-time PCR for the detection and differentiation of equid herpesvirus I (EHV-1) and equid herpesvirus 4 (EHV-4). Vet. Microbiol. 2007, 123, 93–103. [Google Scholar] [CrossRef]
- Hartley, C.A.; Wilks, C.R.; Studdert, M.J.; Gilkerson, J.R. Comparison of antibody detection assays for the diagnosis of equine herpesvirus 1 and 4 infections in horses. Am. J. Vet. Res. 2005, 66, 921–928. [Google Scholar] [CrossRef]
- Wang, L.; Raidal, S.L.; Pizzirani, A.; Wilcox, G.E. Detection of respiratory herpesviruses in foals and adult horses determined by nested multiplex PCR. Vet. Microbiol. 2007, 121, 18–28. [Google Scholar] [CrossRef] [PubMed]
- OIE Guidelines. Available online: https://www.oie.int/app/uploads/2021/03/a-hhp-handbook-oct-2018.pdf (accessed on 17 August 2021).
- Windsor, P. How to implement farm biosecurity: The role of government and private sector. In Proceedings of the 30th Conference of the OIE Regional Commission for Asia, the Far East and Oceania, Putrajaya, Malaysia, 20–24 November 2017. [Google Scholar] [CrossRef]
- Ataseven, V.S.; Dagalp, S.B.; Güzel, M.; Basaran, Z.; Tan, M.T.; Geraghty, B. Prevalence of equine herpesvirus-1 and equine herpesvirus-4 infections in equidae species in Turkey as determined by ELISA and multiplex nested PCR. Res. Vet. Sci. 2009, 86, 339–344. [Google Scholar] [CrossRef]
- Dayaram, A.; Seeber, P.A.; Greenwood, A.D. Environmental Detection and Potential Transmission of Equine Herpesviruses. Pathogens 2021, 10, 423. [Google Scholar] [CrossRef]
- Goehring, L.S.; Landolt, G.A.; Morley, P.S. Detection and management of an outbreak of equine herpesvirus type 1 infection and associated neurological disease in a veterinary teaching hospital. J. Vet. Intern. Med. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Pritchard, J.C.; Lindberg, A.C.; Main, D.C.; Whay, H.R. Assessment of the welfare of working horses, mules and donkeys, using health and behaviour parameters. Prev. Vet. Med. 2005, 69, 265–283. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar, C.; EFSA Panel on Animal Health and Welfare (AHAW); et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Infection with Equine Herpesvirus-1. EFSA J. 2022, 20, e07036. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.B.F.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.M.; Cavalcante, G.T.; Lara, M.; Villalobos, E.M.C.; Cunha, E.M.S.; Okuda, L.H.; Stéfano, E.d.; Nassar, A.F.d.C.; Souza, G.O.; Vasconcellos, S.A.; et al. Seroprevalence of viral and bacterial agents in equids from Monte Negro Municipality, State of Rondônia. Braz. J. Vet. Res. Anim. Sci. 2008, 45, 269–276. [Google Scholar] [CrossRef]
- De Souz, H.L.M.d.C.C.; Torelli, C.S.; Cunha, E.M.S.; Villalobos, E.M.C.; Cunha, M.S.; Bello, A.C.P.P.; Cunha, A.P.; Reis, J.K.P.; Leite, R.C.; Mori, E. Serological survey of equine herpesvirus infection in Minas Gerais state. Braz. J. Vet. Res. Anim. Sci. 2010, 47, 352–356. [Google Scholar]
- Chenchev, I.; Rusenova, N.; Sandev, N. Seroepidemiological studies of donkeys’ blood for detection of some virus infections on ungulates. Trakia J. Sci. 2011, 9, 82–86. [Google Scholar]
- Getachew, M.; Alemayehu, F.; Chala, C.; Amare, B.; Kassa, D.; Burden, F.; Wernery, R.; Wernery, U. A cross-sectional sero-survey of some infectious diseases of working equids in Central Ethiopia. J. Vet. Med. Anim. Health 2014, 6, 231–238. [Google Scholar]
- Abdelgawad, A.; Hermes, R.; Damiani, A.; Lamglait, B.; Czirják, G.Á.; East, M.; Aschenborn, O.; Wenker, C.; Kasem, S.; Osterrieder, N.; et al. Comprehensive Serology Based on a Peptide ELISA to Assess the Prevalence of Closely Related Equine Herpesviruses in Zoo and Wild Animals. PLoS ONE 2015, 10, e138370. [Google Scholar] [CrossRef]
- Pusterla, N.; Mapes, S.; Akana, N.; Barnett, C.; Mackenzie, C.; Gaughan, E.; Craig, B.; Chappell, D.; Vaala, W. Prevalence factors associated with equine herpesvirus type 1 infection in equids with upper respiratory tract infection and/or acute onset of neurological signs from 2008 to 2014. Vet. Rec. 2016, 178, 70. [Google Scholar] [CrossRef]
- Wegdan, H.; Intisar, K.; Shaza, M.; Algezoli, O.; Ballal, A.; Ihsan, H.; Sahar, M.; Baraa, A.; Manal, H.; Muna, E.; et al. Serological Detection of Equine Herpes Virus (EHV) Type 1 and 4 in Sudan. Br. Microbiol. Res. J. 2016, 14, 1–6. [Google Scholar] [CrossRef]
- Ali, S.; Mohammadi, G. Detection of equine herpesvirus-1 and equine herpesvirus-4 in mules and donkeys by real time PCR. Vet. Pract. 2016, 17, 160–163. [Google Scholar]
- Negussie, H.; Gizaw, D.; Tessema, T.S.; Nauwynck, H.J. Equine Herpesvirus-1 Myeloencephalopathy, an Emerging Threat of Working Equids in Ethiopia. Transbound Emerg. Dis. 2017, 64, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Negussie, H.; Gizaw, D.; Tesfaw, L.; Li, Y.; Oguma, K.; Sentsui, H.; Tessema, T.S.; Nauwynck, H.J. Detection of Equine Herpesvirus (EHV) -1, -2, -4 and -5 in Ethiopian Equids with and without Respiratory Problems and Genetic Characterization of EHV-2 and EHV-5 Strains. Transbound Emerg. Dis. 2017, 64, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Bolfa, P.; Jeon, I.; Loftis, A.; Leslie, T.; Marchi, S.; Sithole, F.; Beck, C.; Lecollinet, S.; Zientara, S.; Hans, A.; et al. Detection of West Nile Virus and other common equine viruses in three locations from the Leeward Islands, West Indies. Acta Trop. 2017, 174, 24–28. [Google Scholar] [CrossRef] [PubMed]
- De Souza Hunold Lara, M.D.C.C.; Villalobos, E.M.C.; Cunha, E.M.S.; De Oliveira, J.V.; de Castro Nassar, A.F.; Silva, L.M.P.; Okuda, L.; Nogueira, A.H.C.; Cunha, M.S.; Marques, E.C.; et al. Occurrence of viral diseases in donkeys (Equus asinus) in São Paulo State, Brazil. Braz. J. Vet. Res. Anim. Sci. 2017, 54, 154. [Google Scholar] [CrossRef][Green Version]
- Azab, W.; Bedair, S.; Abdelgawad, A.; Eschke, K.; Farag, G.K.; Abdel-Raheim, A.; Greenwood, A.D.; Osterrieder, N.; Ali, A.A.H. Detection of equid herpesviruses among different Arabian horse populations in Egypt. Vet. Med. Sci. 2019, 5, 361–371. [Google Scholar] [CrossRef]
- Matsumura, T.; Sugiura, T.; Imagawa, H.; Fukunaga, Y.; Kamada, M. Epizootiological Aspects of Type 1 and Type 4 Equine Herpesvirus Infections among Horse Populations. J. Vet. Med. Sci. 1992, 54, 207–211. [Google Scholar] [CrossRef]
- Pavulraj, S.; Eschke, K.; Theisen, J.; Westhoff, S.; Reimers, G.; Andreotti, S.; Osterrieder, N.; Azab, W. Equine Herpesvirus Type 4 (EHV-4) Outbreak in Germany: Virological, Serological, and Molecular Investigations. Pathogens 2021, 10, 810. [Google Scholar] [CrossRef]
- European Parliament. The EU’s Organic Food Market: Facts and Rules; European Parliament: Brussels, Belgium, 2018; pp. 1–6. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20180404STO00909/the-eu-s-organic-food-market-facts-and-rules-infographic (accessed on 17 August 2021).
- Spada, V.; Ferranti, P.; Chianese, L.; Salimei, E.; Addeo, F.; Picariello, G. Antibacterial potential of donkey’s milk disclosed by untargeted proteomics. J. Proteomics. 2021, 231, 104007. [Google Scholar] [CrossRef]
- Duval, E.; von Keyserlingk, M.A.G.; Lecorps, B. Organic Dairy Cattle: Do European Union Regulations Promote Animal Welfare? Animals 2020, 10, 1786. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Webster, J.; Sutherland, I. Animal health and welfare issues facing organic production systems. Animals 2013, 3, 1021–1035. [Google Scholar] [CrossRef]
- Gianfaldoni, C.; Barlozzari, G.; Mancini, S.; Domenico, E.; Maestrini, M.; Perrucci, S. Parasitological investigation in an organic dairy donkey farm. Large Anim. Rev. 2020, 26, 25–30. [Google Scholar]
- Conte, F.; Panebianco, A. Potential Hazards Associated with Raw Donkey Milk Consumption: A Review. Int. J. Food Sci. 2019, 2019, 5782974. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B. Facing the threat of equine parasitic disease. Equine Vet. J. 2011, 43, 126–132. [Google Scholar] [CrossRef]
- Foster, N.; Elsheikha, H.M. The immune response to parasitic helminths of veterinary importance and its potential manipulation for future vaccine control strategies. Parasitol. Res. 2012, 110, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A. The Influence of Parasite Infections on Host Immunity to Co-infection with Other Pathogens. Front. Immunol. 2018, 9, 2579. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.F.; Ahmed, B.M.; Salem, S.A.; El-Sanousi, A.A.; Shalaby, M.A. First Isolation and identification of EHV-4 during abortion outbreak among Arabian horses in Egypt reflects an alteration in pathogenic potentiality of EHV-4. J. Virol. Sci. 2017, 2, 92–101. [Google Scholar]
- Smith, K.C.; Blunden, A.S.; Whitwell, K.E.; Dunn, K.A.; Wales, D. A survey of equine abortion, stillbirth and neonatal death in the UK from 1988 to 1997. Equine Vet. J. 2003, 35, 496–501. [Google Scholar] [CrossRef]
- Laugier, C.; Foucher, N.; Sevin, C.; Leon, A.; Tapprest, J. A 24-year retrospective study of equine abortion in Normandy (France). J. Equine Vet. Sci. 2011, 31, 116–123. [Google Scholar] [CrossRef]
- Ji, C.; Cai, S.; Lu, G.; Zhang, G. Challenges to develop an equine herpesvirus vaccine in China. J. Infect. 2020, 80, 578–606. [Google Scholar] [CrossRef]
- Foote, C.E.; Love, D.N.; Gilkerson, J.R.; Wellington, J.E.; Whalley, J.M. EHV-1 and EHV-4 infection in vaccinated mares and their foals. Vet. Immunol. Immunopathol. 2006, 111, 41–46. [Google Scholar] [CrossRef]
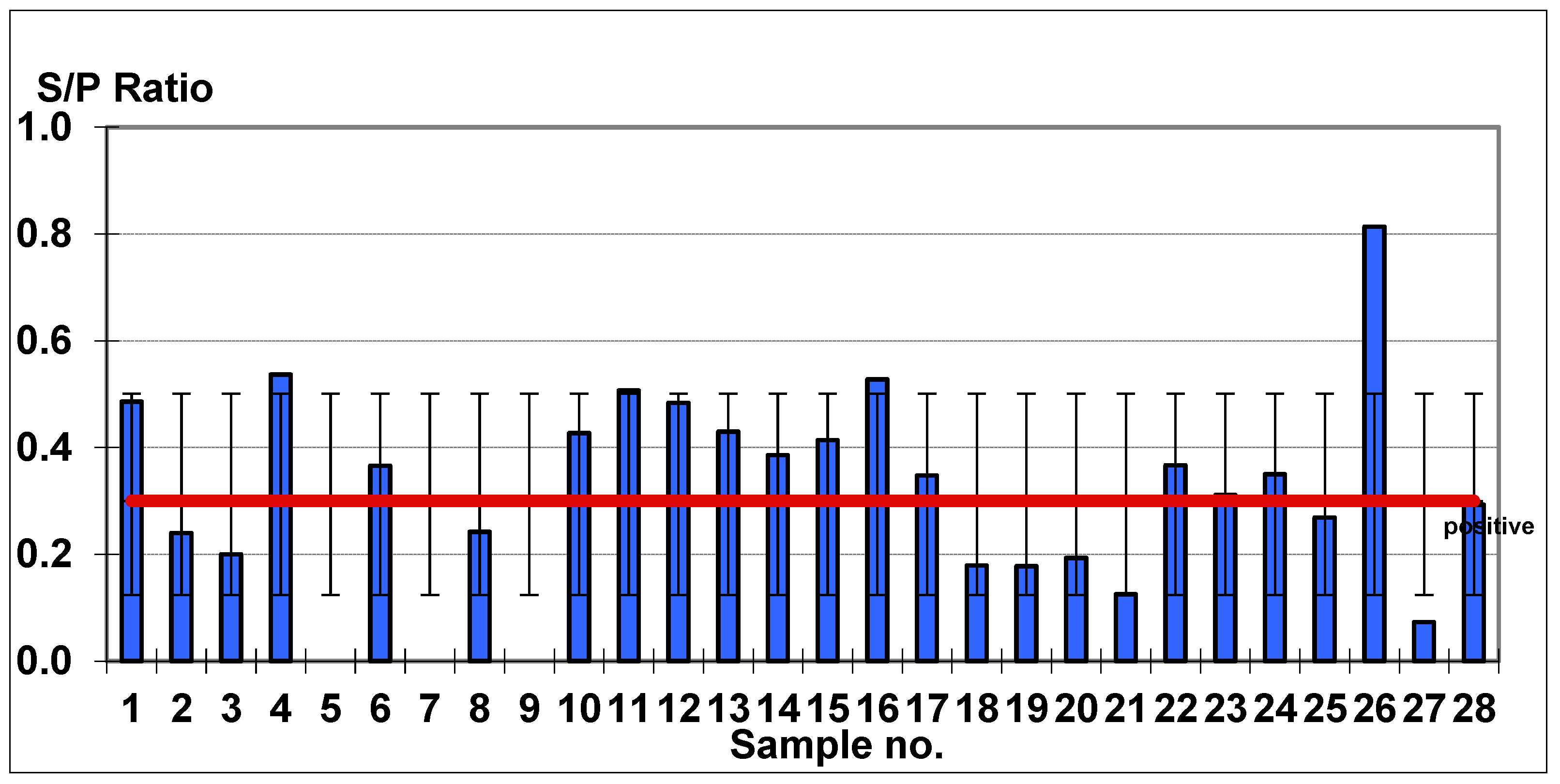
| Mean Age, Age Ranges of Affected Animals | URT Symptoms | Pyrexia | Abortion | EHV Positive Based on Antibody Titer (S/P > 0.3) | EHV Negative Based on Antibody Titer (S/P < 0.3) |
|---|---|---|---|---|---|
| 4.2 years (3–6 years) | 28/28 | 28/28 | 3/28 | 15/28 | 13/28 |
| Report | EHV Type, Symptomatology | Percentage of Positives | Country, Year | Reference |
|---|---|---|---|---|
| 1 | EHV-1 | 6.6% (1/15) | Brazil, 2008 | [26] |
| 2 | EHV-1, both symptomatic and asymptomatic | 24.2% (31/128) | Turkey, 2009 | [19] |
| 3 | EHV-1 and 4, asymptomatic | 0% (0/4) | Brazil, 2010 | [27] |
| 4 | EHV-4, asymptomatic | 69.7% (134/192) | Bulgaria, 2011 | [28] |
| 5 | EHV-1 EHV-4 asymptomatic | 20.2% (21/104) 84.6% (88/104) | Ethiopia, 2014 | [29] |
| 6 | EHV-1, asymptomatic | 33.3% (4/12) | Tanzania, Namibia, 2015 | [30] |
| 7 | EHV-1, symptomatic | 14.28% (2/14) | EUA, 2016 | [31] |
| 8 | EHV-1 EHV-4 Asymptomatic | 51.85% (126/243) 64.2% (156/243) | Turkey, 2015 | [7] |
| 9 | EHV-1 and/or 4, asymptomatic | 69.5% (57/82) | Sudan, 2016 | [32] |
| 10 | EHV-4, asymptomatic | 14.8% (16/108) | Iran, 2016 | [33] |
| 11 | EHV-1, symptomatic | 98.79% (82/83) | Ethiopia, 2017 | [34] |
| 12 | EHV-1 and/or 4 | 74.7% (201/269) | Ethiopia, 2017 | [6] |
| 13 | EHV-1, symptomatic EHV-4, symptomatic EHV-1, asymptomatic EHV-4, asymptomatic | 19.5% (8/41) 9.8% (4/41) 0% (0/10) 0% (0/10) | Ethiopia, 2017 | [35] |
| 14 | EHV-1 EHV-4 asymptomatic | 10% (4/40) 53% (21/40) | West Indies, 2017 | [36] |
| 15 | EHV-1 and/or 4, asymptomatic | 47% (40/85) | Brazil, 2017 | [37] |
| 16 | EHV-1 EHV-4 asymptomatic | 50% (8/16) 6.25% (1/16) | Egypt, 2019 | [38] |
| 17 | EHV-1, symptomatic | 76% (19/25) | Ethiopia, 2021 | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureşan, A.; Mureşan, C.; Siteavu, M.; Avram, E.; Bochynska, D.; Taulescu, M. An Outbreak of Equine Herpesvirus-4 in an Ecological Donkey Milk Farm in Romania. Vaccines 2022, 10, 468. https://doi.org/10.3390/vaccines10030468
Mureşan A, Mureşan C, Siteavu M, Avram E, Bochynska D, Taulescu M. An Outbreak of Equine Herpesvirus-4 in an Ecological Donkey Milk Farm in Romania. Vaccines. 2022; 10(3):468. https://doi.org/10.3390/vaccines10030468
Chicago/Turabian StyleMureşan, Alexandra, Cosmin Mureşan, Madalina Siteavu, Electra Avram, Diana Bochynska, and Marian Taulescu. 2022. "An Outbreak of Equine Herpesvirus-4 in an Ecological Donkey Milk Farm in Romania" Vaccines 10, no. 3: 468. https://doi.org/10.3390/vaccines10030468
APA StyleMureşan, A., Mureşan, C., Siteavu, M., Avram, E., Bochynska, D., & Taulescu, M. (2022). An Outbreak of Equine Herpesvirus-4 in an Ecological Donkey Milk Farm in Romania. Vaccines, 10(3), 468. https://doi.org/10.3390/vaccines10030468






