SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore
Abstract
:1. Background
2. Methods
2.1. Study Participants
2.2. Analytical Methods
2.3. Statistical Analysis
3. Results
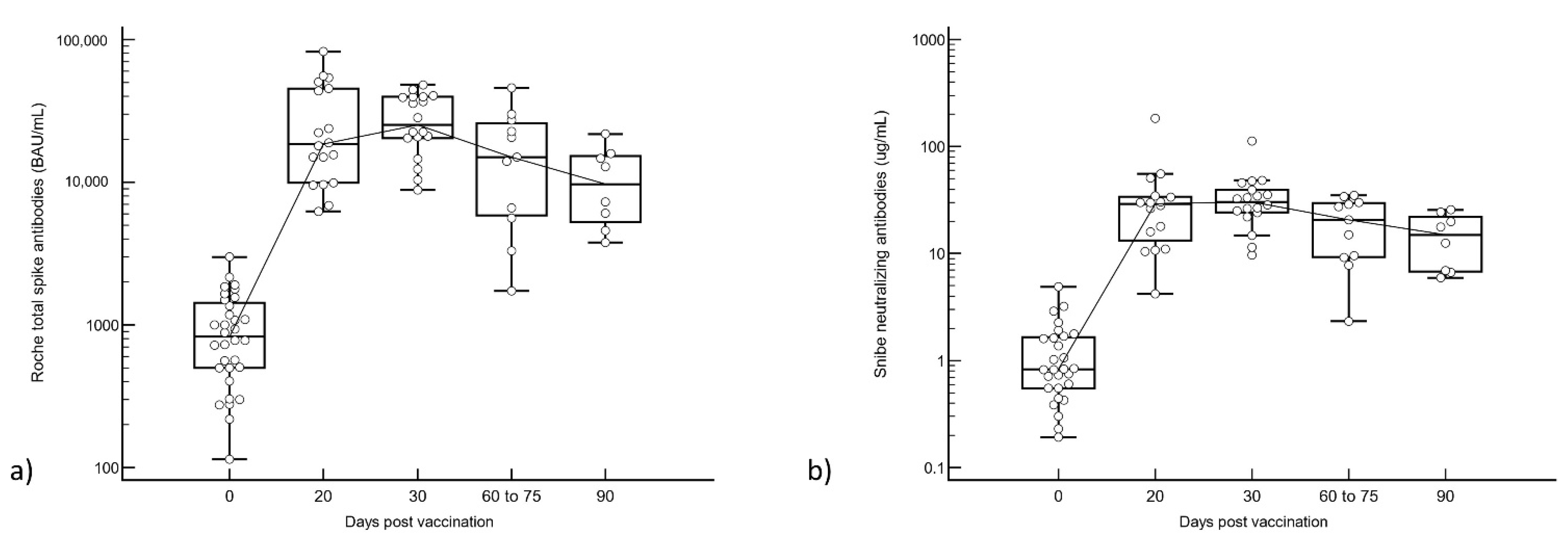
3.1. Total S-Ab vs. N-Ab Responses after Booster Vaccination
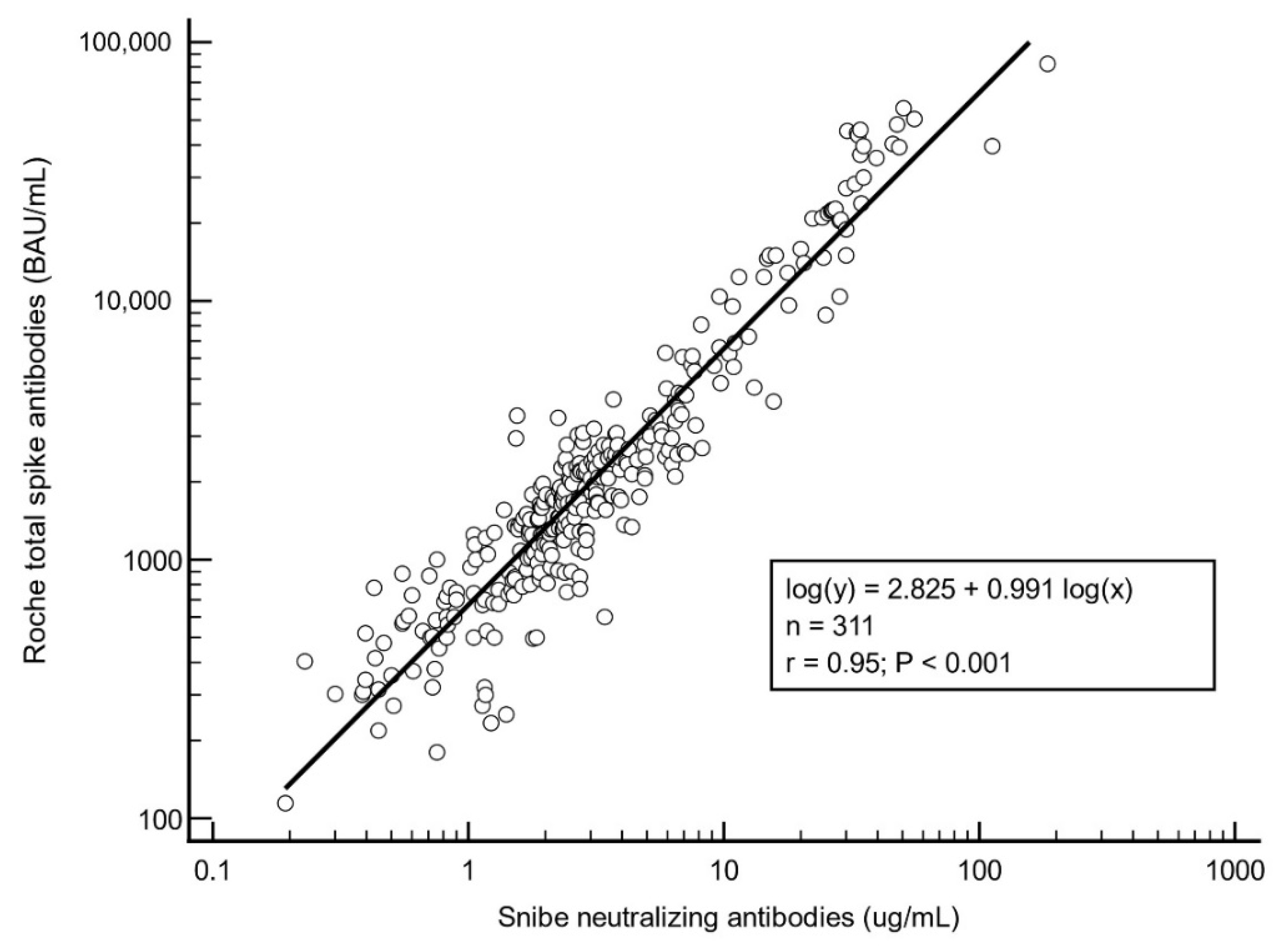
3.2. Regression Analysis
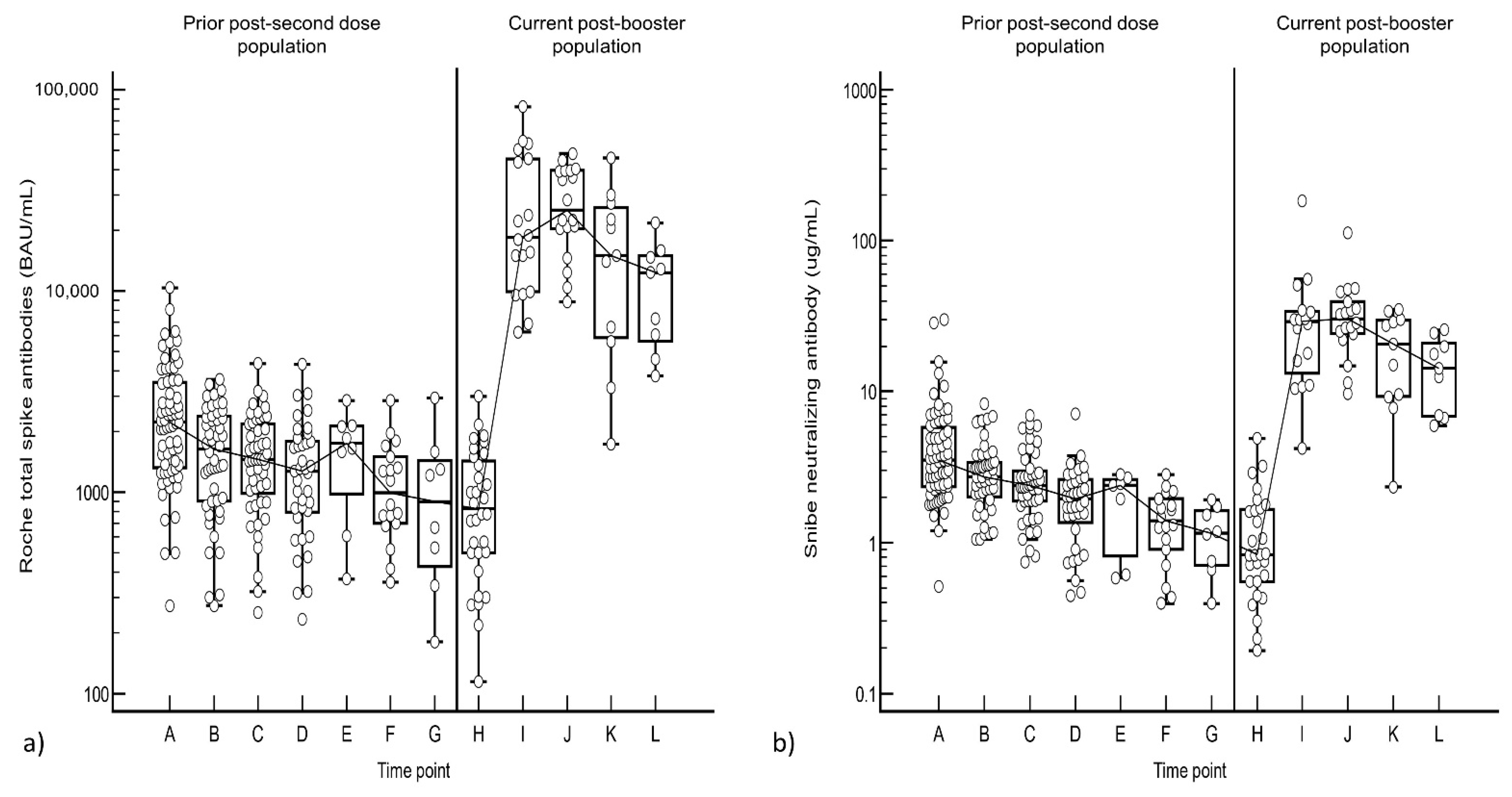
3.3. Comparison between Post-Second Dose and Post-Booster Responses
3.4. Antibody Half-Life Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Acronyms
| S-Ab | Spike antibodies |
| N-Ab | Neutralizing antibodies |
References
- Mbaeyi, S.; Oliver, S.E.; Collins, J.; Godfrey, M.; Goswami, N.D.; Hadler, S.C.; Jones, J.; Moline, H.; Moulia, D.; Reddy, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines—United States, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Sancilio, A.; Vaught, L.A.; Reiser, N.L.; Pesce, L.; McNally, E.M.; McDade, T.W. Antibody titers before and after booster doses of SARS-CoV-2 mRNA vaccines in healthy adults. medRxiv 2021. Preprint. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Leibovici-Weisman, Y.; Stemmer, A.; Ness, A.; Awwad, M.; Ghantous, N.; Stemmer, S.M. Antibody Titers Before and After a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Adults Aged ≥60 Years. JAMA 2021, 326, 2203–2204. [Google Scholar] [CrossRef] [PubMed]
- Ireland, G.; Whitaker, H.; Ladhani, S.N.; Baawuah, F.; Subbarao, S.; Linley, E.; Warrener, L.; O’Brien, M.; Whillock, C.; Martin, O.; et al. Serological responses to COVID-19 Comirnaty booster vaccine, London, United Kingdom, September to December 2021. Euro. Surveill. 2022, 27, 2101114. [Google Scholar] [CrossRef]
- Groβ, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pohlmann, S.; et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2021, 75, 103761. [Google Scholar]
- Peiris, M.; Cheng, S.; Mok, C.K.P.; Leung, Y.; Ng, S.; Chan, K.; Ko, F.; Yiu, K.; Lam, B.; Lau, E.; et al. Neutralizing antibody titres to SARS-CoV-2 Omicron variant and wild-type virus in those with past infection or vaccinated or boosted with mRNA BNT162b2 or inactivated CoronaVac vaccines. Res. Sq. 2022. Preprint. [Google Scholar] [CrossRef]
- Xue, J.; Lai, D.; Jiang, H.; Zhou, J.; Tao, S. One year landscape of the inactivated virus vaccine triggered RBD-specific IgG, IgM and IgA responses against the Omicron variant. medRxiv 2022. Preprint. [Google Scholar] [CrossRef]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Pries, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Lau, C.S.; Wong, M.S.; Hoo, S.P.; Heng, P.Y.; Phua, S.K.; Aw, T.C. Performance of the Roche/Snibe electrochemiluminescent anti-SARS-COV-2 spike assays compared to the Roche/Abbott IgG nucleocapsid and Abbott IgM spike assays. Pract. Lab. Med. 2021, 27, e00257. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Phua, S.K.; Liang, Y.L.; Oh, H.M.L.; Aw, T.C. Robust SARS-CoV-2 Antibody Responses in Asian COVID-Naïve Subjects 180 Days after Two Doses of BNT162b2 mRNA COVID-19 Vaccine. Vaccines 2021, 9, 1241. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Cosma, C.; Bonfante, F.; Rocca, F.D.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing antibody titers six months after Comirnaty vaccination: Kinetics and comparison with SARS-CoV-2 immunoassays. Clin. Chem. Lab. Med. 2021, 60, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Hoo, S.P.; Yew, S.F.; Ong, S.K.; Lum, L.T.; Heng, P.Y.; Tan, J.G.; Wong, M.S.; Aw, T.C. Evaluation of an electrochemiluminescent SARS-CoV-2 antibody assay. J. Appl. Lab. Med. 2020, 5, 1313–1323. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; Nitto, S.D.; Lippi, G. Total Anti-SARS-CoV-2 Antibodies Measured 6 months after Pfizer-BioNTech COVID-19 Vaccination in Healthcare Workers. J. Med. Biochem. 2022, 41, 1–5. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Aldridge, R.W.; Yavlinsky, A.; Nguyen, V.; Eyre, M.T.; Shrotri, M.; Navaratnam, A.M.D.; Beale, S.; Braithwaite, I.; Byrne, T.; Kovar, J.; et al. Waning of SARS-CoV-2 antibodies targeting the Spike protein in individuals post second dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and risk of breakthrough infections: Analysis of the Virus Watch community cohort. medRxiv 2021. Preprint. [Google Scholar] [CrossRef]
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A.; Griggs, E.P.; Gaglani, M.; Klein, N.P.; Grannis, S.J.; DeSilva, M.B.; Stenehjem, E.; et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Vari-ant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Spitzer, A.; Angel, Y.; Marudi, O.; Zeltser, D.; Saiag, E.; Goldshmidt, H.; Goldiner, I.; Stark, M.; Halutz, O.; Gamzu, R.; et al. Association of a Third Dose of BNT162b2 Vaccine with Incidence of SARS-CoV-2 Infection Among Health Care Workers in Israel. JAMA 2022, 327, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Hamady, A.; Lee, J.; Loboda, Z.A. Waning antibody responses in COVID-19: What can we learn from the analysis of other coronaviruses? Infection 2021, 50, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jurjenson, V.; Adamson, A.; Haljasmagi, L.; Rumm, A.P.; Maruste, R.; Karner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 booster vaccines against covid-19 related symptoms, hospitalisation and death in England. Nat. Med. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Booster Vaccination to Reduce SARS-CoV-2 Transmission and Infection. JAMA 2022, 327, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Oved, K.; Olmer, L.; Shemer-Avni, Y.; Wolf, T.; Supino-Rosin, L.; Prajgrod, G.; Shenhar, Y.; Payorsky, I.; Cohen, Y.; Kohn, Y.; et al. Multi-center nationwide comparison of seven serology assays reveals a SARS-CoV-2 non-responding seronegative subpopulation. EClinicalMedicine 2020, 29, 100651. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.S.; Phua, S.K.; Liang, Y.L.; Oh, M.L.H.; Aw, T.C. SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore. Vaccines 2022, 10, 331. https://doi.org/10.3390/vaccines10020331
Lau CS, Phua SK, Liang YL, Oh MLH, Aw TC. SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore. Vaccines. 2022; 10(2):331. https://doi.org/10.3390/vaccines10020331
Chicago/Turabian StyleLau, Chin Shern, Soon Kieng Phua, Ya Li Liang, May Lin Helen Oh, and Tar Choon Aw. 2022. "SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore" Vaccines 10, no. 2: 331. https://doi.org/10.3390/vaccines10020331
APA StyleLau, C. S., Phua, S. K., Liang, Y. L., Oh, M. L. H., & Aw, T. C. (2022). SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore. Vaccines, 10(2), 331. https://doi.org/10.3390/vaccines10020331






