Cellular Immunity—The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohorts
2.2. Analysis of Cellular and Humoral Immunity
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Levels of Cellular and Humoral Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, N.; Klausner, J.D. Protective immunity after recovery from SARS-CoV-2 infection. Lancet Infect. Dis. 2021, 22, 12–14. [Google Scholar] [CrossRef]
- Abbasi, J. The Flawed Science of Antibody Testing for SARS-CoV-2 Immunity. JAMA J. Am. Med. Assoc. 2021, 326, 1781. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Richterman, A.; Cevik, M. COVID-19 vaccination: Evidence of waning immunity is overstated. BMJ 2021, 374, n2320. [Google Scholar] [CrossRef]
- Mohiuddin; Kasahara, K. Investigating the aggressiveness of the COVID-19 Omicron variant and suggestions for possible treatment options. Respir. Med. 2021, 191, 106716. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Do vaccines work against omicron—and other questions answered. BMJ 2021, 375, n3062. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Cui, M.; Sun, S.; Zhou, J.; Du, Z.; Cui, Y.; Fan, H. Genome Characterization and Potential Risk Assessment of the Novel SARS-CoV-2 Variant Omicron (B.1.1.529). Zoonoses 2021, 1. [Google Scholar] [CrossRef]
- Heggestad, J.T.; Britton, R.J.; Kinnamon, D.S.; Wall, S.A.; Joh, D.Y.; Hucknall, A.M.; Olson, L.B.; Anderson, J.G.; Mazur, A.; Wolfe, C.R.; et al. Rapid test to assess the escape of SARS-CoV-2 variants of concern. Sci. Adv. 2021, 7, 7682. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Pavel, A.B.; Glickman, J.W.; Michels, J.R.; Kim-Schulze, S.; Miller, R.L.; Guttman-Yassky, E. Th2/Th1 Cytokine Imbalance Is Associated With Higher COVID-19 Risk Mortality. Front. Genet. 2021, 12, 706902. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, S.; Krawczyk, A.; Knop, D.; Klump, H.; Heinold, A.; Heinemann, F.M.; Thümmler, L.; Temme, C.; Breyer, M.; Witzke, O.; et al. Cellular Immunity in COVID-19 Convalescents with PCR-Confirmed Infection but with Undetectable SARS-CoV-2–Specific IgG. Emerg. Infect. Dis. 2021, 27, 122–129. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Montero-Escribano, P.; Matías-Guiu, J.; Gómez-Iglesias, P.; Porta-Etessam, J.; Pytel, V.; Matias-Guiu, J.A. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Mult. Scler. Relat. Disord. 2020, 42, 102185. [Google Scholar] [CrossRef]
- Soresina, A.; Moratto, D.; Chiarini, M.; Paolillo, C.; Baresi, G.; Focà, E.; Bezzi, M.; Baronio, B.; Giacomelli, M.; Badolato, R. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr. Allergy Immunol. 2020, 31, 565–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huzly, D.; Panning, M.; Smely, F.; Enders, M.; Komp, J.; Steinmann, D. Validation and performance evaluation of a novel interferon-γ release assay for the detection of COVID-19 specific T-cell response. medRxiv 2021. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Leibovici-Weisman, Y.; Stemmer, A.; Ness, A.; Awwad, M.; Ghantous, N.; Stemmer, S.M. Antibody Titers Before and After a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Adults Aged ≥60 Years. JAMA J. Am. Med. Assoc. 2021, 326, 2203–2204. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Virus-Specific Memory CD8 T Cells Provide Substantial Protection from Lethal Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 2014, 88, 11034–11044. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Quan, Y.; Xin, Z.-T.; Wrammert, J.; Ma, M.-J.; Lv, H.; Wang, T.-B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef] [Green Version]
- Redd, A.D.; Nardin, A.; Kared, H.; Bloch, E.M.; Pekosz, A.; Laeyendecker, O.; Abel, B.; Fehlings, M.; Quinn, T.C.; Tobian, A.A.R. CD8+ T-Cell Responses in COVID-19 Convalescent Individuals Target Conserved Epitopes From Multiple Prominent SARS-CoV-2 Circulating Variants. Open Forum Infect. Dis. 2021, 8, ofab143. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; da Silva Antunes, R.; Frazier, A.; et al. Negligible Impact of SARS-CoV-2 Variants on CD4+ and CD8+ T Cell Reactivity in COVID-19 Exposed Donors and Vaccinees. bioRxiv 2021. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Investig. 2021, 131, e149335. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.D.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.; Mumoli, N.; Clerici, P.; De Paschale, M.; Evangelista, I.; Cei, M.; Mazzone, A. Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Intern. Med. 2021, 181, 1407. [Google Scholar] [CrossRef]
- Le Hingrat, Q.; Visseaux, B.; Laouenan, C.; Tubiana, S.; Bouadma, L.; Yazdanpanah, Y.; Duval, X.; Burdet, C.; Ichou, H.; Damond, F.; et al. Detection of SARS-CoV-2 N-antigen in blood during acute COVID-19 provides a sensitive new marker and new testing alternatives. Clin. Microbiol. Infect. 2020, 27, 789.e1–789.e5. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Ishii, Y.; Aoki, K.; Yagi, S.; Maeda, T.; Miyazaki, T.; Yoshizawa, S.; Aoyagi, K.; Tateda, K. Immunochromatographic test for the detection of SARS-CoV-2 in saliva. J. Infect. Chemother. 2021, 27, 384–386. [Google Scholar] [CrossRef]
- Dobaño, C.; Jiménez, A.; Rubio, R.; Alonso, S.; Ramírez-Morros, A.; Vidal, M.; Vidal-Alaball, J.; Ruiz-Comellas, A.; García-Basteiro, A.L.; Izquierdo, L.; et al. Spike-based COVID-19 immunization increases antibodies to nucleocapsid antigen. Transl. Res. 2021, 240, 26–32. [Google Scholar] [CrossRef]
- Yatim, N.; Boussier, J.; Tetu, P.; Smith, N.; Bruel, T.; Charbit, B.; Barnabei, L.; Corneau, A.; Da Meda, L.; Allayous, C.; et al. Immune checkpoint inhibitors increase T cell immunity during SARS-CoV-2 infection. Sci. Adv. 2021, 7, eabg4081. [Google Scholar] [CrossRef]
- Abbasi, J. COVID-19 Vaccine Focused on T-Cell Response Promising in Early Trial. JAMA J. Am. Med. Assoc. 2022, 327, 115. [Google Scholar] [CrossRef] [PubMed]
- Šikić, J.; Planinić, Z.; Matišić, V.; Friščić, T.; Molnar, V.; Jagačić, D.; Vujičić, L.; Tudorić, N.; Gršić, L.P.; Ljubičić, Đ.; et al. COVID-19: The Impact on Cardiovascular System. Biomedicines 2021, 9, 1691. [Google Scholar] [CrossRef] [PubMed]
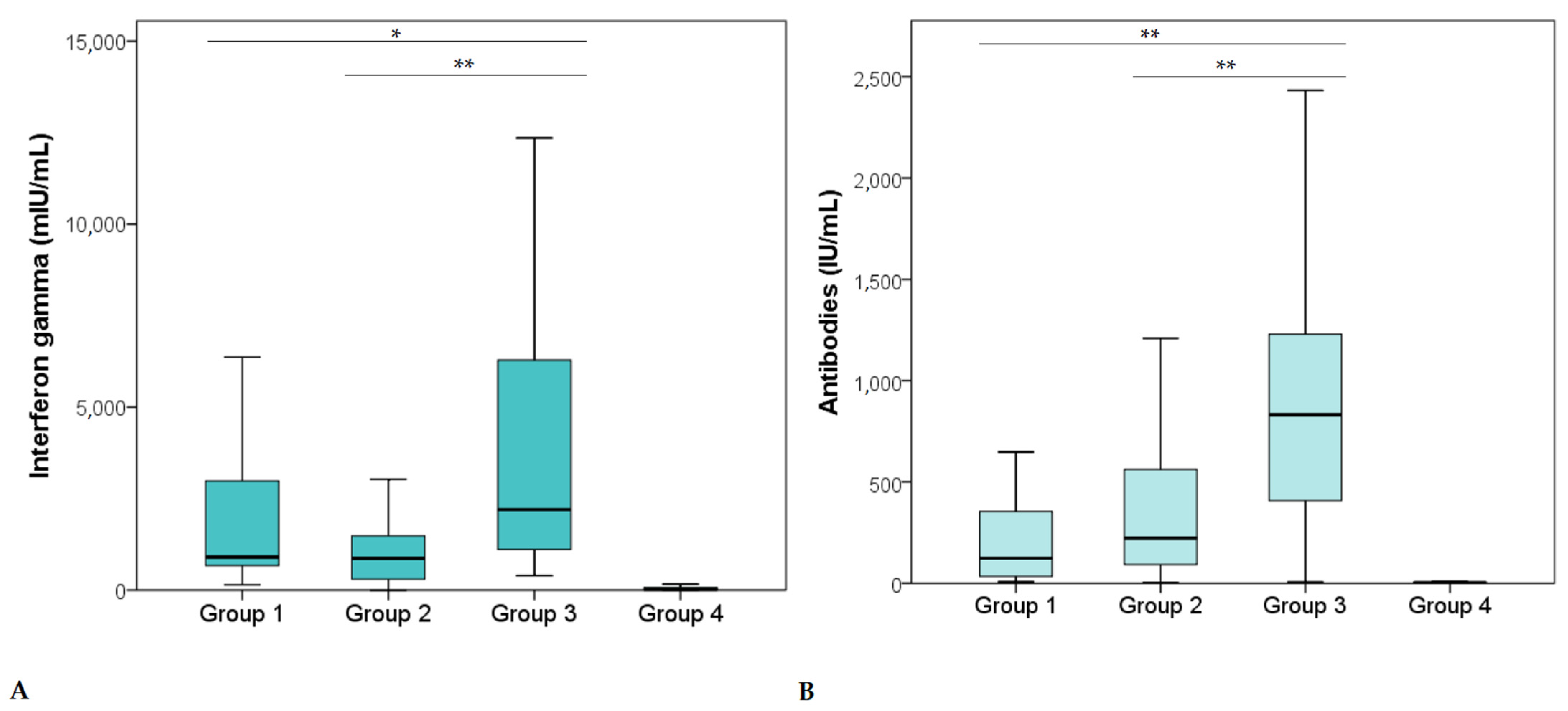
| Group 1 (N = 55) | Group 2 (N = 55) | Group 3 (N = 45) | Group 4 (N = 45) | ||
|---|---|---|---|---|---|
| Cellular immunity (mIU/mL) | MD | 932.0 | 866.0 | 2203.0 | 22.0 |
| IQR | 2514.0 | 1242.0 | 5556.0 | 78.5 | |
| Antibodies (IU/mL) | MD | 128.0 | 222.9 | 831.7 | 3.3 |
| IQR | 320.4 | 470.9 | 906.6 | 2.8 | |
| Age (years) | MD | 46.0 | 52.0 | 49.0 | 43.0 |
| IQR | 15.0 | 13.0 | 20.0 | 14.0 | |
| Sex, No. (%) | M | 58.2 | 52.7 | 48.9 | 42.2 |
| F | 41.8 | 47.3 | 51.1 | 57.8 |
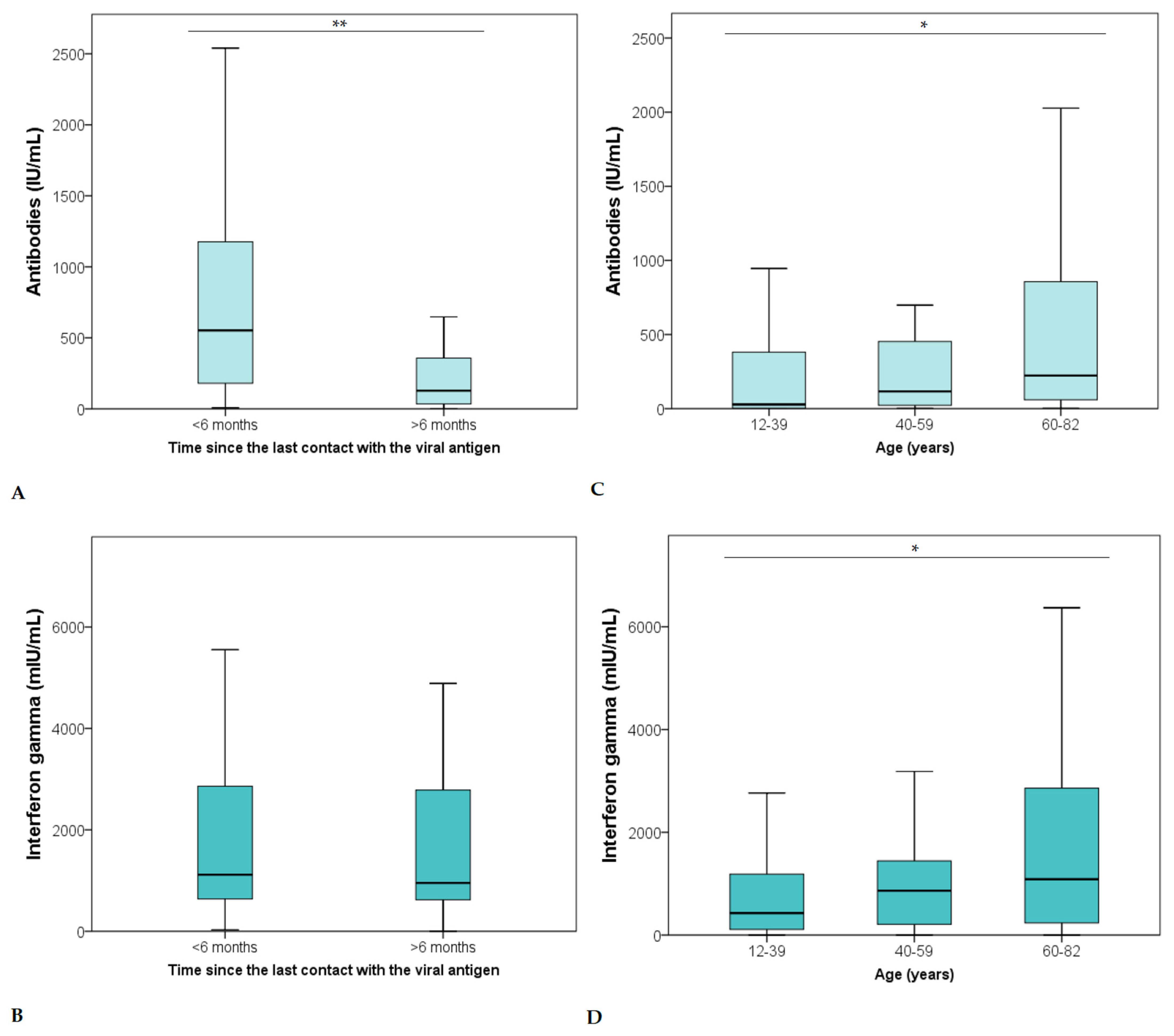
| Cellular Immunity (mIU/mL) | Antibodies (IU/mL) | Age (Years) | Time (Months) | Number of Symptoms | ||
|---|---|---|---|---|---|---|
| Cellular immunity (mIU/mL) | r | 1.000 | 0.801 ** | 0.189 ** | −0.095 | 0.400 ** |
| p | . | <0.001 | 0.007 | 0.240 | <0.001 | |
| N | 200 | 200 | 200 | 155 | 200 | |
| Antibodies (IU/mL) | r | 0.801 ** | 1.000 | 0.225 ** | −0.426 ** | 0.314 ** |
| p | <0.001 | . | 0.001 | <0.001 | <0.001 | |
| N | 200 | 200 | 200 | 155 | 200 | |
| Age (years) | r | 0.189 ** | 0.225 ** | 1.000 | 0.073 | −0.024 |
| p | 0.007 | 0.001 | . | 0.365 | 0.731 | |
| N | 200 | 200 | 200 | 155 | 200 | |
| TIME (months) | r | −0.095 | –0.426 ** | 0.073 | 1.000 | 0.182 * |
| p | 0.240 | <0.001 | 0.365 | . | 0.023 | |
| N | 155 | 155 | 155 | 155 | 155 | |
| Number of symptoms | r | 0.400 ** | 0.314 ** | −0.024 | 0.182 * | 1.000 |
| p | <0.001 | <0.001 | 0.731 | 0.023 | . | |
| N | 200 | 200 | 200 | 155 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primorac, D.; Brlek, P.; Matišić, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parčina, M. Cellular Immunity—The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. https://doi.org/10.3390/vaccines10030442
Primorac D, Brlek P, Matišić V, Molnar V, Vrdoljak K, Zadro R, Parčina M. Cellular Immunity—The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines. 2022; 10(3):442. https://doi.org/10.3390/vaccines10030442
Chicago/Turabian StylePrimorac, Dragan, Petar Brlek, Vid Matišić, Vilim Molnar, Kristijan Vrdoljak, Renata Zadro, and Marijo Parčina. 2022. "Cellular Immunity—The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination" Vaccines 10, no. 3: 442. https://doi.org/10.3390/vaccines10030442
APA StylePrimorac, D., Brlek, P., Matišić, V., Molnar, V., Vrdoljak, K., Zadro, R., & Parčina, M. (2022). Cellular Immunity—The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines, 10(3), 442. https://doi.org/10.3390/vaccines10030442








