Seroprevalence against Diphtheria in Pregnant Women and Newborns in Colombia: New Arguments to Promote Maternal Immunization
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Variables
2.4. Diphtheria Assay
2.5. Statistical Methods
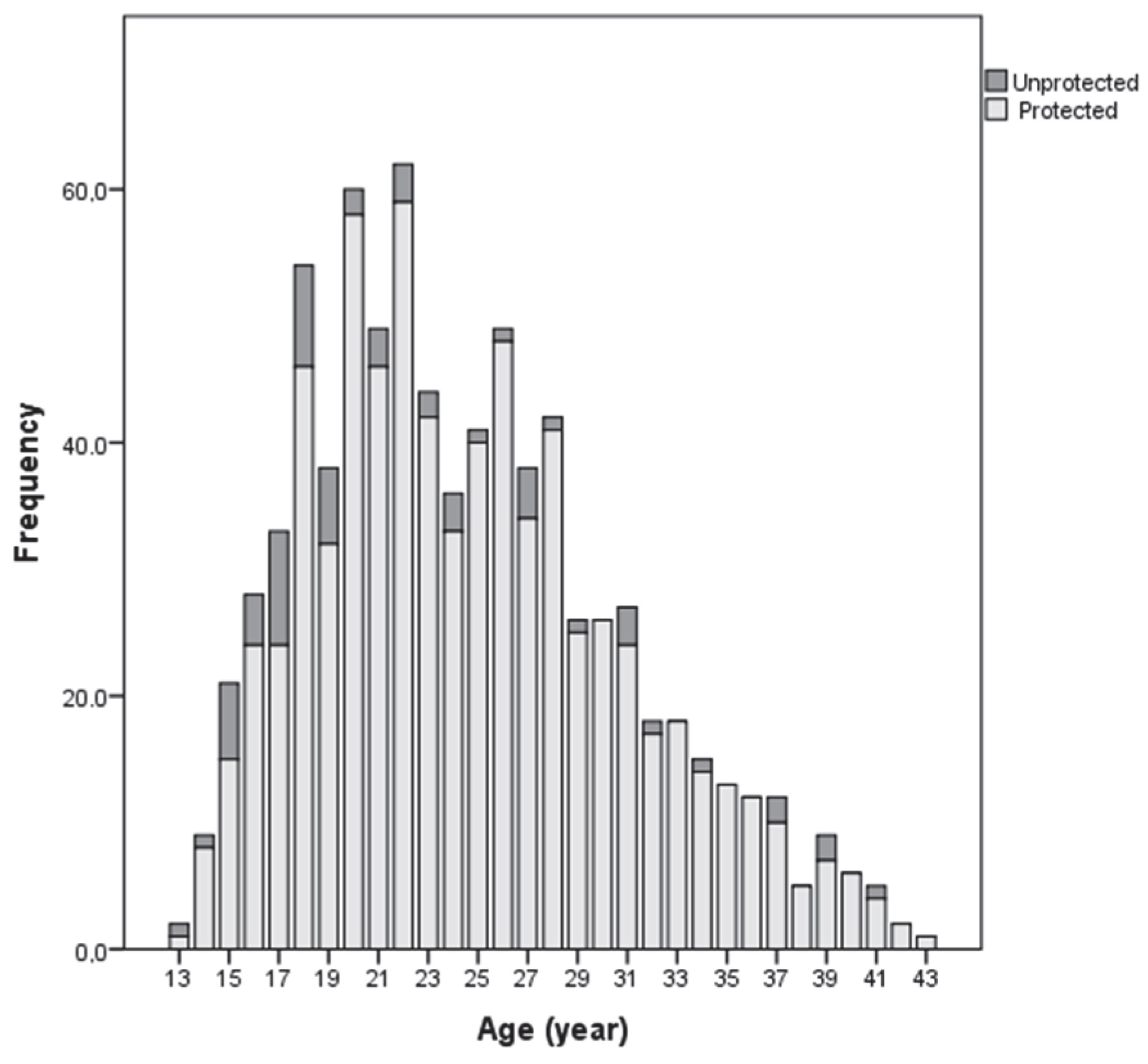
3. Results
3.1. Patients
3.2. Tdap Vaccination Status during the Pregnancy
3.3. Weighted Prevalence of IgG Antibodies to Diphtheria
3.4. Factors That May Influence the Protection of Pregnant Women
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bishai, W.R.; Murphy, J.R. Diphtheria and other infections caused by corynebacteria. In Principles of Internal Medicine, 20th ed.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Sharma, N.; Efstratiou, A.; Mokrousov, I.; Mutreja, A.; Das, B.; Ramamurthy, T. Diphtheria. Nat. Rev. Dis. Prim. 2019, 5, 81. [Google Scholar] [CrossRef]
- Kembabanova, G.; Askarova, J.; Ivanova, R.; Deshevoi, S.; Vitek, C.; McNabb, S. Epidemic investigation of diphtheria, Republic of Kazakhstan, 1990–1996. J. Infect. Dis. 2000, 181 (Suppl. 1), S94–S97. [Google Scholar] [CrossRef][Green Version]
- Clarke, K.E.; MacNeil, A.; Hadler, S.; Scott, C.; Tiwari, T.S.; Cherian, T. Epidemiology of Diphtheria, 2000–2017. Emerg. Infect. Dis. 2019, 25, 1834–1842. [Google Scholar] [CrossRef]
- Pan American Health Organization/World Health Organization. Epidemiological Update: Diphtheria. 2020. Available online: https://iris.paho.org/handle/10665.2/51929 (accessed on 25 October 2020).
- Hincapié-Palacio, D.; Hoyos, M.C.; Ochoa, J.; Montoya, N.; García, D.; Osorio, E.; Pertussis Working Group. Effect of maternal immunization against pertussis in Medellin and the metropolitan area, Colombia, 2016–2017. Vaccine 2018, 36, 3984–3991. [Google Scholar] [CrossRef]
- Hincapié-Palacio, D.; Acevedo, M.; Hoyos, M.C.; Ochoa, J.; González, C.; Pérez, P.A.; Molina, A.; Restrepo, B.I.; Arrubla, M.; Echeverri, A.P.; et al. Serosurveillance for vaccine-preventable diseases: A look inside the pertussis experience. Biomedica 2019, 39 (Suppl. 2), 130–143. [Google Scholar] [CrossRef]
- Cullen, J.; Stone, S.; Phipps, M.; Cypher, R. Immunization for Pregnant Women: A Call to Action. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, e1–e6. [Google Scholar] [CrossRef]
- Andrus, J.K.; Evans-Gilbert, T.; Santos, J.I.; Guzman, M.G.; Rosenthal, P.J.; Toscano, C.; Valenzuela, M.T.; Siqueira, M.; Etienne, C.; Breman, J.G. Perspectives on Battling COVID-19 in Countries of Latin America and the Caribbean. Am. J. Trop. Med. Hyg. 2020, 103, 593–596. [Google Scholar] [CrossRef]
- Drezner, D.; Youngster, M.; Klainer, H.; Youngster, I. Maternal vaccinations coverage and reasons for non-compliance—A cross sectional observational study. BMC Pregnancy Childbirth 2020, 20, 541. [Google Scholar] [CrossRef]
- Ministry of Health and Social Protection of Colombia. Colombia Vaccination Scheme. 2019. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/ET/ficha-esquema-vacunacion-vf2.pdf (accessed on 26 November 2020).
- Guidelines for the Management and Administration of the Expanded Immunization Program—EPI. Colombia; Ministry of Health and Social Protection of Colombia: Bogotá, Colombia, 2016.
- Ministry of Health and Social Protection of Colombia. Strategic Guidelines for the Introduction of the Tdap Vaccine (Diphtheria—Acellular Pertussis—Tétanos) in the Scheme of the Expanded Program of Immunizations—EPI for Pregnant Women of the Cohorts 2013 and 2014. 2013. Available online: https://www.minsalud.gov.co/sites/rid/1/Vacuna%20contra%20difteria,%20tos%20ferina%20y%20t%C3%A9tanos%20(DPT).pdf (accessed on 2 February 2020).
- Zasada, A.A.; Rastawicki, W.; Śmietańska, K.; Rokosz, N.; Jagielski, M. Comparison of seven commercial enzyme-linked immunosorbent assays for the detection of anti-diphtheria toxin antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 891–897. [Google Scholar] [CrossRef]
- Carrasquilla, G.; Porras, A.; Martinez, S.; DeAntonio, R.; Devadiga, R.; Caceres, D.C.; Juliao, P. Incidence and mortality of pertussis disease in infants <12 months of age following introduction of pertussis maternal universal mass vaccination in Bogota, Colombia. Vaccine 2020, 38, 7384–7392. [Google Scholar] [CrossRef]
- National Institute of Health Group of Communicable Diseases. Diphtheria Report, Colombia, First Semester 2019. 2019. Available online: https://www.ins.gov.co/buscadoreventos/Informesdeevento/DIFTERIA SEMESTRE I 2019.pdf (accessed on 20 February 2020).
- de Voer, R.M.; van der Klis, F.R.; Nooitgedagt, J.E.; Versteegh, F.G.; van Huisseling, J.C.; van Rooijen, D.M.; Sanders, E.A.; Berbers, G.A. Seroprevalence and Placental Transportation of Maternal Antibodies Specific for Neisseria meningitidis Serogroup C, Haemophilus influenzae Type B, Diphtheria, Tetanus, and Pertussis. Clin. Infect. Dis. 2009, 49, 58–64. [Google Scholar] [CrossRef]
- Post, A.L.; Li, S.H.; Berry, M.; Itell, H.; Martinez, D.R.; Xie, G.; Permar, S.R.; Swamy, G.K.; Fouda, G.G. Efficiency of placental transfer of vaccine-elicited antibodies relative to prenatal Tdap vaccination status. Vaccine 2020, 38, 4869–4876. [Google Scholar] [CrossRef]
- Cummings, H.; Sadoh, A.; Oviawe, O.; Sadoh, W. Anti-diphtheria immunity in Nigerian mothers and their newborns. Vaccine 2014, 32, 3211–3215. [Google Scholar] [CrossRef]
- Vos, R.; Mollema, L.; Kerkhof, J.; van den Kerkhof, J.; Gerstenbluth, I.; Janga-Jansen, A. Risk of Measles and Diphtheria Introduction and Transmission on Bonaire, Caribbean Netherlands, 2018. Am. J. Trop. Med. Hyg. 2019, 101, 237–241. [Google Scholar] [CrossRef]
- Divino-Goes, K.G.; de Moraes-Pinto, M.I.; Dinelli, M.I.; Casagrande, S.T.; Bonetti, T.C.; Andrade, P.R.; Weckx, L.Y. Prevalence of diphtheria and tetanus antibodies and circulation of Corynebacterium diphtheriae in São Paulo, Brazil. Braz. J. Med. Biol. Res. 2007, 40, 1681–1687. [Google Scholar] [CrossRef][Green Version]
- García, D.A.; Velandia, M.; Pierson, S.; Pedreira, M.C.; Bravo, P.; Danovaro, M.C. Understanding the main barriers to immunization in Colombia to better tailor communication strategies. BMC Public Health 2014, 14, 669. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Maertens, K.; Edwards, K.M.; Omer, S.B.; Englund, J.A.; Flanagan, K.L.; Snape, M.D.; Amirthalingam, G.; Leuridan, E.; Damme, P.V.; et al. Global Perspectives on Immunization During Pregnancy and Priorities for Future Research and Development: An International Consensus Statement. Front. Immunol. 2020, 11, 1282. [Google Scholar] [CrossRef]
- World Health Organization. Diphtheria vaccine: WHO position paper—August 2017. Wkly. Epidemiol. Rec. 2017, 92, 417–435. [Google Scholar]
- Völzke, H.; Kloker, K.M.; Kramer, A.; Guertler, L.; Dören, M.; Baumeister, S.E.; Hoffmann, W.; John, U. Susceptibility to diphtheria in adults: Prevalence and relationship to gender and social variables. Clin. Microbiol. Infect. 2006, 12, 961–967. [Google Scholar] [CrossRef]
- Narváez, J.; Osorio, M.B.; Castañeda-Orjuela, C.; Zakzuk, N.A.; Cediel, N.; Chocontá-Piraquive, L.Á.; de La Hoz-Restrepo, F. Is Colombia reaching the goals on infant immunization coverage? A quantitative survey from 80 municipalities. Vaccine 2017, 35, 1501–1508. [Google Scholar] [CrossRef]
- Gowin, E.; Wysocki, J.; Kałużna, E.; Świątek-Kościelna, B.; Wysocka-Leszczyńska, J.; Michalak, M.; Januszkiewicz-Lewandowska, D. Does vaccination ensure protection? Assessing diphtheria and tetanus antibody levels in a population of healthy children. Medicine 2016, 95, e5571. [Google Scholar] [CrossRef]
- von Hunolstein, C.; Ralli, L.; Pinto, A.; Stickings, P.; Efstratiou, A.; Al, E. Relevance and Criticality in an External Quality Assessment or the Determination of Diphtheria Antitoxin. J. Immunol. Clin. Res. 2014, 2, 1022. [Google Scholar]
- Dinleyici, E.C.; Borrow, R.; Safadi, M.A.P.; van Damme, P.; Munoz, F.M. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum. Vaccine Immunother. 2020, 17, 400–407. [Google Scholar] [CrossRef]

| Variables | n (%) |
|---|---|
| Age (years) | |
| 13–23 | 404 (50.2) |
| 24–43 | 401 (49.8) |
| Marital status | |
| Married/common law | 586 (71.9) |
| Single/divorced | 219 (28.1) |
| Area | |
| Rural | 40 (5.0) |
| Urban | 765 (95) |
| Socioeconomic level | |
| 1 to 3 (low) | 708 (95.3) |
| 4 to 6 (high) | 35 (4.7) |
| Three or more people per room | |
| Yes | 26 (3.2) |
| No | 779 (96.8) |
| Health insurance | |
| Contributive | 385 (47.8) |
| Other affiliation | 420 (52.2) |
| Years of schooling | |
| 1–11 | 614 (76.7) |
| 12–more | 187 (23.3) |
| Birth before 1980 (start of DPT vaccination) | |
| Yes | 49 (6.1) |
| No | 756 (93.9) |
| Childhood immunization | |
| Yes | 582 (96.8) |
| No | 19 (3.2) |
| Vaccination during previous pregnancy | |
| Yes | 83 (26.1) |
| No | 235 (73.9) |
| Protective antibodies (pregnant) | |
| Yes | 736 (91.4) |
| No | 12 (1.5) |
| Yes, but without certainty of protection | 54 (6.7) |
| Missing data | 3 (0.4) |
| Protective antibodies (umbilical cord) | |
| Yes | 701 |
| No | 12 |
| Yes, but without certainty of protection | 42 |
| Missing data | 50 |
| Pregnant Proportion (95% CI) n | Umbilical Cord Proportion (95%CI) n | |||
|---|---|---|---|---|
| Protected | Unprotected | Protection Uncertain | Total | |
| Protected | 84.4 (80.5–87.6) 685 | 0.1 (0–0.3) 1 | 1.3 (0.9–1.9) 9 | 736 (91.0) 695 |
| Unprotected | - | 1.4 (0.9–2.1) 10 | - | 1.8 (1.2–1.8) 10 |
| Protection uncertain | 1.9 (1.2–3.1) 15 | 0.1 (0–0.4) 1 | 4.3 (3.2–5.8) 33 | 7.0 (5.9–82) 49 |
| Total | 86.5 (83.2–89.2) 701 | 1.6 (1.0–2.5) 12 | 5.6 (4.4–7.2) 42 | (100) 805 |
| Variable | Protected n | Not protected n | Crude PR (95% CI) | Adjusted PR (95% CI) |
|---|---|---|---|---|
| Age (years) | ||||
| 13–23 | 375 | 50 | 1.06 (1.02–1.11) | 1.01 (0.97–1.06) |
| 24–43 | 395 | 26 | 1 | 1 |
| Vaccination during the current pregnancy | ||||
| Yes | 532 | 22 | 1 | 1 |
| No | 238 | 54 | 0.85 (0.81–0.90) | 0.87 (0.82–0.93) |
| Health Insurance | ||||
| Contributive | 368 | 30 | 1.02 (0.97–1.06) | 1.00 (0.95–1.05) |
| Other affiliation | 402 | 46 | 1 | 1 |
| Years of schooling | ||||
| 1–11 | 557 | 66 | 0.96 (0.92–1.00) | 0.98 (0.94–1.03) |
| 12–more | 144 | 10 | 1 | 1 |
| Socioeconomic level | ||||
| 1 to 3 (low) | 678 | 34 | 0.94 (0.89–1.00) | 0.99 (0.92–1.06) |
| 4 to 6 (high) | 63 | 1 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Santamaría, L.M.; Hincapié-Palacio, D.; Ochoa, J.; Vargas-Restrepo, F.; Ospina, M.C.; Buitrago-Giraldo, S. Seroprevalence against Diphtheria in Pregnant Women and Newborns in Colombia: New Arguments to Promote Maternal Immunization. Vaccines 2022, 10, 458. https://doi.org/10.3390/vaccines10030458
Rivera-Santamaría LM, Hincapié-Palacio D, Ochoa J, Vargas-Restrepo F, Ospina MC, Buitrago-Giraldo S. Seroprevalence against Diphtheria in Pregnant Women and Newborns in Colombia: New Arguments to Promote Maternal Immunization. Vaccines. 2022; 10(3):458. https://doi.org/10.3390/vaccines10030458
Chicago/Turabian StyleRivera-Santamaría, Laura María, Doracelly Hincapié-Palacio, Jesús Ochoa, Felipe Vargas-Restrepo, Marta C. Ospina, and Seti Buitrago-Giraldo. 2022. "Seroprevalence against Diphtheria in Pregnant Women and Newborns in Colombia: New Arguments to Promote Maternal Immunization" Vaccines 10, no. 3: 458. https://doi.org/10.3390/vaccines10030458
APA StyleRivera-Santamaría, L. M., Hincapié-Palacio, D., Ochoa, J., Vargas-Restrepo, F., Ospina, M. C., & Buitrago-Giraldo, S. (2022). Seroprevalence against Diphtheria in Pregnant Women and Newborns in Colombia: New Arguments to Promote Maternal Immunization. Vaccines, 10(3), 458. https://doi.org/10.3390/vaccines10030458






