Adverse Events Reporting Quality of Randomized Controlled Trials of COVID-19 Vaccine Using the CONSORT Criteria for Reporting Harms: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Risk of Bias Assessment
2.3. Quality of Reporting of Harm Data
2.4. Data Extraction and Analysis
2.5. Statistical Analysis
3. Results
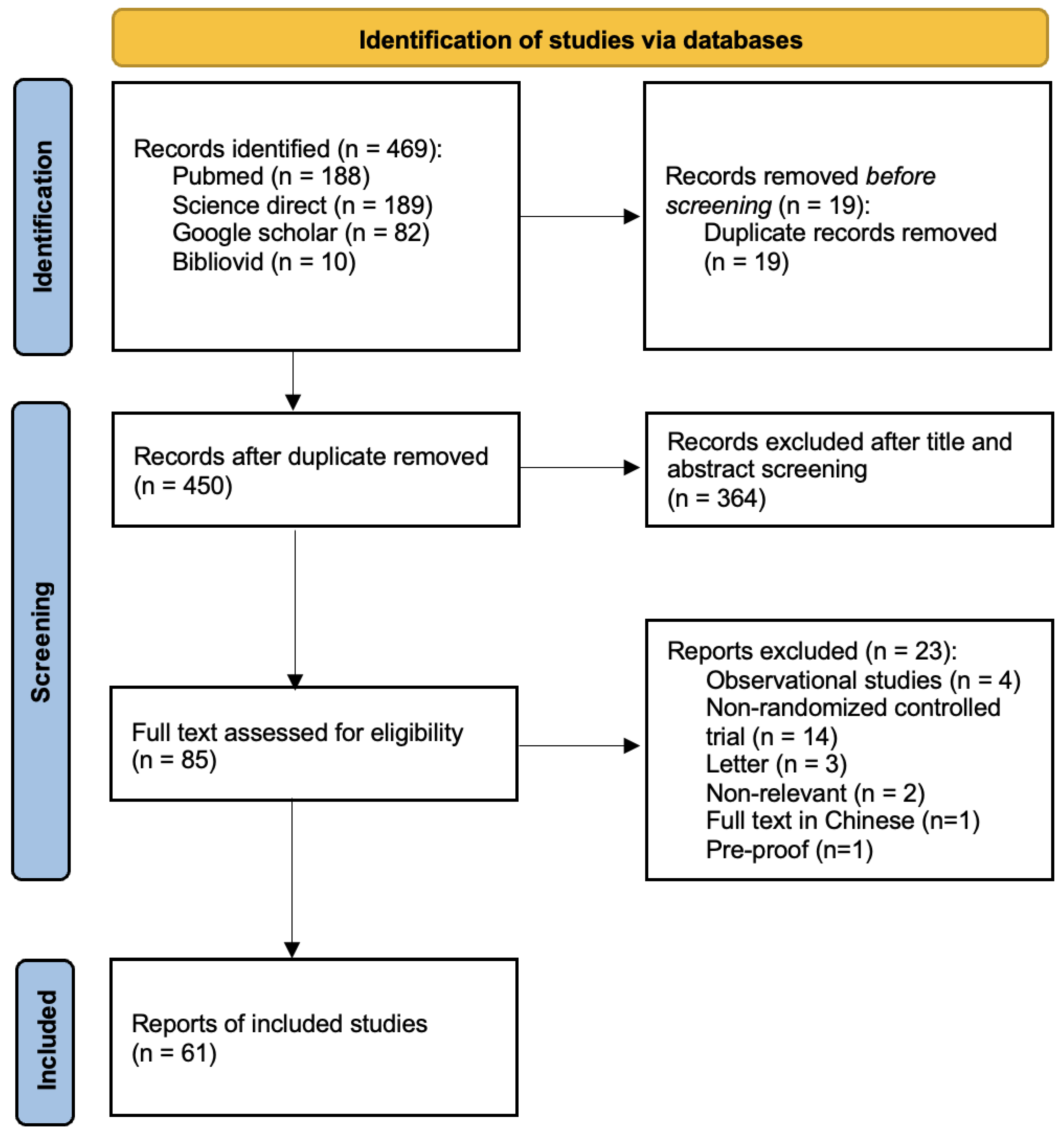
3.1. Result of the Search
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Adherence to CONSORT Recommendations
3.5. Determinant Factors of Reporting Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitworth, J. COVID-19: A Fast Evolving Pandemic. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Li, J.; Pang, Z. Recent Insights for the Emerging COVID-19: Drug Discovery, Therapeutic Options and Vaccine Development. Asian J. Pharm. Sci. 2021, 16, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, F.; Hadi, M.A.; Kow, C.S.; Marran, A.M.N.; Merchant, H.A.; Hasan, S.S. Use of Hydroxychloroquine and Chloroquine in COVID-19: How Good Is the Quality of Randomized Controlled Trials? Int. J. Infect. Dis. 2020, 101, 107–120. [Google Scholar] [CrossRef]
- BBC. COVID-19: First Vaccine given in US as Roll-out Begins. BBC News, 14 December 2020. [Google Scholar]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus (COVID-19) Vaccinations—Statistics and Research—Our World in Data. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 19 October 2021).
- Wagner, A.L.; Gordon, A.; Tallo, V.L.; Simaku, A.; Porter, R.M.; Edwards, L.J.; Duka, E.; Abu-Khader, I.; Gresh, L.; Sciuto, C.; et al. Intent to Obtain Pediatric Influenza Vaccine among Mothers in Four Middle Income Countries. Vaccine 2020, 38, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 Vaccine Acceptance and Hesitancy in Low- and Middle-Income Countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- France24. WHO Urges Countries to Continue the Roll-out of AstraZeneca Vaccine. France24, 19 March 2021. [Google Scholar]
- European Medical Agency. AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets; European Medicines Agency: Amsterdam, The Netherlands, 2021.
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA—J. Am. Med. Assoc. 2021, 325, 2448–2456. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events after COVID-19 MRNA Vaccination. JAMA—J. Am. Med. Assoc. 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 MRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Shukralla, A.A.; Tudur-Smith, C.; Powell, G.A.; Williamson, P.R.; Marson, A.G. Reporting of Adverse Events in Randomised Controlled Trials of Antiepileptic Drugs Using the CONSORT Criteria for Reporting Harms. Epilepsy Res. 2011, 97, 20–29. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Evans, S.J.W.; Gøtzsche, P.C.; O’Neill, R.T.; Altman, D.G.; Schulz, K.; Moher, D. Better Reporting of Harms in Randomized Trials: An Extension of the CONSORT Statement. Ann. Intern. Med. 2004, 141, 781–788. [Google Scholar] [CrossRef]
- Cornelius, V.R.; Sauzet, O.; Williams, J.E.; Ayis, S.; Farquhar-Smith, P.; Ross, J.R.; Branford, R.A.; Peacock, J.L. Adverse Event Reporting in Randomised Controlled Trials of Neuropathic Pain: Considerations for Future Practice. Pain 2013, 154, 213–220. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liampas, I.; Chlinos, A.; Siokas, V.; Brotis, A.; Dardiotis, E. Assessment of the Reporting Quality of RCTs for Novel Oral Anticoagulants in Venous Thromboembolic Disease Based on the CONSORT Statement. J. Thromb. Thrombolysis 2019, 48, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Liu, X.; Pu, Y.; Zhou, M.; Zhao, Z.; Jiang, R.; Yin, Z.; Xu, M.; Yin, Q.; Wang, J.; et al. Randomized, Double-Blinded, Placebo-Controlled Phase 2 Trial of an Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in Healthy Adults. Clin. Infect. Dis. 2020, 73, e3949–e3955. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBV152: Interim Results from a Double-Blind, Randomised, Multicentre, Phase 2 Trial, and 3-Month Follow-up of a Double-Blind, Randomised Phase 1 Trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (CoronaVac) in Healthy Children and Adolescents: A Double-Blind, Randomised, Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 NCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J.; et al. Safety and Immunogenicity of S-Trimer (SCB-2019), a Protein Subunit Vaccine Candidate for COVID-19 in Healthy Adults: A Phase 1, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and Safety of a Recombinant Adenovirus Type-5-Vectored COVID-19 Vaccine in Healthy Adults Aged 18 Years or Older: A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Healthy Adults Aged 18–59 Years: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and Immunogenicity of a Recombinant Tandem-Repeat Dimeric RBD-Based Protein Subunit Vaccine (ZF2001) against COVID-19 in Adults: Two Randomised, Double-Blind, Placebo-Controlled, Phase 1 and 2 Trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and Immunogenicity of an Inactivated COVID-19 Vaccine, BBIBP-CorV, in People Younger than 18 Years: A Randomised, Double-Blind, Controlled, Phase 1/2 Trial. Lancet Infect. Dis. 2021, 22, 196–208. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA—J. Am. Med. Assoc. 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (CoronaVac) in Healthy Adults Aged 60 Years and Older: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Pan, H.X.; Liu, J.K.; Huang, B.Y.; Li, G.F.; Chang, X.Y.; Liu, Y.F.; Wang, W.L.; Chu, K.; Hu, J.L.; Li, J.X.; et al. Immunogenicity and Safety of a Severe Acute Respiratory Syndrome Coronavirus 2 Inactivated Vaccine in Healthy Adults: Randomized, Double-Blind, and Placebo-Controlled Phase 1 and Phase 2 Clinical Trials. Chin. Med. J. 2021, 134, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, P.A.; Fu, B.; Chabanon, A.L.; Bonaparte, M.I.; Davis, M.G.; Essink, B.J.; Frank, I.; Haney, O.; Janosczyk, H.; Keefer, M.C.; et al. Safety and Immunogenicity of SARS-CoV-2 Recombinant Protein Vaccine Formulations in Healthy Adults: Interim Results of a Randomised, Placebo-Controlled, Phase 1–2, Dose-Ranging Study. Lancet Infect. Dis. 2021, 21, 1257–1270. [Google Scholar] [CrossRef]
- Li, J.; Hui, A.; Zhang, X.; Yang, Y.; Tang, R.; Ye, H.; Ji, R.; Lin, M.; Zhu, Z.; Türeci, Ö.; et al. Safety and Immunogenicity of the SARS-CoV-2 BNT162b1 MRNA Vaccine in Younger and Older Chinese Adults: A Randomized, Placebo-Controlled, Double-Blind Phase 1 Study. Nat. Med. 2021, 27, 1062–1070. [Google Scholar] [CrossRef]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 NCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Chappell, K.J.; Mordant, F.L.; Li, Z.; Wijesundara, D.K.; Ellenberg, P.; Lackenby, J.A.; Cheung, S.T.M.; Modhiran, N.; Avumegah, M.S.; Henderson, C.L.; et al. Safety and Immunogenicity of an MF59-Adjuvanted Spike Glycoprotein-Clamp Vaccine for SARS-CoV-2: A Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Infect. Dis. 2021, 21, 1383–1394. [Google Scholar] [CrossRef]
- Chu, L.; McPhee, R.; Huang, W.; Bennett, H.; Pajon, R.; Nestorova, B.; Leav, B. A Preliminary Report of a Randomized Controlled Phase 2 Trial of the Safety and Immunogenicity of MRNA-1273 SARS-CoV-2 Vaccine. Vaccine 2021, 39, 2791–2799. [Google Scholar] [CrossRef]
- Sadoff, J.; le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBV152: A Double-Blind, Randomised, Phase 1 Trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Mann, P.; Kroidl, A.; Leroux-Roels, I.; Schindler, C.; Gabor, J.J.; Schunk, M.; Leroux-Roels, G.; Bosch, J.J.; Fendel, R.; et al. Safety and Immunogenicity of an MRNA-Lipid Nanoparticle Vaccine Candidate against SARS-CoV-2: A Phase 1 Randomized Clinical Trial. Wien. Klin. Wochenschr. 2021, 133, 931–941. [Google Scholar] [CrossRef]
- Meng, F.Y.; Gao, F.; Jia, S.Y.; Wu, X.H.; Li, J.X.; Guo, X.L.; Zhang, J.L.; Cui, B.P.; Wu, Z.M.; Wei, M.W.; et al. Safety and Immunogenicity of a Recombinant COVID-19 Vaccine (Sf9 Cells) in Healthy Population Aged 18 Years or Older: Two Single-Center, Randomised, Double-Blind, Placebo-Controlled, Phase 1 and Phase 2 Trials. Signal Transduct. Target. Ther. 2021, 6, 271. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and Immunogenicity of Heterologous versus Homologous Prime-Boost Schedules with an Adenoviral Vectored and MRNA COVID-19 Vaccine (Com-COV): A Single-Blind, Randomised, Non-Inferiority Trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Shu, Y.J.; He, J.F.; Pei, R.J.; He, P.; Huang, Z.H.; Chen, S.M.; Ou, Z.Q.; Deng, J.L.; Zeng, P.Y.; Zhou, J.; et al. Immunogenicity and Safety of a Recombinant Fusion Protein Vaccine (V-01) against Coronavirus Disease 2019 in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Phase II Trial. Chin. Med. J. 2021, 134, 1967–1976. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and Safety of an Inactivated Whole-Virion SARS-CoV-2 Vaccine (CoronaVac): Interim Results of a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Pu, J.; Yu, Q.; Yin, Z.; Zhang, Y.; Li, X.; Yin, Q.; Chen, H.; Long, R.; Zhao, Z.; Mou, T.; et al. The Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Chinese Adults Aged 18–59 Years: A Phase I Randomized, Double-Blinded, Controlled Trial. Vaccine 2021, 39, 2746–2754. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, Tolerability, and Immunogenicity of an Aerosolised Adenovirus Type-5 Vector-Based COVID-19 Vaccine (Ad5-NCoV) in Adults: Preliminary Report of an Open-Label and Randomised Phase 1 Clinical Trial. Lancet Infect. Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Zakarya, K.; Kutumbetov, L.; Orynbayev, M.; Abduraimov, Y.; Sultankulova, K.; Kassenov, M.; Sarsenbayeva, G.; Kulmagambetov, I.; Davlyatshin, T.; Sergeeva, M.; et al. Safety and Immunogenicity of a QazCovid-In® Inactivated Whole-Virion Vaccine against COVID-19 in Healthy Adults: A Single-Centre, Randomised, Single-Blind, Placebo-Controlled Phase 1 and an Open-Label Phase 2 Clinical Trials with a 6 Months Follow-up in Kazakhstan. EClinicalMedicine 2021, 39, 101078. [Google Scholar] [CrossRef] [PubMed]
- Fadlyana, E.; Rusmil, K.; Tarigan, R.; Rahmadi, A.R.; Prodjosoewojo, S.; Sofiatin, Y.; Khrisna, C.V.; Sari, R.M.; Setyaningsih, L.; Surachman, F.; et al. A Phase III, Observer-Blind, Randomized, Placebo-Controlled Study of the Efficacy, Safety, and Immunogenicity of SARS-CoV-2 Inactivated Vaccine in Healthy Adults Aged 18–59 Years: An Interim Analysis in Indonesia. Vaccine 2021, 39, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 NCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Hsieh, S.M.; Liu, M.C.; Chen, Y.H.; Lee, W.-S.; Hwang, S.J.; Cheng, S.H.; Ko, W.C.; Hwang, K.P.; Wang, N.C.; Lee, Y.L.; et al. Safety and Immunogenicity of CpG 1018 and Aluminium Hydroxide-Adjuvanted SARS-CoV-2 S-2P Protein Vaccine MVC-COV1901: Interim Results of a Large-Scale, Double-Blind, Randomised, Placebo-Controlled Phase 2 Trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Asano, M.; Okada, H.; Itoh, Y.; Hirata, H.; Ishikawa, K.; Yoshida, E.; Matsui, A.; Kelly, E.J.; Shoemaker, K.; Olsson, U.; et al. Immunogenicity and Safety of AZD1222 (ChAdOx1 NCoV-19) against SARS-CoV-2 in Japan: A Double-Blind, Randomized Controlled Phase 1/2 Trial. Int. J. Infect. Dis. 2022, 114, 165–174. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, Safety, and Lot-to-Lot Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (BBV152): Interim Results of a Randomised, Double-Blind, Controlled, Phase 3 Trial. Lancet 2021, 398, 2173–2184. [Google Scholar] [CrossRef]
- Haranaka, M.; Baber, J.; Ogama, Y.; Yamaji, M.; Aizawa, M.; Kogawara, O.; Scully, I.; Lagkadinou, E.; Türeci, Ö.; Şahin, U.; et al. A Randomized Study to Evaluate Safety and Immunogenicity of the BNT162b2 COVID-19 Vaccine in Healthy Japanese Adults. Nat. Commun. 2021, 12, 7105. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and Immunogenicity of Seven COVID-19 Vaccines as a Third Dose (Booster) Following Two Doses of ChAdOx1 NCov-19 or BNT162b2 in the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Y.; Pei, R.; Fang, X.; Zeng, P.; Fan, R.; Ou, Z.; Deng, J.; Zhou, J.; Guan, W.; et al. Safety and Immunogenicity of a Recombinant Interferon-Armed RBD Dimer Vaccine (V-01) for COVID-19 in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Phase I Trial. Emerg. Microbes Infect. 2021, 10, 1589–1597. [Google Scholar] [CrossRef]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Muñoz Navarro, S.R.; et al. Final Efficacy Analysis, Interim Safety Analysis, and Immunogenicity of a Single Dose of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) in Adults 18 Years and Older: An International, Multicentre, Randomised, Double-Blinded, Placebo-Controlled Phase 3 Trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Intapiboon, P.; Seepathomnarong, P.; Ongarj, J.; Surasombatpattana, S.; Uppanisakorn, S.; Mahasirimongkol, S.; Sawaengdee, W.; Phumiamorn, S.; Sapsutthipas, S.; Sangsupawanich, P.; et al. Immunogenicity and Safety of an Intradermal BNT162b2 MRNA Vaccine Booster after Two Doses of Inactivated SARS-CoV-2 Vaccine in Healthy Population. Vaccines 2021, 9, 1375. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Ahuad Guerrero, R.A.; Arana-Arri, E.; Aroca Martinez, G.J.; Bonten, M.; Chandler, R.; Corral, G.; de Block, E.J.L.; Ecker, L.; Gabor, J.J.; et al. Efficacy and Safety of the CVnCoV SARS-CoV-2 MRNA Vaccine Candidate in Ten Countries in Europe and Latin America (HERALD): A Randomised, Observer-Blinded, Placebo-Controlled, Phase 2b/3 Trial. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Guo, W.; Duan, K.; Zhang, Y.; Yuan, Z.; Zhang, Y.B.; Wang, Z.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Healthy Adults Aged 18 Years or Older: A Randomized, Double-Blind, Placebo-Controlled, Phase 1/2 Trial. EClinicalMedicine 2021, 38, 101010. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Padmapriyadarsini, C.; Vekemans, J.; Bavdekar, A.; Gupta, M.; Kulkarni, P.; Garg, B.S.; Gogtay, N.J.; Tambe, M.; Lalwani, S.; et al. A Phase 2/3, Participant-Blind, Observer-Blind, Randomised, Controlled Study to Assess the Safety and Immunogenicity of SII-ChAdOx1 NCoV-19 (COVID-19 Vaccine) in Adults in India. eClinicalMedicine 2021, 42, 101218. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and Safety of a Third Dose of CoronaVac, and Immune Persistence of a Two-Dose Schedule, in Healthy Adults: Interim Results from Two Single-Centre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Clinical Trials. Lancet Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of MRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef]
- el Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Formica, N.; Mallory, R.; Albert, G.; Robinson, M.; Plested, J.S.; Cho, I.; Robertson, A.; Dubovsky, F.; Glenn, G.M.; 2019nCoV-101 Study Group. Different Dose Regimens of a SARS-CoV-2 Recombinant Spike Protein Vaccine (NVXCoV2373) in Younger and Older Adults: A Phase 2 Randomized Placebo-Controlled Trial. PLoS Med. 2021, 18, e1003769. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Yao, T.; Chang, Y.; Li, X.; Xing, R.; Li, H.; Xie, R.; Zhang, X.; Wei, Z.; et al. Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccine in High-Risk Occupational Population: A Randomized, Parallel, Controlled Clinical Trial. Infect. Dis. Poverty 2021, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.Z.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and Immunogenicity of SARS-CoV-2 Variant MRNA Vaccine Boosters in Healthy Adults: An Interim Analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.S.; Yuki, E.F.N.; Pedrosa, T.; Fusco, S.R.G.; Rojo, P.T.; Pereira, R.M.R.; Shinjo, S.K.; Andrade, D.C.O.; et al. Immunogenicity and Safety of the CoronaVac Inactivated Vaccine in Patients with Autoimmune Rheumatic Diseases: A Phase 4 Trial. Nat. Med. 2021, 27, 1744–1751. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBIBP-CorV: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Singh, S.; Loke, Y.K. Drug Safety Assessment in Clinical Trials: Methodological Challenges and Opportunities. Trials 2012, 13, 138. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.; Hazell, L.; Sauzet, O.; Cornelius, V. Analysis and Reporting of Adverse Events in Randomised Controlled Trials: A Review. BMJ Open 2019, 9, e024537. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.B.; Duan, N.; Raneses, E.; Suttner, L.; Ciarametaro, M.; Cooney, E.; Dubois, R.W.; Halpern, S.D.; Kravitz, R.L. No Improvement in the Reporting of Clinical Trial Subgroup Effects in High-Impact General Medical Journals. Trials 2016, 17, 320. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, O. Statistical Methods to Analyze Adverse Events Data of Randomized Clinical Trials. J. Biopharm. Stat. 2009, 19, 889–899. [Google Scholar] [CrossRef]
- Hays, M.; Andrews, M.; Wilson, R.; Callender, D.; O’Malley, P.G.; Douglas, K. Reporting Quality of Randomised Controlled Trial Abstracts among High-Impact General Medical Journals: A Review and Analysis. BMJ Open 2016, 6, e011082. [Google Scholar] [CrossRef] [Green Version]

| Study Characteristic | No. (%) |
|---|---|
| Total, No. | 61 |
| Type of vaccine | |
| Inactivated vaccine | 18 (29.5) |
| mRNA | 18 (29.5) |
| Protein subunit | 7 (11.5) |
| Viral vector | 18 (29.5) |
| No. of subjects | |
| 1–500 | 22 (36.1) |
| 500–1000 | 16 (26.2) |
| >1000 | 23 (37.3) |
| Age of participants | |
| <18 | 4 (6.6) |
| ≥12 | 1 (1.7) |
| ≥18 | 56 (96.7) |
| Type of study | |
| Multi-center | 35 (57.4) |
| Single-center | 26 (42.6) |
| Study design | |
| Open-label | 6 (9.8) |
| Single-blind | 15 (24.6) |
| Double-blind | 33 (54.1) |
| Triple blind | 6 (9.8) |
| Not stated | 1 (1.7) |
| Clinical trial phase | |
| Phase I | 11 (18) |
| Phase I/II | 19 (31.1) |
| Phase II | 12 (19.7) |
| Phase II/III | 4 (6.6) |
| Phase III | 9 (14.7) |
| Phase I–III | 4 (6.6) |
| Phase IV | 2 (3.3) |
| Impact Factor | |
| <50 | 45 (73.8) |
| ≥50 | 16 (26.2) |
| Number of authors | |
| <20 | 16 (26.2) |
| 20–50 | 40 (65.6) |
| >50 | 5 (8.2) |
| Funding | |
| Industry | 31 (50.8) |
| Non-industry | 30 (49.2) |
| Participants flowchart | |
| Yes | 54 (88.5) |
| No | 7 (11.5) |
| Section of Paper | CONSORT Harm Recommendation | Detailed Items | Compliance of Trials, n (%) |
|---|---|---|---|
| Title and abstract |
| 1. Adverse events mentioned in title or abstract | 57 (93) |
| Introduction |
| 2. Information on adverse events mentioned in the introduction | 56 (92) |
| Methods |
| 3a. Definitions of AEs mentioned | 50 (82) |
| 3b. If article mentioned all or selected sample of AE | 45 (74) | ||
| 3c. If article mentioned the use of a validated instrument to report adverse event severity | 25 (41) | ||
| 4a. Describe the mode of data collection (e.g., diaries, phone interviews, face-to-face interviews) | 52 (85) | |
| 4b. Stated the timing of collection of AE data | 59 (97) | ||
| 4c. Description of how AE were attributed to trial drugs | 39 (64) | ||
| 4d. Described the plan for monitoring for harms and rules for stopping the trial because of harms | 19 (31) | ||
| 5a. Described the methods for presenting and/or analyzing adverse events | 53 (87) | |
| 5b. Description of approach for the handling of recurrent AEs | 6 (10) | ||
| Result |
| 6a. Reported withdrawals because of AE in each arm | 30 (49) |
| 6b. Reported deaths and serious AEs | 61 (100) | ||
| 7a. Provided denominators for adverse events | 44 (72) | |
| 7b. Provided definitions used for analysis set (intention to treat, per protocol, safety data available, unclear) | 25 (41) | ||
| 8a. Reported results separately for each treatment arm | 55 (90) | |
| 8b. Severity and grading of AEs | 56 (92) | ||
| 8c. Provided both number of adverse events and number of patients with adverse events | 57 (93) | ||
| 9. Described subgroup analysis and exploratory analysis for harms | 15 (25) | |
| Discussion |
| 10a. Provided a balanced view that puts benefits and harms into perspective | 55 (90) |
| 10b. Included limitations of study with respect to harms (e.g., lack of power, short duration of exposure, inconclusive findings, post hoc analysis, generalisability of AE info as dependent on clinical setting) | 57 (93) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuniar, C.T.; Pratiwi, B.; Ihsan, A.F.; Laksono, B.T.; Risfayanti, I.; Fathadina, A.; Jeong, Y.; Kim, E. Adverse Events Reporting Quality of Randomized Controlled Trials of COVID-19 Vaccine Using the CONSORT Criteria for Reporting Harms: A Systematic Review. Vaccines 2022, 10, 313. https://doi.org/10.3390/vaccines10020313
Yuniar CT, Pratiwi B, Ihsan AF, Laksono BT, Risfayanti I, Fathadina A, Jeong Y, Kim E. Adverse Events Reporting Quality of Randomized Controlled Trials of COVID-19 Vaccine Using the CONSORT Criteria for Reporting Harms: A Systematic Review. Vaccines. 2022; 10(2):313. https://doi.org/10.3390/vaccines10020313
Chicago/Turabian StyleYuniar, Cindra Tri, Bhekti Pratiwi, Ardika Fajrul Ihsan, Bambang Tri Laksono, Iffa Risfayanti, Annisa Fathadina, Yeonseon Jeong, and Eunyoung Kim. 2022. "Adverse Events Reporting Quality of Randomized Controlled Trials of COVID-19 Vaccine Using the CONSORT Criteria for Reporting Harms: A Systematic Review" Vaccines 10, no. 2: 313. https://doi.org/10.3390/vaccines10020313
APA StyleYuniar, C. T., Pratiwi, B., Ihsan, A. F., Laksono, B. T., Risfayanti, I., Fathadina, A., Jeong, Y., & Kim, E. (2022). Adverse Events Reporting Quality of Randomized Controlled Trials of COVID-19 Vaccine Using the CONSORT Criteria for Reporting Harms: A Systematic Review. Vaccines, 10(2), 313. https://doi.org/10.3390/vaccines10020313






