Systemic and Lower Respiratory Tract Immunity to SARS-CoV-2 Omicron and Variants in Pediatric Severe COVID-19 and Mis-C
Abstract
:1. Introduction
2. Methods
2.1. Pediatric Samples
2.2. Quantification of Total IgM, IgG, and IgA in Pediatric Samples
2.3. SARS-CoV-2 Neutralization Assay
2.4. Quantification and Statistical Analysis
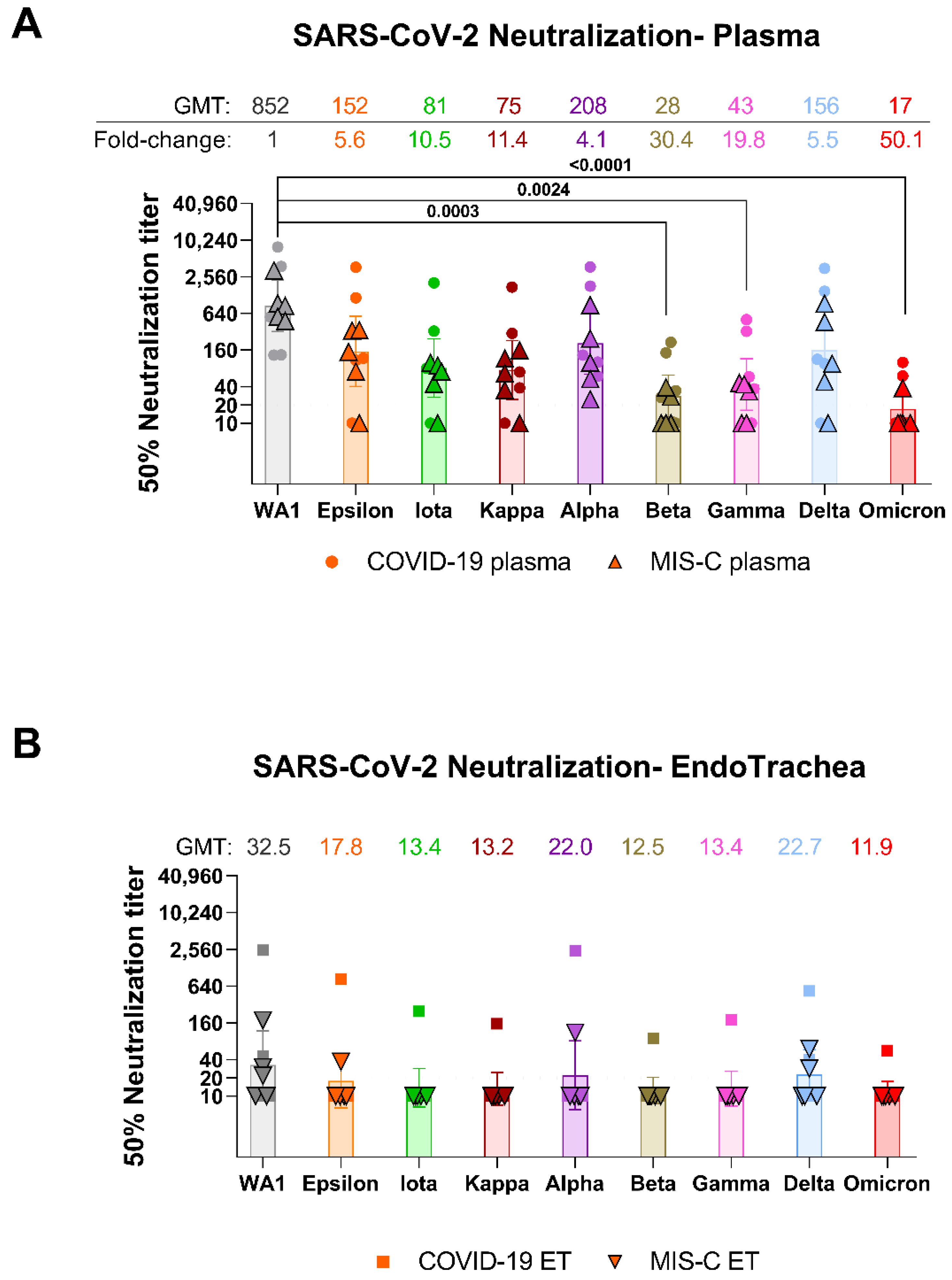
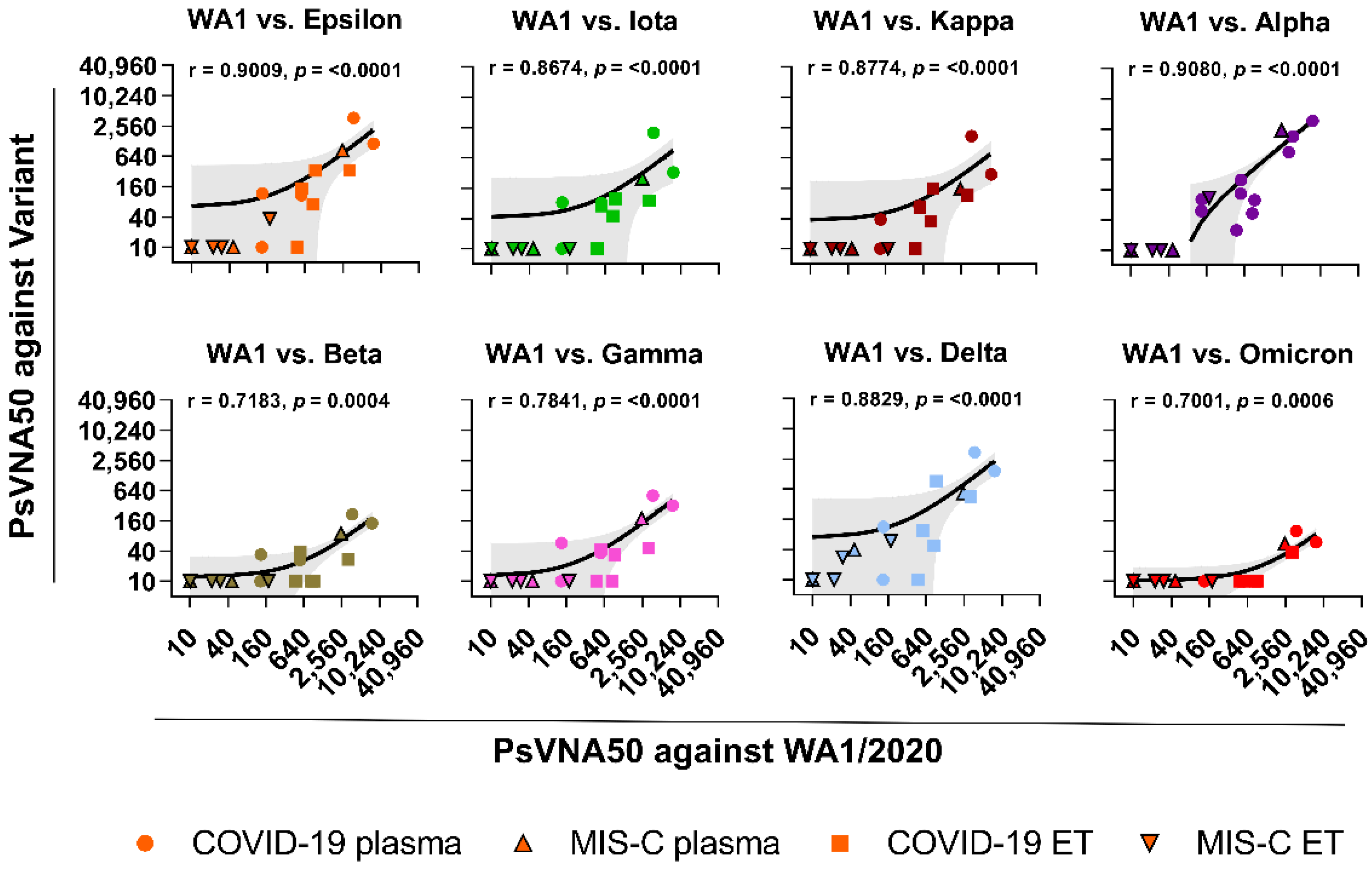
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
Acronyms
| CDC | Centers for Disease Control and Prevention |
| COVID-19 | coronavirus disease 2019 (COVID-19) |
| ET | endotracheal aspirates |
| IQR | interquartile range |
| MIS-C | multisystem inflammatory syndrome in children |
| PCR | polymerase chain reaction |
| PsVNA | pseudovirion neutralization assay |
| VOCs | variants of concern |
| VOIs | variants of interest |
References
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 4, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Wang, C.; Zohar, T.; Fischinger, S.; Atyeo, C.; Burke, J.S.; Kang, J.; Edlow, A.G.; Fasano, A.; Baden, L.R.; et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021, 3, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Tang, J.; Grubbs, G.; Lee, Y.; Pourhashemi, S.; Hussaini, L.; Lapp, S.A.; Jerris, R.C.; Singh, V.; Chahroudi, A.; et al. SARS-CoV-2 immune repertoire in MIS-C and pediatric COVID-19. Nat. Immunol. 2021, 22, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Where did Omicron come from? Three key theories. Nature 2022, 7895, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Sigal, A. Milder disease with Omicron: Is it the virus or the pre-existing immunity? Nat. Rev. Immunol. 2022, 22, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lee, Y.; Ravichandran, S.; Grubbs, G.; Huang, C.; Stauft, C.B.; Wang, T.; Golding, B.; Golding, H.; Khurana, S. Epitope diversity of SARS-CoV-2 hyperimmune intravenous human immunoglobulins and neutralization of variants of concern. IScience 2021, 24, 103006. [Google Scholar] [CrossRef]
- Ravichandran, S.; Coyle, E.M.; Klenow, L.; Tang, J.; Grubbs, G.; Liu, S.; Wang, T.; Golding, H.; Khurana, S. Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci. Transl. Med. 2020, 12, eabc3539. [Google Scholar] [CrossRef]
- Neerukonda, S.N.; Vassell, R.; Herrup, R.; Liu, S.; Wang, T.; Takeda, K.; Yang, Y.; Lin, T.L.; Wang, W.; Weiss, C.D. Establishment of a well-characterized SARS-CoV-2 lentiviral pseudovirus neutralization assay using 293T cells with stable expression of ACE2 and TMPRSS2. PLoS ONE 2021, 3, e0248348. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Grubbs, G.; Tang, J.; Lee, Y.; Huang, C.; Golding, H.; Khurana, S. Systemic and mucosal immune profiling in asymptomatic and symptomatic SARS-CoV-2-infected individuals reveal unlinked immune signatures. Sci. Adv. 2021, 7, eabi6533. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A.F.; Cherian, T.; Chow, J.; Shahid-Salles, S.A.; Laxminarayan, R.; John, T.J. Acute Respiratory Infections in Children. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Bittmann, S. Role of Omicron variant of SARS-CoV-2 in children in Germany. World J. Pediatr. 2022, 18, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, P. SARS-CoV-2 variant Omicron (B.1.1.529) is in a rising trend of mutations increasing the positive electric charge in crucial regions of the spike protein S. Acta Biochim. Pol. 2021, 68. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claer, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Di Giuda, D.; Sigfrid, L.; Pizzuto, D.A.; Di Sante, G.; De Rose, C.; Lazzareschi, I.; Sali, M.; Baldi, F.; Chieffo, D.P.R.; et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc. Health 2021, 5, 677–680. [Google Scholar] [CrossRef]
| ANTIBODY ISOTYPES | ACUTE COVID-19 (n = 5) | MIS-C (n = 5) | ||
|---|---|---|---|---|
| PLASMA | TRACHEA | PLASMA | TRACHEA | |
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | |
| IgM | 2.06 ± 0.58 | 0.051 ± 0.036 | 1.72 ± 0.34 | 0.039 ± 0.018 |
| IgG | 12.49 ± 3.27 | 0.231 ± 0.104 | 13.18 ± 2.72 | 0.078 ± 0.042 |
| IgA | 2.51 ± 1.04 | 0.047 ± 0.029 | 2.41 ± 0.69 | 0.032 ± 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Randolph, A.G.; Novak, T.; Walker, T.C.; Loftis, L.L.; Zinter, M.S.; Irby, K.; Khurana, S. Systemic and Lower Respiratory Tract Immunity to SARS-CoV-2 Omicron and Variants in Pediatric Severe COVID-19 and Mis-C. Vaccines 2022, 10, 270. https://doi.org/10.3390/vaccines10020270
Tang J, Randolph AG, Novak T, Walker TC, Loftis LL, Zinter MS, Irby K, Khurana S. Systemic and Lower Respiratory Tract Immunity to SARS-CoV-2 Omicron and Variants in Pediatric Severe COVID-19 and Mis-C. Vaccines. 2022; 10(2):270. https://doi.org/10.3390/vaccines10020270
Chicago/Turabian StyleTang, Juanjie, Adrienne G. Randolph, Tanya Novak, Tracie C. Walker, Laura L. Loftis, Matt S. Zinter, Katherine Irby, and Surender Khurana. 2022. "Systemic and Lower Respiratory Tract Immunity to SARS-CoV-2 Omicron and Variants in Pediatric Severe COVID-19 and Mis-C" Vaccines 10, no. 2: 270. https://doi.org/10.3390/vaccines10020270
APA StyleTang, J., Randolph, A. G., Novak, T., Walker, T. C., Loftis, L. L., Zinter, M. S., Irby, K., & Khurana, S. (2022). Systemic and Lower Respiratory Tract Immunity to SARS-CoV-2 Omicron and Variants in Pediatric Severe COVID-19 and Mis-C. Vaccines, 10(2), 270. https://doi.org/10.3390/vaccines10020270






