SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Peripheral Blood Mononuclear Cell (PBMC) Isolation
2.3. Peptide Pools
2.4. INF-γ ELISPOT Assay
2.5. Intracellular Cytokine Staining by Flow Cytometry
3. Results
3.1. Patients
3.2. Humoral and Cellular Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Robinson, P.C.; Uldrick, T.S.; Ljungman, P. COVID-19 in immunocompromised populations: Implications for prognosis and repurposing of immunotherapies. J. Immunother. Cancer 2021, 9, e002630. [Google Scholar] [CrossRef] [PubMed]
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021, 32, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.E.; Blake, M.; Chilleo, C.; Wells, A.; Haidar, G. Suboptimal Response to Coronavirus Disease 2019 Messenger RNA Vaccines in Patients With Hematologic Malignancies: A Need for Vigilance in the Postmasking Era. Open Forum Infect. Dis. 2021, 8, ofab353. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv-Baran, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Ou, M.T.; Boyarsky, B.J.; Motter, J.D.; Greenberg, R.S.; Teles, A.T.; Ruddy, J.A.; Krach, M.R.; Jain, V.S.; Werbel, W.A.; Avery, R.K.; et al. Safety and reactogenicity of 2 Doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation 2021, 105, 2170–2174. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Beckerich, F.; Fourati, S.; Maury, S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet 2021, 398, 298–299. [Google Scholar] [CrossRef]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Dhakal, B.; Abedin, S.; Fenske, T.; Chhabra, S.; Ledeboer, N.; Hari, P.; Hamadani, M. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR-T cell therapy. Blood 2021, 138, 1278–1281. [Google Scholar] [CrossRef]
- Le Bourgeois, A.; Coste-Burel, M.; Guillaume, T.; Peterlin, P.; Garnier, A.; Béné, M.C.; Chevallier, P. Safety and antibody response after 1 and 2 doses of BNT162b2 mRNA vaccine in recipients of allogeneic hematopoietic stem cell transplant. JAMA Netw. Open 2021, 4, e2126344. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Einarsdottir, S.; Cesaro, S.; Di Blasi, R.; Mikulska, M.; Rieger, C.; de Lavallade, H.; Gallo, G.; Lehrnbecher, T.; Engelhard, D.; et al. Vaccination of haemopoietic stem cell transplant recipients: Guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 2019, 19, e200–e212. [Google Scholar] [CrossRef]
- Engelhard, D.; Zakay-Rones, Z.; Shapira, M.Y.; Resnick, I.; Averbuch, D.; Grisariu, S.; Dray, L.; Djian, E.; Strauss-Liviatan, N.; Grotto, I.; et al. The humoral immune response of hematopoietic stem cell transplantation recipients to AS03-adjuvanted A/California/7/2009 (H1N1)v-like virus vaccine during the 2009 pandemic. Vaccine 2011, 29, 1777–1782. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA vaccines: Immunological mechanism and beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Ferreira, V.H.; Ierullo, M.; Ku, T.; Marinelli, T.; Majchrzak-Kita, B.; Yousuf, A.; Kulasingam, V.; Humar, A.; Kumar, D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3980–3989. [Google Scholar] [CrossRef]
- Sharma, A.; Bhatt, N.S.; Martin, A.S.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.-A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-based CART therapy—A Single-Center Prospective Cohort Study. Transplant. Cell. Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Saha, C.; Saunders, J.; Child, F.; Dillon, R.; Saglam, S.; Raj, K.; McLornan, D.; Avenoso, D.; et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell 2021, 39, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Ngo, D.; Aribi, A.; Arslan, S.; Dadwal, S.; Marcucci, G.; Nakamura, R.; Forman, S.J.; Chen, J.; Al Malki, M.M. Safety and tolerability of SARS-CoV2 emergency-use authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplant. Cell. Ther. 2021, 27, e1–e938. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.R.; Huang, L.; Dedeoglu, B.; Meijers, R.W.J.; Kwekkeboom, J.; Betjes, M.G.H. Protective Cytomegalovirus (CMV)-Specific T-Cell Immunity Is Frequent in Kidney Transplant Patients without Serum Anti-CMV Antibodies. Front. Immunol. 2017, 8, 1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestan, J.; Digel, W.; Mittnacht, S.; Hillen, H.; Blohm, D.; Möller, A.; Jacobsen, H.; Kirchner, H. Antiviral effects of recombinant tumour necrosis factor in vitro. Nature 1986, 323, 816–819. [Google Scholar] [CrossRef]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. Nature 2022, 1–5. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2021, 602, 671–675. [Google Scholar] [CrossRef]
- Maillard, A.; Redjoul, R.; Klemencie, M.; Labussière, H.; Le Bourgeois, A.; D’Aveni, M.; Berceanu, A.; Chantepie, S.; Botella, C.; Loschi, M.; et al. Antibody Response to 2-Dose Sars-Cov-2 mRNA Vaccine in Allogeneic Hematopoietic Cell Transplant Recipients. Blood 2021, 138, 336. [Google Scholar] [CrossRef]
- Jullien, M.; Bourgeois, A.L.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Rimbert, M.; Imbert, B.M.; Gouill, S.L.; Moreau, P.; Mahe, B.; et al. B-cell aplasia is the most powerful predictive marker for poor humoral response after BNT162b2 mRNA SARS-CoV-2 vaccines in recipients of allogeneic hematopoietic stem cell transplantation. Transplant. Cell. Ther. 2022, 23, S2666–S6367. [Google Scholar] [CrossRef]
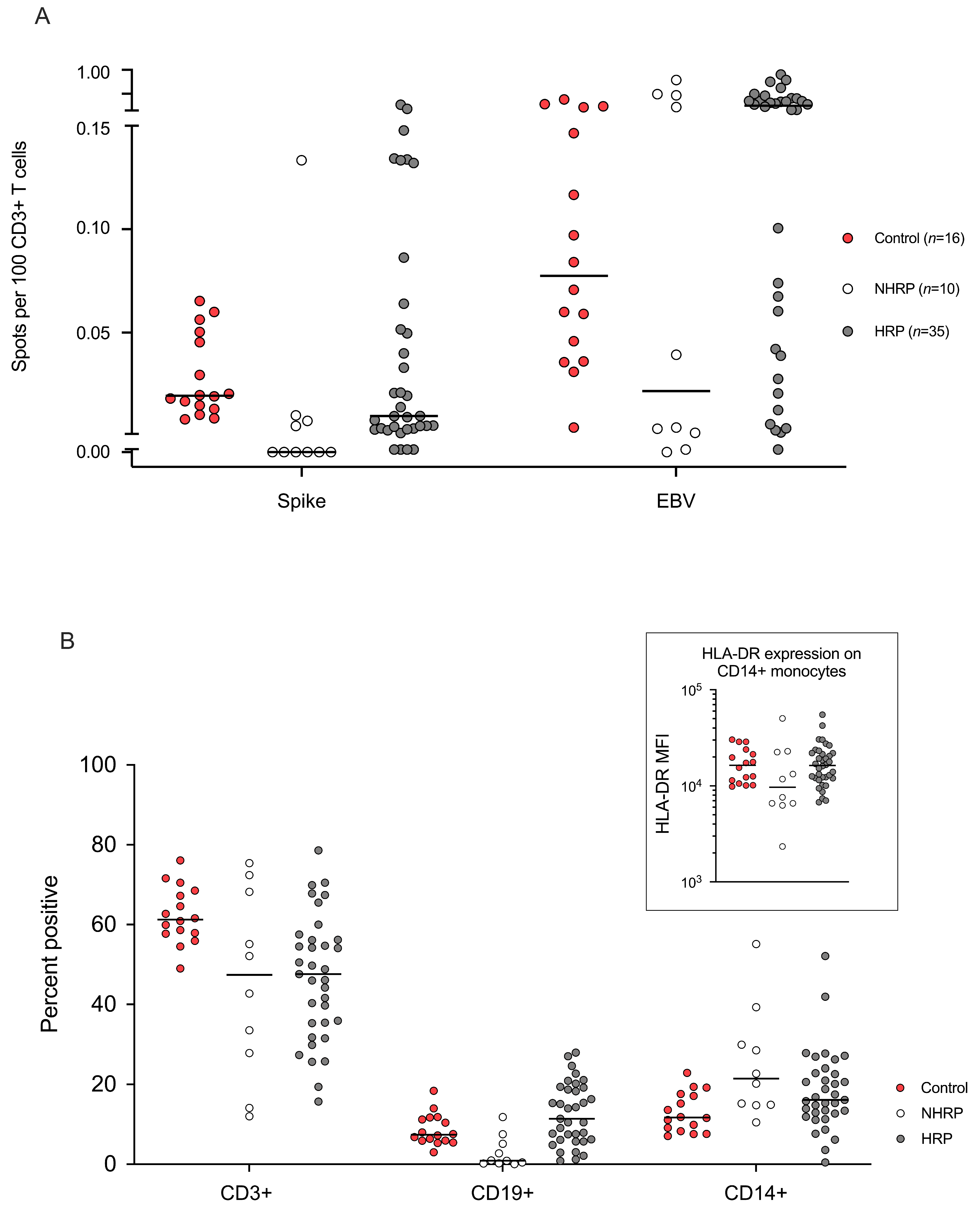

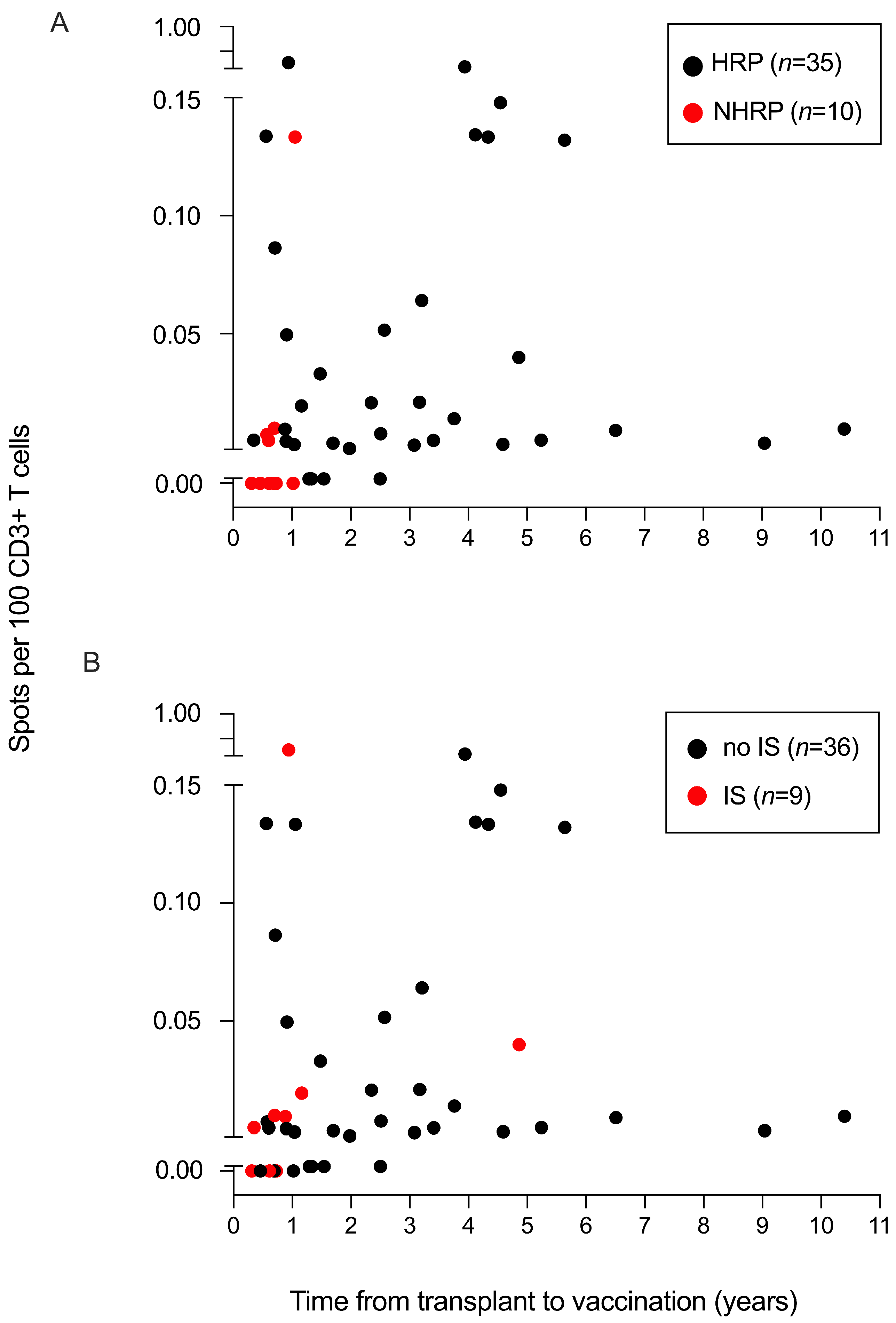
| Allogeneic Hematopoietic Stem-Cell Recipients (N = 45) | Healthy Controls (N = 16) | ||||
|---|---|---|---|---|---|
| Antibody response after two doses of BNT162b2 vaccination | Yes (HR) a 35 (78%) | No (NHR) b 10 (22%) | Yes 16 (100%) | ||
| T-cell response after two doses of BNT162b2 vaccination | Yes 31 (89%) | No 4 (11%) | Yes 4 (40%) | No 6 (60%) | Yes 16 (100%) |
| Median time from transplant to vaccination (days) | 1026 | 523 | 236 | 237 | NA |
| Range | (126–3796) | (471–914) | (208–384) | (112–372) | |
| Median time from first to second vaccination (days) | 21 | 28 | 23 | 21 | 24 |
| Range | (19–35) | (21–29) | (16–29) | (21–29) | (18–32) |
| Median time from second vaccination to T cells response analyses (days) | 32 | 36 | 62 | 45 | 58 |
| Range | (22–67) | (25–69) | (56–70) | (26–56) | (32–70) |
| Median time from first vaccination to T cells response analyses (days) | 56 | 64 | 85 | 66 | 81 |
| Range | (43–95) | (52–90) | (85–86) | (47–85) | (62–91) |
| Underlying disease | 18 AML 13 MDS | 4 MDS | 3 AML 1MDS | 5 AML 1MDS | NA |
| Median age: years | 62 | 58 | 66 | 57 | 52 |
| (range) | (30–75) | (49–72) | (41–70) | (44–66) | (37–63) |
| Gender | |||||
| Male | 18 | 2 | 2 | 4 | 3 |
| Female | 13 | 2 | 1 | 2 | 13 |
| Donor type | |||||
| Geno-identical | 6 | 1 | 1 | 0 | NA |
| MUD | 15 | 2 | 0 | 2 | |
| Haploidentical | 9 | 1 | 3 | 4 | |
| 9/10 mis-MUD | 1 | 0 | 0 | 0 | |
| Conditioning | |||||
| Myeloablative | 1 | 0 | 0 | 0 | NA |
| Reduced-intensity | 29 | 4 | 4 | 6 | |
| Sequential | 1 | 0 | 0 | 0 | |
| GVHD prophylaxis | |||||
| CsA + MMF + ATG | 15 | 1 | 0 | 1 | NA |
| CsA + MMF + PTCY | 7 | 3 | 4 | 5 | |
| PTCY only | 10 | 0 | 0 | 0 | |
| Previous GVHD | |||||
| Yes | 19 (61%) | 2 (50%) | 2 (50%) | 3 (50%) | NA |
| No | 12 (39%) | 2 (50%) | 2 (50%) | 3 (50%) | |
| Ongoing treatment * | |||||
| No | 26 (84%) | 4 (100%) | 3 (75%) | 3 (50%) | NA |
| Yes | 5 (16%) | 0 | 1 (25%) | 3 (50%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clémenceau, B.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Jullien, M.; Ollier, J.; Grain, A.; Béné, M.C.; et al. SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 448. https://doi.org/10.3390/vaccines10030448
Clémenceau B, Guillaume T, Coste-Burel M, Peterlin P, Garnier A, Le Bourgeois A, Jullien M, Ollier J, Grain A, Béné MC, et al. SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine. Vaccines. 2022; 10(3):448. https://doi.org/10.3390/vaccines10030448
Chicago/Turabian StyleClémenceau, Béatrice, Thierry Guillaume, Marianne Coste-Burel, Pierre Peterlin, Alice Garnier, Amandine Le Bourgeois, Maxime Jullien, Jocelyn Ollier, Audrey Grain, Marie C. Béné, and et al. 2022. "SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine" Vaccines 10, no. 3: 448. https://doi.org/10.3390/vaccines10030448
APA StyleClémenceau, B., Guillaume, T., Coste-Burel, M., Peterlin, P., Garnier, A., Le Bourgeois, A., Jullien, M., Ollier, J., Grain, A., Béné, M. C., Vié, H., & Chevallier, P. (2022). SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine. Vaccines, 10(3), 448. https://doi.org/10.3390/vaccines10030448







