Vaccines, Microbiota and Immunonutrition: Food for Thought
Abstract
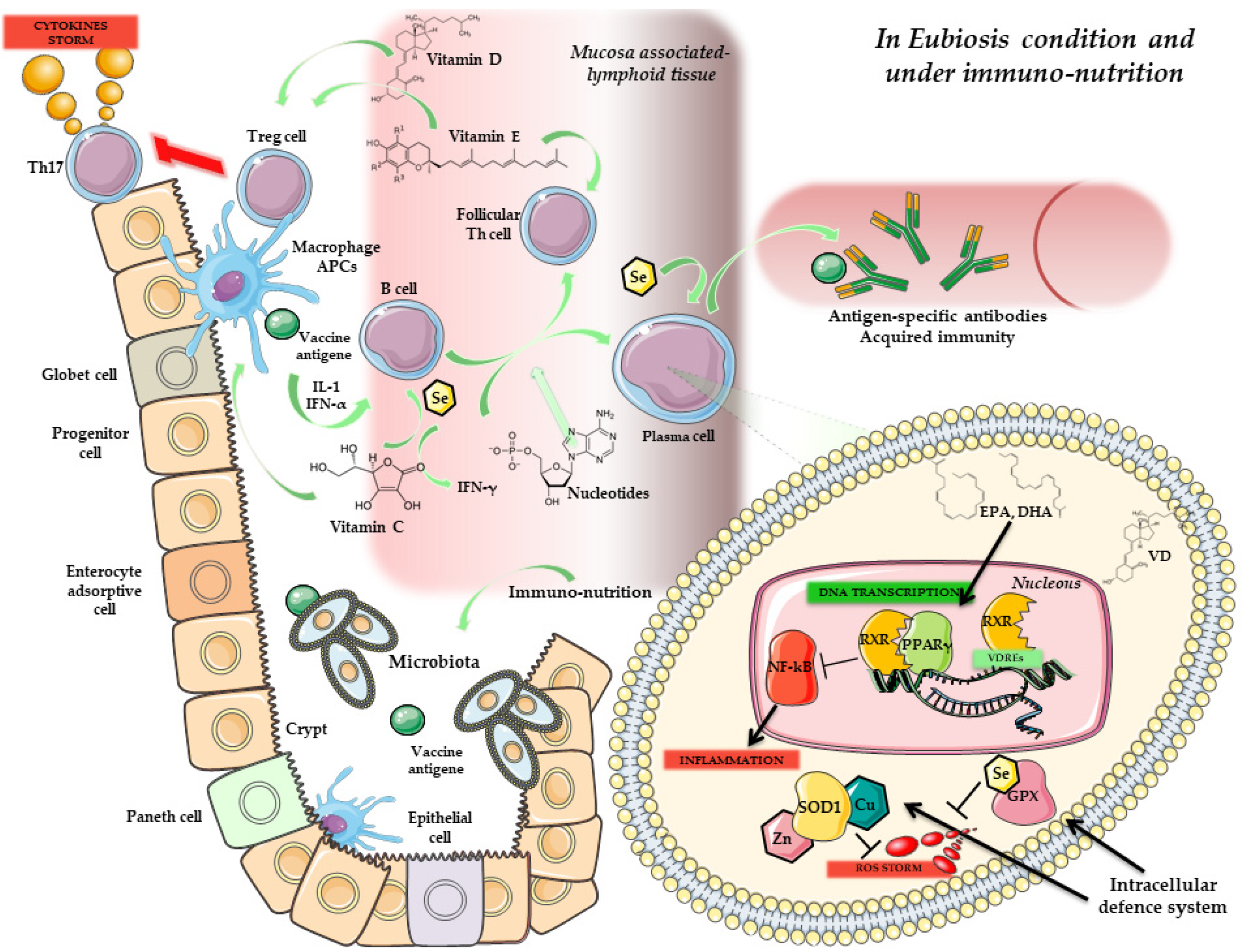
:1. Introduction
2. Microbiota and Nutritional Status
2.1. Gut Microbiota Composition
2.2. Impact of Diet on Gut Microbiota
2.3. Gut Microbiota and Inflammatory Status
2.4. Diet, Gut Microbiota and Human Health
3. Immunonutrition: An Overview
3.1. Nutritional Status in Human Health
3.2. Nutrients and Immune System: An Intricate Relationship
3.3. Immunonutrition: The Key Actors
3.3.1. Glutamine
3.3.2. Arginine
3.3.3. Omega-3
3.3.4. Alfa-Linolenic Acid
3.3.5. Vitamin D
3.3.6. Vitamin E
3.3.7. Vitamin C
3.3.8. Selenium
3.3.9. Zinc
4. Immunity, Vaccines and Microbiota
Microbiota Composition and Response to Vaccines
5. Microbiota, Immunonutrition and Probiotics: Improving Vaccine Response
Prebiotics, Probiotics and Vaccination
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Callegaro, A.; Curran, D.; Matthews, S. Burden-of-illness vaccine efficacy. Pharm. Stat. 2020, 19, 636–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.L.; Pellicane, A.J.; Tyring, S.K. Vaccine immunology. Dermatol. Ther. 2009, 22, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Sadighi Akha, A.A. Aging and the immune system: An overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef]
- Cianci, R.; Franza, L.; Massaro, M.G.; Borriello, R.; De Vito, F.; Gambassi, G. The Interplay between Immunosenescence and Microbiota in the Efficacy of Vaccines. Vaccines 2020, 8, 636. [Google Scholar] [CrossRef]
- Wiedermann, U.; Garner-Spitzer, E.; Wagner, A. Primary vaccine failure to routine vaccines: Why and what to do? Hum. Vaccines Immunother. 2016, 12, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharmacal Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Schenten, D.; Medzhitov, R. The control of adaptive immune responses by the innate immune system. Adv. Immunol. 2011, 109, 87–124. [Google Scholar]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [Green Version]
- Lynn, D.J.; Pulendran, B. The potential of the microbiota to influence vaccine responses. J. Leukoc. Biol. 2018, 103, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciabattini, A.; Olivieri, R.; Lazzeri, E.; Medaglini, D. Role of the Microbiota in the Modulation of Vaccine Immune Responses. Front. Microbiol. 2019, 10, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. MMBR 2017, 81, e00036-17. [Google Scholar] [CrossRef] [Green Version]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Merra, G.; Noce, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef]
- Di Renzo, L.; Cinelli, G. Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women. Nutrients 2020, 12, 3456. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.T.; Shih, P.C.; Liu, S.J. Effect of Probiotics and Prebiotics on Immune Response to Influenza Vaccination in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 1175. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean diet and health: Food effects on gut microbiota and disease control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Franza, L.; Carusi, V.; Nucera, E.; Pandolfi, F. Luteolin, inflammation and cancer: Special emphasis on gut microbiota. BioFactors 2021, 47, 181–189. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Casey, D.; Coates, M.M.; Delwiche, K.; Estep, K.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013, a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef] [Green Version]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; Pivari, F. COVID-19, Is there a role for immunonutrition in obese patient? J. Transl. Med. 2020, 18, 415. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Feeding the immune system. Proc. Nutr. Soc. 2013, 72, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Noncommunicable Diseases Country Profiles 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Van Loveren, H.; Van Amsterdam, J.G.; Vandebriel, R.J.; Kimman, T.G.; Rümke, H.C.; Steerenberg, P.S.; Vos, J.G. Vaccine-induced antibody responses as parameters of the influence of endogenous and environmental factors. Environ. Health Perspect. 2001, 109, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Fuel utilization by cells of the immune system. Proc. Nutr. Soc. 1995, 54, 65–82. [Google Scholar] [CrossRef] [Green Version]
- Sallusto, F.; Lanzavecchia, A. Heterogeneity of CD4+ memory T cells: Functional modules for tailored immunity. Eur. J. Immunol. 2009, 39, 2076–2082. [Google Scholar] [CrossRef]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef]
- Di Renzo, L.; Galvano, F.; Orlandi, C.; Bianchi, A.; Di Giacomo, C.; La Fauci, L.; Acquaviva, R.; De Lorenzo, A. Oxidative stress in normal-weight obese syndrome. Obesity 2010, 18, 2125–2130. [Google Scholar] [CrossRef]
- Uauy, R. Academic-industry partnerships in addressing nutrition–[infection-immunity-inflammation] interactions. Br. J. Nutr. 2007, 98 (Suppl. 1), S17–S23. [Google Scholar] [CrossRef]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Grimble, R.F. Nutritional modulation of immune function. Proc. Nutr. Soc. 2001, 60, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zapatera, B.; Prados, A.; Gómez-Martínez, S.; Marcos, A. Immunonutrition: Methodology and applications. Nutr. Hosp. 2015, 31 (Suppl. 3), 145–154. [Google Scholar] [PubMed]
- Marik, P.E.; Zaloga, G.P. Immunonutrition in critically ill patients: A systematic review and analysis of the literature. Intensive Care Med. 2008, 34, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [Green Version]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I.H., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef]
- Hiscock, N.; Petersen, E.W.; Krzywkowski, K.; Boza, J.; Halkjaer-Kristensen, J.; Pedersen, B.K. Glutamine supplementation further enhances exercise-induced plasma IL-6. J. Appl. Physiol. 2003, 95, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Curi, R.; Lagranha, C.J.; Doi, S.Q.; Sellitti, D.F.; Procopio, J.; Pithon-Curi, T.C. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem. Funct. 2005, 23, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Bittencourt, A.; Scomazzon, S.P.; Leite, J.S.; de Bittencourt, P.I., Jr.; Tirapegui, J. Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition 2014, 30, 602–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenders, C.M.; Liu, S.; Wilmore, D.W.; Sampson, L.; Dougherty, L.W.; Spiegelman, D.; Willett, W.C. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur. J. Clin. Nutr. 2009, 63, 1433–1439. [Google Scholar] [CrossRef]
- Furst, P.; Alteheld, B.; Stehle, P. Why should a single nutrien—glutamine—improve outcome?: The remarkable story of glutamine dipeptides. Clin. Nutr. Suppl. 2004, 1, 3–15. [Google Scholar]
- Kim, H. Glutamine as an immunonutrient. Yonsei Med. J. 2011, 52, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Lecleire, S.; Hassan, A.; Marion-Letellier, R.; Antonietti, M.; Savoye, G.; Bôle-Feysot, C.; Lerebours, E.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Combined glutamine and arginine decrease proinflammatory cytokine production by biopsies from Crohn’s patients in association with changes in nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways. J. Nutr. 2008, 138, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Budani, M.C.; Tiboni, G.M. Novel Insights on the Role of Nitric Oxide in the Ovary: A Review of the Literature. Int. J. Environ. Res. Public Health 2021, 18, 980. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Impact of Arginine Nutrition and Metabolism during Pregnancy on Offspring Outcomes. Nutrients 2019, 11, 1452. [Google Scholar] [CrossRef] [Green Version]
- Albaugh, V.L.; Pinzon-Guzman, C.; Barbul, A. Arginine-Dual roles as an onconutrient and immunonutrient. J. Surg. Oncol. 2017, 115, 273–280. [Google Scholar] [CrossRef]
- Jabłońska, B.; Mrowiec, S. The Role of Immunonutrition in Patients Undergoing Pancreaticoduodenectomy. Nutrients 2020, 12, 2547. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Carrott, P.W.; Patel, J.; Kiraly, L.; Martindale, R.G. Parenteral or Enteral Arginine Supplementation Safety and Efficacy. J. Nutr. 2016, 146, 2594S–2600S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.H.; Cha, H.J. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, L.; Molinari, R. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [Green Version]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-León, A.M.; Lapuente, M.; Estruch, R.; Casas, R. Clinical Advances in Immunonutrition and Atherosclerosis: A Review. Front. Immunol. 2019, 10, 837. [Google Scholar] [CrossRef]
- Thies, F.; Garry, J.M.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Tamblyn, J.A.; Hewison, M.; Wagner, C.L.; Bulmer, J.N.; Kilby, M.D. Immunological role of vitamin D at the maternal-fetal interface. J. Endocrinol. 2015, 224, R107–R121. [Google Scholar] [CrossRef] [Green Version]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3, a helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, F.; Franza, L.; Mandolini, C.; Conti, P. Immune Modulation by Vitamin D: Special Emphasis on Its Role in Prevention and Treatment of Cancer. Clin. Ther. 2017, 39, 884–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Ganini, D.; Mason, R.P. Absence of an effect of vitamin E on protein and lipid radical formation during lipoperoxidation of LDL by lipoxygenase. Free Radic. Biol. Med. 2014, 76, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, M.; Hernanz, A.; Guayerbas, N.; Victor, V.M.; Arnalich, F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008, 42, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Wu, D.; Meydani, M.; Beharka, A.A.; Serafini, M.; Martin, K.R.; Meydani, S.N. In vitro supplementation with different tocopherol homologues can affect the function of immune cells in old mice. Free Radic. Biol. Med. 2000, 28, 643–651. [Google Scholar] [CrossRef]
- Radhakrishnan, A.K.; Mahalingam, D.; Selvaduray, K.R.; Nesaretnam, K. Supplementation with natural forms of vitamin E augments antigen-specific TH1-type immune response to tetanus toxoid. BioMed Res. Int. 2013, 2013, 782067. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Meydani, S.N.; Barklund, M.P.; Liu, S.; Meydani, M.; Miller, R.A.; Cannon, J.G.; Morrow, F.D.; Rocklin, R.; Blumberg, J.B. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am. J. Clin. Nutr. 1990, 52, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13’-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R. Ascorbate-mediated stimulation of neutrophil motility and lymphocyte transformation by inhibition of the peroxidase/H2O2/halide system in vitro and in vivo. Am. J. Clin. Nutr. 1981, 34, 1906–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boretti, A.; Banik, B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020, 12, 100190. [Google Scholar] [CrossRef]
- Wilson, R.; Willis, J. SunGold Kiwifruit Supplementation of Individuals with Prediabetes Alters Gut Microbiota and Improves Vitamin C Status, Anthropometric and Clinical Markers. Nutrients 2018, 10, 895. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Rahmat, A.; Patimah, I.; Khaza’ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug design, development and therapy. Drug Des. Dev. Ther. 2015, 9, 3405–3412. [Google Scholar] [CrossRef] [Green Version]
- Habibi, N.; Grieger, J.A. A Review of the Potential Interaction of Selenium and Iodine on Placental and Child Health. Nutrients 2020, 12, 2678. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Zago, M.P.; Oteiza, P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radic. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef]
- Beigi Harchegani, A.; Dahan, H.; Tahmasbpour, E.; Bakhtiari Kaboutaraki, H.; Shahriary, A. Effects of zinc deficiency on impaired spermatogenesis and male infertility: The role of oxidative stress, inflammation and apoptosis. Hum. Fertil. 2020, 23, 5–16. [Google Scholar] [CrossRef]
- Kudsk, K.A. Immunonutrition in surgery and critical care. Annu. Rev. Nutr. 2006, 26, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Abunnaja, S.; Cuviello, A.; Sanchez, J.A. Enteral and parenteral nutrition in the perioperative period: State of the art. Nutrients 2013, 5, 608–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizock, B.A. Immunonutrition and critical illness: An update. Nutrition 2010, 26, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef] [Green Version]
- Valdez, Y.; Brown, E.M.; Finlay, B.B. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014, 35, 526–537. [Google Scholar] [CrossRef]
- Littman, D.R. Do the Microbiota Influence Vaccines and Protective Immunity to Pathogens? If So, Is There Potential for Efficacious Microbiota-Based Vaccines? Cold Spring Harbor perspectives in biology. Cold Spring Harb. Perspect. Biol. 2018, 10, a029355. [Google Scholar] [CrossRef] [Green Version]
- Sonnenberg, G.F.; Monticelli, L.A.; Elloso, M.M.; Fouser, L.A.; Artis, D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011, 34, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Longman, R.S.; Diehl, G.E.; Victorio, D.A.; Huh, J.R.; Galan, C.; Miraldi, E.R.; Swaminath, A.; Bonneau, R.; Scherl, E.J.; Littman, D.R. CX₃CR1⁺ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J. Exp. Med. 2014, 211, 1571–1583. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Perez-Chanona, E.; Trinchieri, G. The role of microbiota in cancer therapy. Curr. Opin. Immunol. 2016, 39, 75–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallander, H.O.; Paniagua, M.; Espinoza, F.; Askelöf, P.; Corrales, E.; Ringman, M.; Storsaeter, J. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 2002, 21, 138–145. [Google Scholar] [CrossRef]
- Jiang, V.; Jiang, B.; Tate, J.; Parashar, U.D.; Patel, M.M. Performance of rotavirus vaccines in developed and developing countries. Hum. Vaccines 2010, 6, 532–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassly, N.C.; Jafari, H.; Bahl, S.; Durrani, S.; Wenger, J.; Sutter, R.W.; Aylward, R.B. Mucosal immunity after vaccination with monovalent and trivalent oral poliovirus vaccine in India. J. Infect. Dis. 2009, 200, 794–801. [Google Scholar] [CrossRef]
- Humphrey, J.H. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009, 374, 1032–1035. [Google Scholar] [CrossRef]
- Korpe, P.S.; Petri, W.A., Jr. Environmental enteropathy: Critical implications of a poorly understood condition. Trends Mol. Med. 2012, 18, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Campbell, D.I.; Murch, S.H.; Elia, M.; Sullivan, P.B.; Sanyang, M.S.; Jobarteh, B.; Lunn, P.G. Chronic T cell-mediated enteropathy in rural west African children: Relationship with nutritional status and small bowel function. Pediatric Res. 2003, 54, 306–311. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, U.C.; Ghoshal, U.; Ayyagari, A.; Ranjan, P.; Krishnani, N.; Misra, A.; Aggarwal, R.; Naik, S.; Naik, S.R. Tropical sprue is associated with contamination of small bowel with aerobic bacteria and reversible prolongation of orocecal transit time. J. Gastroenterol. Hepatol. 2003, 18, 540–547. [Google Scholar] [CrossRef]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F. Stool microbiota and vaccine responses of infants. Pediatrics 2014, 134, e362–e372. [Google Scholar] [CrossRef] [Green Version]
- Eloe-Fadrosh, E.A.; McArthur, M.A.; Seekatz, A.M.; Drabek, E.F.; Rasko, D.A.; Sztein, M.B.; Fraser, C.M. Impact of oral typhoid vaccination on the human gut microbiota and correlations with s. Typhi-specific immunological responses. PLoS ONE 2013, 8, e62026. [Google Scholar] [CrossRef]
- Kim, A.H.; Armah, G.; Dennis, F.; Wang, L.; Rodgers, R.; Droit, L.; Baldridge, M.T.; Handley, S.A.; Harris, V.C. Enteric virome negatively affects seroconversion following oral rotavirus vaccination in a longitudinally sampled cohort of Ghanaian infants. Cell Host Microbe 2022, 30, 110–123.e5. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.K.; Bronowski, C. Impact of maternal antibodies and microbiota development on the immunogenicity of oral rotavirus vaccine in African, Indian, and European infants. Nat. Commun. 2021, 12, 7288. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes 2018, 9, 93–101. [Google Scholar] [CrossRef]
- Robertson, R.C.; Church, J.A.; Edens, T.J.; Mutasa, K.; Min Geum, H.; Baharmand, I.; Gill, S.K.; Ntozini, R.; Chasekwa, B.; Carr, L.; et al. The fecal microbiome and rotavirus vaccine immunogenicity in rural Zimbabwean infants. Vaccine 2021, 39, 5391–5400. [Google Scholar] [CrossRef] [PubMed]
- Borey, M.; Blanc, F. Links between fecal microbiota and the response to vaccination against influenza A virus in pigs. Npj Vaccines 2021, 6, 92. [Google Scholar] [CrossRef]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.Y.; et al. Antibiotics-Driven Gut Microbiome Perturbation Alters Immunity to Vaccines in Humans. Cell 2019, 178, 1313–1328.e13. [Google Scholar] [CrossRef]
- Nothaft, H.; Perez-Muñoz, M.E.; Yang, T.; Murugan, A.V.M.; Miller, M.; Kolarich, D.; Plastow, G.S.; Walter, J.; Szymanski, C.M. Improving Chicken Responses to Glycoconjugate Vaccination Against Campylobacter jejuni. Front. Microbiol. 2021, 12, 734526. [Google Scholar] [CrossRef]
- Chac, D.; Bhuiyan, T.R.; Saha, A.; Alam, M.M.; Salma, U.; Jahan, N.; Chowdhury., F.; Khan, A.I.; Ryan, E.T.; LaRocque, R.; et al. Gut Microbiota and Development of Vibrio cholerae-Specific Long-Term Memory B Cells in Adults after Whole-Cell Killed Oral Cholera Vaccine. Infect. Immun. 2021, 89, e0021721. [Google Scholar] [CrossRef]
- Gonçalves, E.; Guillén, Y.; Lama, J.R.; Sanchez, J.; Brander, C.; Paredes, R.; Combadiere, B. Host Transcriptome and Microbiota Signatures Prior to Immunization Profile Vaccine Humoral Responsiveness. Front. Immunol. 2021, 12, 657162. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Praharaj, I.; John, S.M.; Bandyopadhyay, R.; Kang, G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015, 370, 20140144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, I.K.; Iwasaki, A. Control of antiviral immunity by pattern recognition and the microbiome. Immunol. Rev. 2012, 245, 209–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.W.; Myers, L.E.; Ray, L.; Song, S.C.; Nasr, T.R.; Berardinelli, A.J.; Kundu, K.; Murthy, N.; Hansen, J.M.; Neish, A.S. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 2009, 47, 1205–1211. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, C1018–C1030. [Google Scholar] [CrossRef] [Green Version]
- Woo, P.C.; Tsoi, H.W.; Wong, L.P.; Leung, H.C.; Yuen, K.Y. Antibiotics modulate vaccine-induced humoral immune response. Clin. Diagn. Lab. Immunol. 1999, 6, 832–837. [Google Scholar] [CrossRef] [Green Version]
- Tauber, S.C.; Nau, R. Immunomodulatory properties of antibiotics. Curr. Mol. Pharmacol. 2008, 1, 68–79. [Google Scholar]
- Lamousé-Smith, E.S.; Tzeng, A.; Starnbach, M.N. The intestinal flora is required to support antibody responses to systemic immunization in infant and germ free mice. PLoS ONE 2011, 6, e27662. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, R.; Chassaing, B.; Zhang, B.; Gewirtz, A.T. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J. Infect. Dis. 2014, 210, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, S.; Klein, S.L. Host Factors Impact Vaccine Efficacy: Implications for Seasonal and Universal Influenza Vaccine Programs. J. Virol. 2019, 93, e00797-19. [Google Scholar] [CrossRef] [PubMed]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, L.E.; Fiorino, A.M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Puyenbroeck, K.; Hens, N.; Coenen, S.; Michiels, B.; Beunckens, C.; Molenberghs, G.; Van Royen, P.; Verhoeven, V. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: A randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 2012, 95, 1165–1171. [Google Scholar] [CrossRef]
- Enani, S.; Przemska-Kosicka, A.; Childs, C.E.; Maidens, C.; Dong, H.; Conterno, L.; Tuohi, K.; Todd, S.; Gosney, M.; Yaqoob, P. Impact of ageing and a synbiotic on the immune response to seasonal influenza vaccination; a randomised controlled trial. Clin. Nutr. 2018, 37, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Langkamp-Henken, B.; Bender, B.S.; Gardner, E.M.; Herrlinger-Garcia, K.A.; Kelley, M.J.; Murasko, D.M.; Shaller, J.P.; Stechmiller, J.K.; Thomas, D.J.; Wood, S.M. Nutritional formula enhanced immune function and reduced days of symptoms of upper respiratory tract infection in seniors. J. Am. Geriatr. Soc. 2004, 52, 3–12. [Google Scholar] [CrossRef]
- Bunout, D.; Hirsch, S.; de la Maza, M.P.; Muñoz, C.; Haschke, F.; Steenhout, P.; Klassen, P.; Barrera, G.; Gattas, V.; Petermann, M. Effects of prebiotics on the immune response to vaccination in the elderly. JPEN J. Parenter. Enter. Nutr. 2002, 26, 372–376. [Google Scholar] [CrossRef]
- Lomax, A.R.; Cheung, L.V.; Noakes, P.S.; Miles, E.A.; Calder, P.C. Inulin-Type β2-1 Fructans have Some Effect on the Antibody Response to Seasonal Influenza Vaccination in Healthy Middle-Aged Humans. Front. Immunol. 2015, 6, 490. [Google Scholar] [CrossRef] [Green Version]
- Boge, T.; Rémigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef]
- Isolauri, E.; Joensuu, J.; Suomalainen, H.; Luomala, M.; Vesikari, T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 1995, 13, 310–312. [Google Scholar] [CrossRef]
- Wang, H.; Gao, K.; Wen, K.; Allen, I.C.; Li, G.; Zhang, W.; Kocher, J.; Yang, X.; Giri-Rachman, E.; Li, G.; et al. Lactobacillus rhamnosus GG modulates innate signaling pathway and cytokine responses to rotavirus vaccine in intestinal mononuclear cells of gnotobiotic pigs transplanted with human gut microbiota. BMC Microbiol. 2016, 16, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, K.; Liu, F.; Li, G.; Bai, M.; Kocher, J.; Yang, X.; Wang, H.; Clark-Deener, S.; Yuan, L. Lactobacillus rhamnosus GG Dosage Affects the Adjuvanticity and Protection Against Rotavirus Diarrhea in Gnotobiotic Pigs. J. Pediatric Gastroenterol. Nutr. 2015, 60, 834–843. [Google Scholar] [CrossRef]
- Alqazlan, N.; Astill, J.; Taha-Abdelaziz, K.; Nagy, É.; Bridle, B.; Sharif, S. Probiotic Lactobacilli Enhance Immunogenicity of an Inactivated H9N2 Influenza Virus Vaccine in Chickens. Viral Immunol. 2021, 34, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lesourd, B. Protein undernutrition as the major cause of decreased immune function in the elderly: Clinical and functional implications. Nutr. Rev. 1995, 53, S86–S91, discussion S2–S4. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Martin, P.; Jadoul, M.; Messa, P. Meta-analysis: The impact of nutritional status on the immune response to hepatitis B virus vaccine in chronic kidney disease. Dig. Dis. Sci. 2012, 57, 1366–1372. [Google Scholar] [CrossRef]
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2011, 60, 1–64. [Google Scholar]
- Yaqoob, P. Ageing alters the impact of nutrition on immune function. Proc. Nutr. Soc. 2017, 76, 347–351. [Google Scholar] [CrossRef]
- Untersmayr, E.; Kallay, E. Insights in Immuno-Nutrition: Vitamin D as a Potent Immunomodulator. Nutrients 2020, 12, 3554. [Google Scholar] [CrossRef]
- Stachowska, E.; Folwarski, M.; Jamioł-Milc, D.; Maciejewska, D.; Skonieczna-Żydecka, K. Nutritional Support in Coronavirus 2019 Disease. Medicina 2020, 56, 289. [Google Scholar] [CrossRef]
| Author | Setting | Number of Subjects | Type of Vaccine | Outcome Measure | Reference |
|---|---|---|---|---|---|
| Huda et al., 2019 | Infants | 291 | Bacillus Calmette-Guérin, oral polio virus, tetanus toxoid, hepatitis B virus | Bifidobacterium richness is associated with the efficacy of vaccines. | [110] |
| Eloe-Fadrosh et al., 2013 | Infants | 17 | Oral live-attenuated typhoid vaccine Ty21a | Cell-mediated immune response was associated with more diverse and complex gut microbiota populations. | [111] |
| Kim et al., 2022 | Infants | 122 | Oral Rotavirus Vaccine | Association of Streptococcus and Enterobacteriaceae with seroconversion | [112] |
| Parker et al., 2021 | Infants | 486 | Oral Rotavirus Vaccine | Negative correlation between microbiota diversity and vaccine immunogenicity | [113] |
| Harris et al., 2018 | Infants | 30 | Oral Rotavirus Vaccine | Association between vaccine response and higher abundance of Gammaproteobacteria (Serratia and E. coli) | [114] |
| Robertson et al., 2021 | Infants | 158 | Oral Rotavirus Vaccine | No association observed with the microbiota composition | [115] |
| Borey et al., 2021 | Pigs | 98 | Influenza A virus | Positive immune response is associated with Prevotella and Muribaculaceae, while the negative response is linked with Helicobacter and Bacteroides | [116] |
| Hagan et al., 2019 | Adults | 22 | Seasonal influenza vaccination | Association between impairment of immune response after antibiotics treatment | [117] |
| Nothaft et al., 2021 | Chicken | 60 | Glycoconjugate vaccination against Campylobacter jejuni | Involvement of Clostridium spp., Ruminococcaceae and Lachnospiraceae in the responders | [118] |
| Chac et al., 2021 | Adults | 69 | Oral cholera vaccine | Positive association with an abundance of Clostridiales. Enterobacterales are dominant in poor response | [119] |
| Goncalves et al., 2021 | Adults | 10 | MVA-HIV clade B vaccine | Abundance of Eubacterium in stool and Prevotella in the skin was associated with a positive immune response | [120] |
| Reference | Setting | Number of Subjects | Intervention | Duration | Type of Vaccine | Outcome Measure | Reference |
|---|---|---|---|---|---|---|---|
| Van Puyenboreck et al. | Human Clinical trial | 737 | L. casei | 3 weeks | H1N1: Solomon Islands /3/2006 IVR-145 H3N2: Wisconsin/67/2005 B: Malaysia/2506/2004 | Univariate and multivariate modelling showed no effect of the probiotic on clinical outcome parameters. | [136] |
| Rizzardini et al. | Human Clinical trial | 211 | BB-12® and L. casei | 6 weeks | H1N1: Brisbane/59/2007 H3N2: Uruguay/716/2007 B: Florida/4/2006 | Improved immune function by augmenting systemic and mucosal immune responses to challenge. | [134] |
| Enani et al. | Human Clinical trial | 112 | B. longum with GOS | 8 weeks | H1N1: California/7/2009 H3N2: Perth/16/2009 B: Brisbane/60/2008 | Improved IgA memory, IgG memory and total IgG in young subjects, but in elderly no significant changes were evaluated. | [137] |
| Langkamp-Henken et al. | Human Clinical trial | 157 | Antioxidants, B vitamins, selenium, zinc, FOS | 10 weeks | H1N1: Caledonia/20/99 H3N2: Panama/2007/99 B: Hong Kong/1434/2002 | Lymphocyte proliferation to influenza vaccine components was greater in the treated than the control group. | [138] |
| Bunout et al. | Human Clinical trial | 66 | FOS | 28 weeks | PPSV 23 H1N1: Caledonia A: Moscow (subtype AC3N2), Sydney B: Belgium (code 184-93) | Antibodies against influenza A did not increase. | [139] |
| Lomax et al. | Human Clinical trial | 49 | 50:50 mixture of long-chain inulin and FOS | 8 weeks | H1N1: Brisbane/59/2007 H3N2: Brisbane/10/2007 B: Florida/4/2006 | Supplementation can enhance some aspects of the immune response in healthy middle-aged adults, but that is not a global effect. | [140] |
| Boge et al. | Human Clinical trial | 222 | L. casei | 13 weeks | H1N1: New Caledonia/20/99 H3N2: California/7/2004 B:Shanghai/361/2002a B:Jiangsu/10/2003a | The influenza-specific antibodies in the treated group were increased after vaccination rather than the placebo group. | [141] |
| Isolauri et al. | Human Clinical trial | 28 | L.casei | 1 week | D x RRV rhesus-human reassortant live oral rotavirus vaccine | Enhanced IgA seronversion for the treated group. | [142] |
| Wang et al. | Animal model (pigs) | 34 | Lactobacillus spp. | 3 weeks | Att-HRV attenuated human rotavirus | Enhanced innate immune response after vaccination in the treated group. | [143] |
| Wen et al. | Animal model (pigs) | 23 | Lactobacillus rhamnosus | 2 weeks | Att-HRV attenuated human rotavirus. | Enhanced immune response through IgA production. | [144] |
| Alqazlan et al. | Animal model (chickens) | 84 | Lactobacillus spp. | 5 weeks | (WIV) vaccine of inactivated virus H9N2 | Increase of efficacy of vaccinations in chickens using Lactobacilli administration. | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Renzo, L.; Franza, L.; Monsignore, D.; Esposito, E.; Rio, P.; Gasbarrini, A.; Gambassi, G.; Cianci, R.; De Lorenzo, A. Vaccines, Microbiota and Immunonutrition: Food for Thought. Vaccines 2022, 10, 294. https://doi.org/10.3390/vaccines10020294
Di Renzo L, Franza L, Monsignore D, Esposito E, Rio P, Gasbarrini A, Gambassi G, Cianci R, De Lorenzo A. Vaccines, Microbiota and Immunonutrition: Food for Thought. Vaccines. 2022; 10(2):294. https://doi.org/10.3390/vaccines10020294
Chicago/Turabian StyleDi Renzo, Laura, Laura Franza, Diego Monsignore, Ernesto Esposito, Pierluigi Rio, Antonio Gasbarrini, Giovanni Gambassi, Rossella Cianci, and Antonino De Lorenzo. 2022. "Vaccines, Microbiota and Immunonutrition: Food for Thought" Vaccines 10, no. 2: 294. https://doi.org/10.3390/vaccines10020294
APA StyleDi Renzo, L., Franza, L., Monsignore, D., Esposito, E., Rio, P., Gasbarrini, A., Gambassi, G., Cianci, R., & De Lorenzo, A. (2022). Vaccines, Microbiota and Immunonutrition: Food for Thought. Vaccines, 10(2), 294. https://doi.org/10.3390/vaccines10020294











