Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized
Abstract
1. Introduction
2. Materials and Methods
2.1. Objectives
- 1.
- What type of thermostable vaccines have been developed for veterinary use?
- 2.
- What are the characteristics of these thermostable vaccines?
- 3.
- How immunogenic and effective are these thermostable vaccines?
2.2. Eligibility Criteria
2.3. Information Sources
- •
- Authors may not specify that DNA vaccines do not need the cold-chain, a thermostability is an intrinsic characteristic of these vaccines. Thus, the computerized search would not be able to retrieve the manuscripts if it only used general keywords;
- •
- The use of a unique complex search strategy, combining multiple different terms, would not be an efficient way to identify relevant articles.
2.4. Data Collection Process and Data
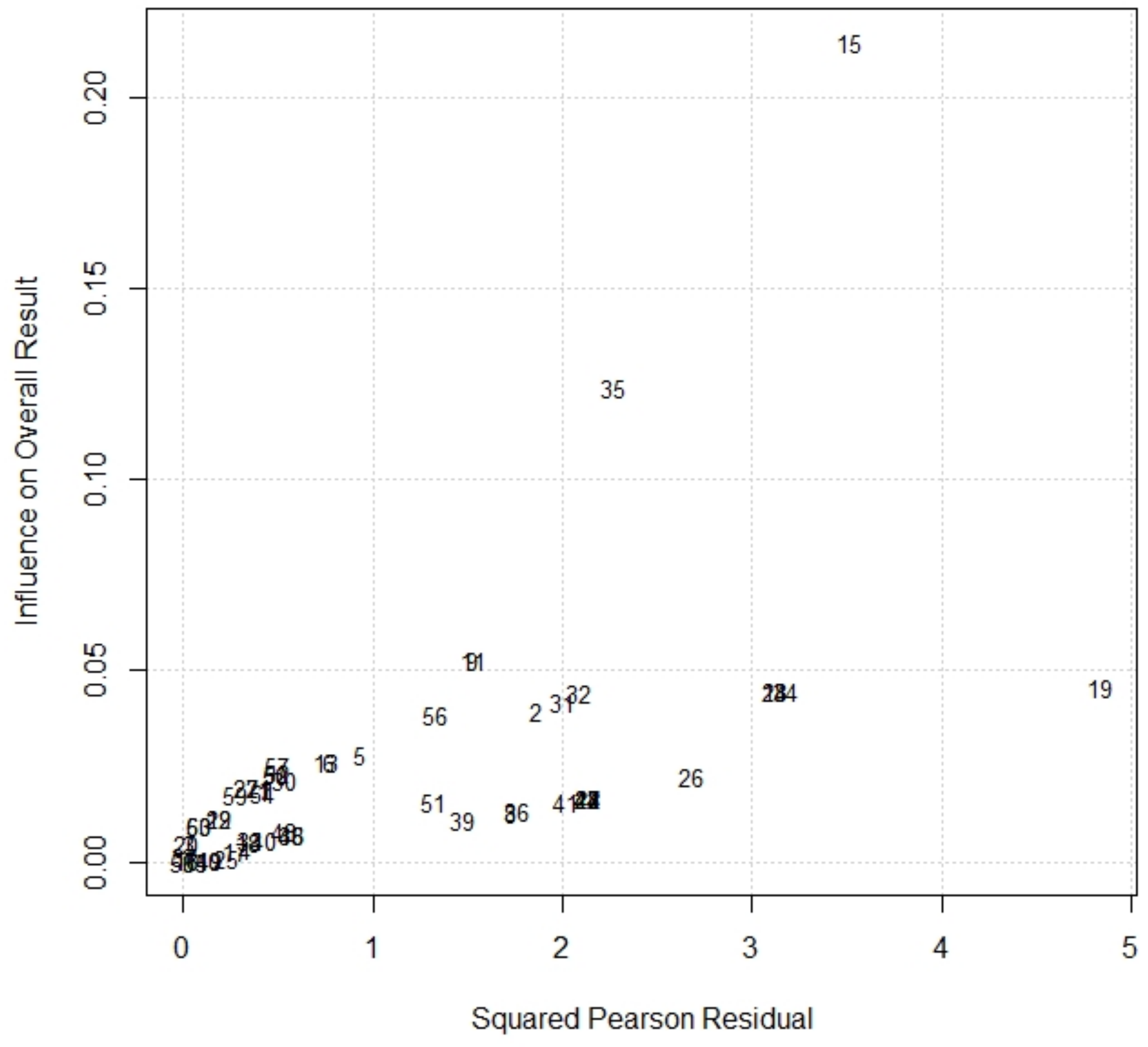
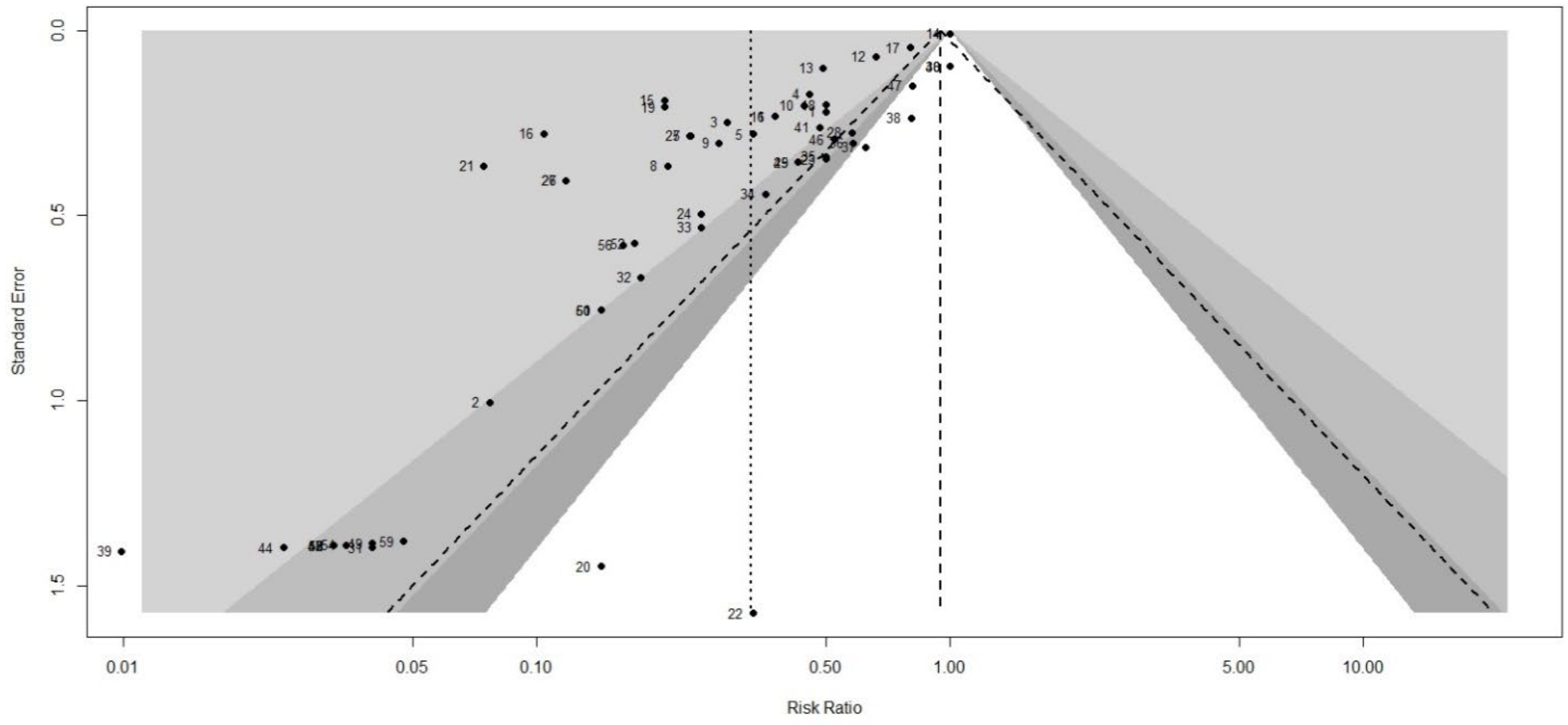
2.5. Risk of Bias (Quality Assessment)
2.6. Method of Analysis
3. Results
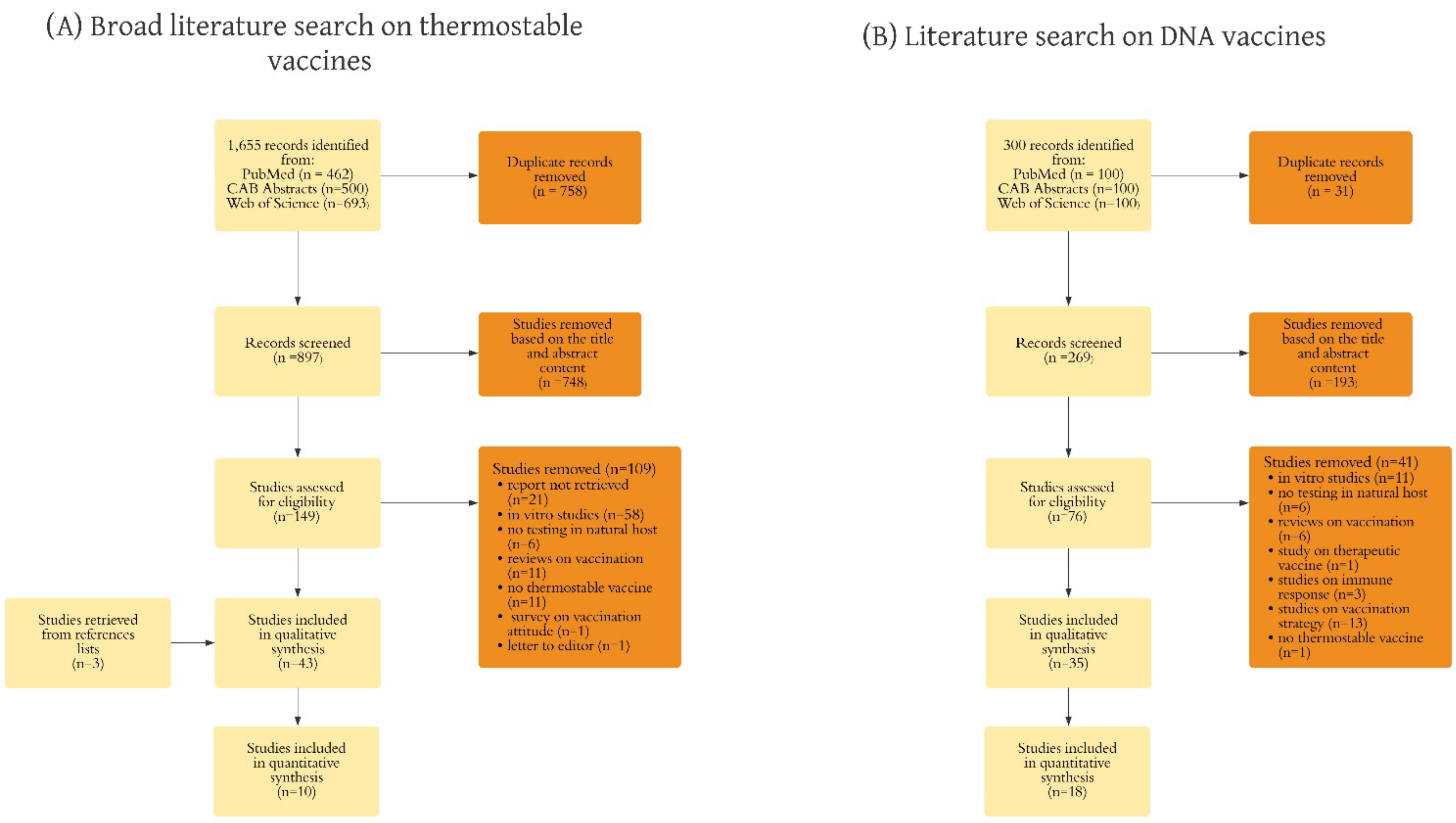
3.1. Study Selection
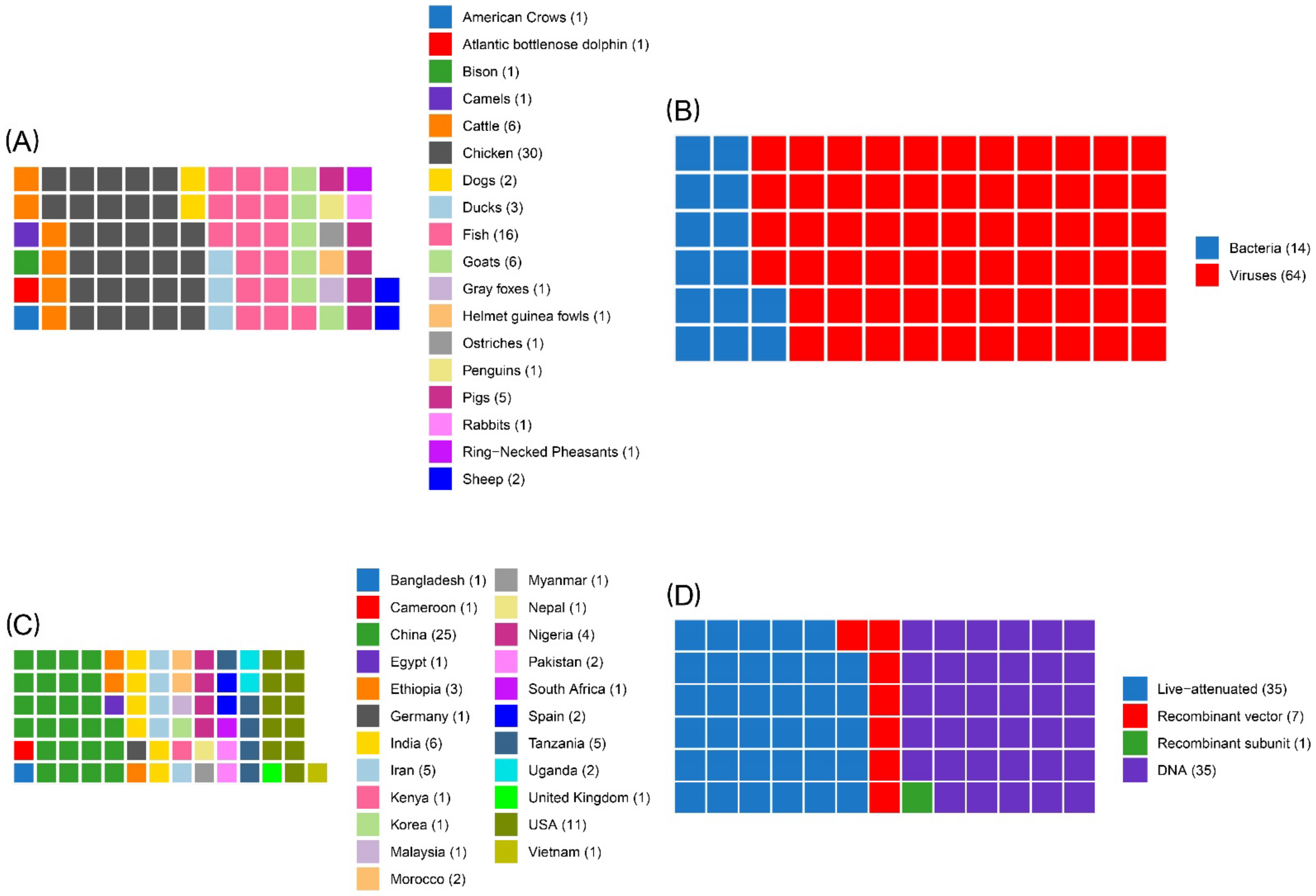
3.2. Study Characteristics
- •
- Two studies performing both clinical and field trials (one using vaccinated and control groups, and one with all animals vaccinated);
- •
- Thirteen studies performing field trials (eight using vaccinated and control groups, and five with all animals vaccinated);
- •
- Sixty-three studies performing clinical trials (60 using vaccinated and control groups, and three with all animals vaccinated).
- •
- •
- •
- •
- Study on a vaccine against Rinderpest virus (n = 1), describing a recombinant heat stable vaccinia virus [80].
3.3. Risk of Bias (Quality) Assessment
3.4. Synthesis of Results
4. Discussion
4.1. Summary of Evidence
- •
- Three against viral diseases;
- ◦
- Two for fish (one against infectious hematopoietic necrosis virus (IHNV), and one against salmon alphavirus subtype 3);
- ◦
- One for horses against WNV, but used also in several avian species;
- •
- One to treat cancer melanoma in dogs;
- •
- One growth hormone-releasing hormone (GHRH) gene therapy for swine.
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name | Causative Agent | Disease Group |
|---|---|---|
| Anthrax | Bacillus anthracis | Multiple species diseases |
| Aujeszky’s disease | Suid herpesvirus 1 (SuHV-1) | Multiple species diseases |
| Brucellosis | Brucella abortus/B. melitensis | Multiple species diseases |
| Bluetongue | Bluetongue virus (BTV) | Multiple species diseases |
| Echinococcosis/hydatidosis | Echinococcus granulosus/E. multilocularis | Multiple species diseases |
| Epizootic haemorrhagic disease | Epizootic haemorrhagic disease virus (EHDV) | Multiple species diseases |
| Foot and mouth disease | Foot and mouth disease virus (FMDV) | Multiple species diseases |
| Heartwater (cowdriosis) | Ehrlichia ruminantium (formerly Cowdria ruminantium) | Multiple species diseases |
| Tuberculosis | Mycobacterium tuberculosis complex | Multiple species diseases |
| Japanese encephalitis | Japanese encephalitis virus (JEV) | Multiple species diseases |
| Paratuberculosis | Mycobacterium avium subsp. paratuberculosis (MAP) | Multiple species diseases |
| Q fever (or Coxiellosis) | Coxiella burnetii | Multiple species diseases |
| Rabies | Rabies virus (RABV) and other lyssaviruses | Multiple species diseases |
| Rift Valley fever | Rift Valley fever virus (RVF) | Multiple species diseases |
| Rinderpest | Rinderpest virus (RPV) | Multiple species diseases |
| Tularemia | Francisella tularensis | Multiple species diseases |
| West Nile Fever | West Nile virus (WNV) | Multiple species diseases |
| Bovine anaplasmosis | Anaplasma marginale/A. centrale | Bovinae |
| Bovine babesiosis | Babesia bovis/B. bigemina/B. divergens | Bovinae |
| Bovine genital campylobacteriosis (bovine venereal campylobacteriosis) | Campylobacter fetus subsp. Venerealis | Bovinae |
| Bovine viral diarrhoea | Bovine viral diarrhoea virus (BVDV) | Bovinae |
| Contagious bovine pleuropneumonia | Mycoplasma mycoides subsp. Mycoides | Bovinae |
| Haemorrhagic septicaemia | Pasteurella multocida | Bovinae |
| Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis (IPV) | Bovine herpesvirus 1 (BoHV-1) | Bovinae |
| Lumpy skin disease virus | Lumpy skin disease virus (LSDV) | Bovinae |
| Theileriosis | Theileria annulata and T. parva | Bovinae |
| Trichomonosis | Tritrichomonas foetus | Bovinae |
| Enzootic abortion of ewes (ovine chlamydiosis) | Chlamydia abortus | Caprinae |
| Contagious agalactia | Mycoplasma agalactiae (Ma) | Caprinae |
| Contagious caprine pleuropneumonia | Mycoplasma capricolum subsp. capripneumoniae (Mccp) | Caprinae |
| Nairobi sheep disease | Nairobi sheep disease virus (NSDV) | Caprinae |
| Peste des petits ruminants virus | Small Ruminant Morbillivirus (SRMV) | Caprinae |
| Salmonellosis | Salmonella abortusovis | Caprinae |
| Sheep pox and goat pox | Sheeppox virus (SPPV) and goatpox virus (GTPV) | Caprinae |
| African horse sickness (AHS) | African horse sickness virus (AHSV) | Equidae |
| Equine rhinopneumonitis | Equid herpesvirus-1 | Equidae |
| Equine viral arteritis (EVA) | Equine arteritis virus (EAV) | Equidae |
| Equine encephalomyelitis (Eastern, Western, Venezuelan) (EEE, WEE and VEE) | Equine encephalomyelitis viruses (Eastern, Western, Venezuelan) (EEEV, WEEV and VEEV) | Equidae |
| Equine influenza | Equine influenza viruses (H7N7, formerly equi-1, and H3N8, formerly equi2) | Equidae |
| Classical swine fever virus | Classical swine fever virus (CSFV) | Suidae |
| Nipah virus encephalitis | Nipah virus (NiV) | Suidae |
| Porcine reproductive and respiratory syndrome (PRRS) | Porcine reproductive and respiratory syndrome virus (PRRSV) | Suidae |
| Porcine cysticercosis | Taenia solium | Suidae |
| Transmissible gastroenteritis (TGE) | Transmissible gastroenteritis virus (TGEV) | Suidae |
| Camelpox | Camelpox virus | Other diseases |
| Leishmaniosis | Leishmania species (approximately 20 recognised) | Other diseases |
| Infectious salmon anaemia virus (Inf. with) (HPR-deleted or HPR0 genotypes) | Infectious salmon anaemia virus (ISAV) | Diseases of fish |
| Koi herpesvirus (Inf. with) | Koi herpesvirus (KHV) | Diseases of fish |
| Red sea bream iridovirus (Inf. with) | Red sea bream iridovirus RSIVD | Diseases of fish |
| Salmonid alphavirus (Inf. with) | Salmonid alphavirus (SAV) | Diseases of fish |
| Avian infectious bronchitis | Gammacoronavirus infectious bronchitis virus (IBV) | Aves |
| Avian infectious laryngotracheitis | Gallid alphaherpesvirus 1 | Aves |
| Avian influenza | Low and High pathogenicity avian influenza viruses | Aves |
| Avian mycoplasmosis (M.synoviae) | Mycoplasma synoviae | Aves |
| Avian mycoplasmosis (Mycoplasma gallisepticum) | Mycoplasma gallisepticum | Aves |
| Duck virus hepatitis | Duck hepatitis A virus (DHAV) | Aves |
| Fowl typhoid | Salmonella Gallinarum | Aves |
| Infectious bursal disease (Gumboro disease) | Infectious bursal disease virus (IBDV) | Aves |
| Newcastle disease | Newcastle disease virus (NDV) | Aves |
| Pullorum disease | Salmonella Pullorum | Aves |
| Turkey rhinotracheitis | Avian metapneumovirus (Ampv) | Aves |
| Myxomatosis | Myxoma virus (MYXV) | Leporidae |
| Rabbit haemorrhagic disease | Rabbit haemorrhagic disease virus (RHDV) | Leporidae |
| Disease | Conjugate Vaccine | DNA Vaccine | Inactivated Vaccine | Live Attenuated Vaccine | Recombinant Vector Vaccine | Subunit Vaccine |
|---|---|---|---|---|---|---|
| African horse sickness | × | |||||
| Anthrax | × | × | ||||
| Aujeszky’s disease | × | × | × | × | ||
| Avian infectious bronchitis | × | × | × | × | ||
| Avian infectious laryngotracheitis | × | × | × | × | ||
| Avian mycoplasmosis (M. gallisepticum) | × | × | ||||
| Bluetongue | × | × | ||||
| Bovine anaplasmosis | × | × | ||||
| Bovine babesiosis | × | × | ||||
| Bovine brucellosis | × | × | ||||
| Bovine viral diarrhoea | × | × | ||||
| Brucellosis (Brucella abortus) | × | |||||
| Brucellosis (Brucella melitensis) | × | × | ||||
| Camelpox | × | × | ||||
| Caprine and ovine brucellosis (excluding B. ovis) | × | × | ||||
| Classical swine fever | × | × | × | × | ||
| Contagious agalactia | × | × | ||||
| Contagious bovine pleuropneumonia | × | |||||
| Contagious caprine pleuropneumonia | × | × | ||||
| Duck virus enteritis | × | × | ||||
| Duck virus hepatitis | × | × | ||||
| Enterovirus encephalomyelitis | × | × | ||||
| Enzootic abortion of ewes (ovine chlamydiosis) | × | |||||
| Equid herpesvirus-X (EHV-X) (Infection with) | × | |||||
| Equine encephalomyelitis (Eastern) | × | × | ||||
| Equine encephalomyelitis (Western) | × | |||||
| Equina influenza | × | × | ||||
| Equine rhinopneumonitis | × | |||||
| Equine viral arteritis | × | |||||
| Foot and mouth disease | × | × | × | |||
| Fowl cholera | × | × | ||||
| Fowl typhoid | × | × | ||||
| Haemorrhagic septicaemia | × | × | ||||
| Highly pathogenic avian influenza | × | × | × | |||
| Highly pathogenic influenza A viruses (infection with) (non-poultry incluiding wild birds) | × | |||||
| Infection with salmonid alphavirus | × | |||||
| Infectious bovine rhinotracheitis/infectious pustular vulvovaginitis | × | × | × | × | ||
| Infectious bursal disease (Gumboro disease) | × | × | × | × | ||
| Infectious haematopoietic necrosis | × | |||||
| Infectious pancreatic necrosis | × | × | ||||
| Infectious salmon anaemia | × | |||||
| Japanese encephalitis | × | |||||
| Low pathogenic avian influenza (poultry) | × | |||||
| Lumpy skin disease | × | × | ||||
| Marek’s disease | × | × | ||||
| Myxomatosis | × | |||||
| Newcastle disease | × | × | × | |||
| Ovine epididymitis (Brucella ovis) | × | |||||
| Peste des petits ruminants | × | × | ||||
| Porcine reproductive and respiratory syndrome | × | × | × | × | ||
| Pullorum disease | × | |||||
| Rabbit haemorrhagic disease | × | × | ||||
| Rabies | × | × | × | |||
| Red sea bream iridoviral disease | × | |||||
| Rift Valley fever | × | × | ||||
| Rinderpest | × | × | ||||
| Salmonellosis (S. abortusovis) | × | × | ||||
| Sheep pox and goat pox | × | × | ||||
| Theileriosis | × | |||||
| Transmissible gastroenteritis | × | × | ||||
| Trichomonosis | × | |||||
| Turkey rhinotracheitis | × | × | ||||
| Venezuelan equine encephalomyelitis | × | × | ||||
| Vesicular stomatitis | × | |||||
| West Nile Fever | × |

References
- People’s Vaccine Alliance Open Letter: Former Heads of State and Nobel Laureates Call on President Biden To Waive Intellectual Property Rules for COVID Vaccines. Available online: https://en.emergency.it/press-releases/former-heads-of-state-and-nobel-laureates-call-on-president-biden-to-waive-intellectual-property-rules-for-covid-vaccines/ (accessed on 25 November 2021).
- Capua, I.; Giaquinto, C. The unsung virtue of thermostability. Lancet 2021, 397, 1346. [Google Scholar] [CrossRef]
- Global Health Summit The Rome Declarations. May 2021. Available online: https://global-health-summit.europa.eu/rome-declaration_en (accessed on 25 November 2021).
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef]
- Holm, M.R.; Poland, G.A. Critical aspects of packaging, storage, preparation, and administration of mRNA and adenovirus-vectored COVID-19 vaccines for optimal efficacy. Vaccine 2020, 39, 457. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef]
- CDC Janssen COVID-19 Vaccine (Johnson & Johnson). Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/janssen/downloads/janssen-storage-handling-summary.pdf (accessed on 25 November 2021).
- Chen, D.; Kristensen, D. Opportunities and challenges of developing thermostable vaccines. Expert Rev. Vaccines 2009, 8, 547–557. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Vaccine Action Plan; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Meeusen, E.N.T.; Walker, J.; Peters, A.; Pastoret, P.P.; Jungersen, G. Current status of veterinary vaccines. Clin. Microbiol. Rev. 2007, 20, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; de Clercq, K.; Greiner, M.; Dekker, A.; Brocchi, E.; Bergmann, I.; Sammin, D.J.; Gubbins, S.; Parida, S. Application of non-structural protein antibody tests in substantiating freedom from Foot-and-mouth disease virus infection after emergency vaccination of cattle. Vaccine 2006, 24, 6503–6512. [Google Scholar] [CrossRef]
- OIE Manual of Diagnostic Tests for Aquatic Animals. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-manual-online-access/ (accessed on 25 November 2021).
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; OIE: Paris, France, 2021. [Google Scholar]
- Brandau, D.T.; Jones, L.S.; Wiethoff, C.M.; Rexroad, J.; Middaugh, C.R. Thermal stability of vaccines. J. Pharm. Sci. 2003, 92, 218–231. [Google Scholar] [CrossRef] [PubMed]
- OIE. Chapter 4.18 Vaccination. In Terrestrial Animal Health Code; OIE: Paris, France, 2021; pp. 1–8. [Google Scholar]
- Dumpa, N.; Goel, K.; Guo, Y.; McFall, H.; Pillai, A.R.; Shukla, A.; Repka, M.A.; Murthy, S.N. Stability of Vaccines. Am. Assoc. Pharm. Sci. 2019, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.; Chen, D.; Cummings, R. Vaccine stabilization: Research, commercialization, and potential impact. Vaccine 2011, 29, 7122–7124. [Google Scholar] [CrossRef]
- OIE Rinderpest. Available online: https://www.oie.int/en/disease/Rinderpest/ (accessed on 8 August 2021).
- Henderson, D.A. The eradication of smallpox—An overview of the past, present, and future. Vaccine 2011, 29, D7–D9. [Google Scholar] [CrossRef]
- Matthias, D.M.; Robertson, J.; Garrison, M.M.; Newland, S.; Nelson, C. Freezing temperatures in the vaccine cold chain: A systematic literature review. Vaccine 2007, 25, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Cakouros, B.E.; Assi, T.M.; Connor, D.L.; Welling, J.; Kone, S.; Djibo, A.; Wateska, A.R.; Pierre, L.; Brown, S.T. The impact of making vaccines thermostable in Niger’s vaccine supply chain. Vaccine 2012, 30, 5637–5643. [Google Scholar] [CrossRef]
- Lee, B.Y.; Wedlock, P.T.; Haidari, L.A.; Elder, K.; Potet, J.; Manring, R.; Connor, D.L.; Spiker, M.L.; Bonner, K.; Rangarajan, A.; et al. Economic impact of thermostable vaccines. Vaccine 2017, 35, 3135–3142. [Google Scholar] [CrossRef]
- FAO. Livestock. Available online: http://www.fao.org/rural-employment/agricultural-sub-sectors/livestock/en/ (accessed on 7 August 2021).
- Porphyre, T.; Rich, K.M.; Auty, H.K. Assessing the economic impact of vaccine availability when controlling foot and mouth disease outbreaks. Front. Vet. Sci. 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.D.; Rushton, J. The economic impacts of foot and mouth disease—What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- Boklund, A.; Halasa, T.; Christiansen, L.E.; Enøe, C. Comparing control strategies against Foot-and-mouth disease: Will vaccination be cost-effective in Denmark? Prev. Vet. Med. 2013, 111, 206–219. [Google Scholar] [CrossRef]
- Berentsen, P.B.M.; Dijkhuizen, A.A.; Oskam, A.J. A dynamic model for cost-benefit analyses of foot-and-mouth disease control strategies. Prev. Vet. Med. 1992, 12, 229–243. [Google Scholar] [CrossRef]
- WHO. Assessing the Programmatic Suitability of Vaccine Candidates for WHO Prequalification Immunization, Vaccines and Biologicals; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- OIE. Mission. Available online: https://www.oie.int/en/who-we-are/mission/ (accessed on 18 October 2021).
- PAHO. Immunization. Available online: https://www.paho.org/en/topics/immunization (accessed on 25 November 2021).
- FAO. Veterinary Vaccines Protect Humans as Well as Animals, says Director-General. Available online: https://www.fao.org/news/story/it/item/1398970/icode/ (accessed on 25 November 2021).
- Redding, L.; Weiner, D.B. DNA vaccines in veterinary use. Expert Rev. Vaccines 2009, 8, 1251–1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yuan, C.; Song, X.; Xu, L.; Yan, R.; Shah, M.A.A.; Guo, C.; Zhu, S.; Li, X. Optimization of Immunization Procedure for Eimeria tenella DNA Vaccine pVAX1-pEtK2-IL-2 and Its Stability. Acta Parasitol. 2019, 64, 745–752. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vaccine Efficacy, Effectiveness and Protection. Available online: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (accessed on 6 October 2021).
- Orenstein, W.A.; Bernier, R.H.; Dondero, T.J.; Hinman, A.R.; Marks, J.S.; Bart, K.J.; Sirotkin, B. Field evaluation of vaccine efficacy. Bull. World Health Organ. 1985, 63, 1055–1068. [Google Scholar] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Higgins, J.; Thompson, S. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Fanelli, A.; Battisti, E.; Zanet, S.; Trisciuoglio, A.; Ferroglio, E. A systematic review and meta-analysis of Toxoplasma gondii in roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Europe. Zoonoses Public Health 2021, 68, 182–193. [Google Scholar] [CrossRef]
- Fanelli, A.; Tizzani, P.; Buonavoglia, D. A systematic review and meta-analysis of hepatitis E virus (HEV) in wild boars. Res. Vet. Sci. 2022, 142, 54–69. [Google Scholar] [CrossRef]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Publication Bias in Meta-Analysis. J. Am. Med. Assoc. 2006, 295, 1–7. [Google Scholar] [CrossRef]
- Abah, H.O.; Abdu, P.A.; Adamu, J. Newcastle disease virus antibody in serum and feather pulp of chickens vaccinated with thermostable vaccine coated on grains and brans in Zaria, Northern Nigeria. J. Vet. Med. Anim. Health 2017, 9, 264–272. [Google Scholar] [CrossRef]
- Abdi, R.D.; Amsalu, K.; Merera, O.; Asfaw, Y.; Gelaye, E.; Yami, M.; Sori, T. Serological response and protection level evaluation in chickens exposed to grains coated with I2 Newcastle disease virus for effective oral vaccination of village chickens. BMC Vet. Res. 2016, 12, 150. [Google Scholar] [CrossRef]
- Acharya, M.P.; Adhikari, S.K.; Awasthi, H.; Jha, A.; Singh, U.M. Field Verification Trial of ND I-2 Vaccine in Nepal. Nepal. Vet. J. 2019, 36, 15–22. [Google Scholar] [CrossRef]
- Asl Najjari, A.H.; Nili, H.; Asasi, K.; Mosleh, N.; Rohollahzadeh, H.; Mokhayeri, S. Efficacy of thermostable I-2 Newcastle disease vaccine compared to B1 commercial vaccine in broiler chicken. Iran. J. Vet. Res. 2017, 18, 103–107. [Google Scholar] [CrossRef]
- Awa, D.N.; Ngo Tama, A.C.; Njoya, A.; Jumbo, S.D.; Mefomdjo, P. The potential role of an inactivated thermostable vaccine in the control of Newcastle disease in traditionally free-roaming poultry in Central and West Africa. Trop. Anim. Health Prod. 2009, 41, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Sen, A.; Venkatesan, G.; Bhanuprakash, V.; Singh, R.K. Protective immune response of live attenuated thermo-adapted Peste des petits ruminants vaccine in goats. VirusDisease 2014, 25, 350–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Riyesh, T.; Balamurugan, V.; Sen, A.; Bhanuprakash, V.; Venkatesan, G.; Yadav, V.; Singh, R.K. Evaluation of efficacy of stabilizers on the thermostability of live attenuated thermo-adapted Peste des petits ruminants vaccines. Virol. Sin. 2011, 26, 324–337. [Google Scholar] [CrossRef]
- Daouam, S.; Ghzal, F.; Arkam, A.E.; Jazouli, M.; Ennaji, M.M.; Tadlaoui, K.O.; Oura, C.; Elharrak, M. Evaluation of the Safety and Efficacy of a Live Attenuated Thermostable Rift Valley Fever Vaccine in Sheep, Goats and Cattle. J. Vaccines Vaccinatio 2015, 6, 1000295. [Google Scholar] [CrossRef]
- Daouam, S.; Ghzal, F.; Naouli, Y.; Tadlaoui, K.O.; Ennaji, M.M.; Oura, C.; El Harrak, M. Safety and immunogenecity of a live attenuated Rift Valley fever vaccine (CL13T) in camels. BMC Vet. Res. 2016, 12, 154. [Google Scholar] [CrossRef]
- Dulal, P.; Wright, D.; Ashfield, R.; Hill, A.V.S.; Charleston, B.; Warimwe, G.M. Potency of a thermostabilised chimpanzee adenovirus Rift Valley Fever vaccine in cattle. Vaccine 2016, 34, 2296–2298. [Google Scholar] [CrossRef] [PubMed]
- Echeonwu, B.C.; Ngele, M.B.; Echeonwu, G.O.N.; Joannis, T.M.; Onovoh, E.M.; Paul, G. Response of chickens to oral vaccination with Newcastle disease virus vaccine strain I2 coated on maize offal. Afr. J. Biotechnol. 2008, 7, 1594–1599. [Google Scholar] [CrossRef]
- Foster, H.A.; Chitukuro, H.R.; Tuppa, E.; Mwanjala, T.; Kusila, C. Thermostable newcastle disease vaccines in Tanzania. Vet. Microbiol. 1999, 68, 127–130. [Google Scholar] [CrossRef]
- Habibi, H.; Nili, H.; Asasi, K.; Mosleh, N.; Firouzi, S.; Mohammadi, M. Efficacy and transmissibility of Newcastle disease I-2 vaccine strain against a field isolate of virulent ND virus (JF820294.1) in village chicken. Trop. Anim. Health Prod. 2015, 47, 73–78. [Google Scholar] [CrossRef]
- Habibi, H.; Firouzi, S.; Nili, H.; Asasi, K.; Mosleh, N. Efficacy of thermostable Newcastle disease virus strain I-2 in broiler chickens challenged with highly virulent newcastle virus. Arch. Razi Inst. 2020, 75, 31–37. [Google Scholar] [CrossRef]
- Henning, J.; Morton, J.; Pym, R.; Hla, T.; Meers, J. Evaluation of strategies to improve village chicken production-controlled field trials to assess effects of Newcastle disease vaccination and altered chick rearing in Myanmar. Prev. Vet. Med. 2009, 90, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Illango, J.; Olaho-Mukani, W.; Mukiibi-Muka, G.; Abila, P.P.; Etoori, A. Immunogenicity of a locally produced Newcastle disease I-2 thermostable vaccine in chickens in Uganda. Trop. Anim. Health Prod. 2005, 37, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, D.H.; Kim, B.Y.; Choi, S.W.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Immunization with a thermostable Newcastle disease virus K148/08 strain originated from wild mallard duck confers protection against lethal viscerotropic velogenic Newcastle disease virus infection in chickens. PLoS ONE 2013, 8, e83161. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Giavedoni, L.; Saliki, J.T.; Brown, C.; Mebus, C.; Yilma, T. Protection of goats against peste des petits ruminants with a vaccinia virus double recombinant expressing the F and H genes of Rinderpest virus. Vaccine 1993, 11, 961–964. [Google Scholar] [CrossRef]
- Khandelwal, A.; Renukaradhya, G.J.; Rajasekhar, M.; Sita, G.L.; Shaila, M.S. Immune responses to hemagglutinin-neuraminidase protein of Peste des petits ruminants virus expressed in transgenic peanut plants in sheep. Vet. Immunol. Immunopathol. 2011, 140, 291–296. [Google Scholar] [CrossRef]
- Lankester, F.J.; Wouters, P.A.W.M.; Czupryna, A.; Palmer, G.H.; Mzimbiri, I.; Cleaveland, S.; Francis, M.J.; Sutton, D.J.; Sonnemans, D.G.P. Thermotolerance of an inactivated rabies vaccine for dogs. Vaccine 2016, 34, 5504–5511. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hu, S.; Xiao, Y.; Xu, Q.; Li, Z.; Bi, D.; Sun, M. Displaying the protein of Mycoplasma gallisepticum agglutinin on the cell surface of Bacillus thuringiensis with the S-layer protein. Vet. Microbiol. 2008, 130, 99–106. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Hu, S.; Zhao, C.; Bi, D.; Sun, M. Display of avian influenza virus nucleoprotein on Bacillus thuringiensis cell surface using CTC as a fusion partner. Appl. Microbiol. Biotechnol. 2008, 78, 669–676. [Google Scholar] [CrossRef]
- Lv, F.; Lu, Y.; Hao, Z.l.; Zhao, Y.H.; Zhang, L.H.; Feng, L.; Chen, J.; Wang, L.l.; Rui, R.; Hou, J.B. Preparation and heat resistance study of porcine reproductive and respiratory syndrome virus sugar glass vaccine. Vaccine 2016, 34, 3746–3750. [Google Scholar] [CrossRef]
- Mariner, J.C.; Van Den Ende, M.C.; House, J.A.; Mebus, C.A.; Salifou, S.; Stem, C. The serological response to a thermostable vero cell-adapted Rinderpest vaccine under field conditions in Niger. Vet. Microbiol. 1990, 22, 119–127. [Google Scholar] [CrossRef]
- Mariner, J.C.; House, J.A.; Mebus, C.A.; Van Den Ende, M.C. The use of thermostable Vero cell-adapted Rinderpest vaccine as a heterologous vaccine against Peste des petits ruminants. Res. Vet. Sci. 1993, 54, 212–216. [Google Scholar] [CrossRef]
- Mehrabadi, F.; Hajloo, A.; Shoushtari, A.; Tehrani, F.; Masoudi, S.; Abdoshah, M.; Amir Hajloo, S.; Shabani, M. Effectiveness of Thermostable Vaccine for Newcastle Disease Produced by the Razi Institute on Backyard Poultry in Iran during 2015. Arch. Razi Inst. 2020, 75, 1–7. [Google Scholar] [CrossRef]
- Murr, M.; Hoffmann, B.; Grund, C.; Römer-Oberdörfer, A.; Mettenleiter, T.C. A novel recombinant Newcastle disease virus vectored DIVA vaccine against Peste des petits ruminants in goats. Vaccines 2020, 8, 205. [Google Scholar] [CrossRef]
- Nega, M.; Moges, F.; Mazengia, H.; Zeleke, G.; Tamir, S. Evaluation of I2 thermostable Newcastle disease vaccine on local chickens in selected districts of western Amhara. J. Anim. Feed Res. 2012, 2, 244–248. [Google Scholar]
- Nwanta, J.A.; Umoh, J.U.; Abdu, P.A.; Ajogi, I.; Egege, S.; Adeiza, A.A. Field trial of Malaysian thermostable Newcastle disease vaccine in village chickens in Kaduna State, Nigeria. Sokoto J. Vet. Sci. 2008, 3, 45–47. [Google Scholar]
- Omony, J.B.; Wanyana, A.; Kirunda, H.; Mugimba, K.K.; Nakavuma, J.L.; Otim-Onapa, M.; Byarugaba, D.K. Immunogenicity and protection efficacy evaluation of avian paramyxovirus serotype-1 (APMV-1) isolates in experimentally infected chickens. Avian Pathol. 2017, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Bhuiyan, A.R.; Parvin, R.; Giasuddin, M.; Haque, M.E.; Sayem, S.M.; Islam, M.R.; Chowdhury, E.H. Immune response of goats to thermostable PPR vaccine in Bangladesh. SAARC J. Agric. 2011, 9, 73–81. [Google Scholar]
- Ruan, B.; Liu, Q.; Chen, Y.; Niu, X.; Wang, X.; Zhang, C.; Guo, M.; Zhang, X.; Cao, Y.; Wu, Y. Generation and evaluation of a vaccine candidate of attenuated and heat-resistant genotype VIII Newcastle disease virus. Poult. Sci. 2020, 99, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Shendy, M.; El-Dakhly, A.T.; Youssef, M.M. Preparation of a thermostable bovine ephermeral fever virus vaccine inactivated on the time of use. Egypt. J. Agric. Res. 2017, 95, 2. [Google Scholar]
- Siddique, F.; Mahmood, M.S.; Hussain, I.; Deeba, F. Evaluation of efficacy of Vero cell-adapted, thermostable Newcastle disease vaccine in broilers. J. Appl. Poult. Res. 2017, 26, 145–153. [Google Scholar] [CrossRef]
- Siddique, F.; Abbas, R.Z.; Iqbal, A.; Rabbani, M.; Rafique, A.; Hussain, I.; Ahmed, R.; Mahmood, M.S.; Lotfi, A. Development of humoral immune response to thermostable newcastle disease vaccine strain i-2 in ring-necked pheasant (Phasianus colchicus). Kafkas Univ. Vet. Fak. Derg. 2021, 27, 253–258. [Google Scholar] [CrossRef]
- Smith, T.G.; Wu, X.; Ellison, J.A.; Wadhwa, A.; Franka, R.; Langham, G.L.; Skinner, B.L.; Hanlon, C.A.; Bronshtein, V.L. Assessment of the immunogenicity of rabies vaccine preserved by vaporization and delivered to the duodenal mucosa of gray foxes (Urocyon cinereoargenteus). Am. J. Trop. Med. Hyg. 2017, 78, 752–756. [Google Scholar] [CrossRef]
- Tan, L.; Wen, G.; Yuan, Y.; Huang, M.; Sun, Y.; Liao, Y.; Song, C.; Liu, W.; Shi, Y.; Shao, H.; et al. Development of a recombinant thermostable newcastle disease virus (Ndv) vaccine express infectious bronchitis virus (ibv) multiple epitopes for protecting against ibv and ndv challenges. Vaccines 2020, 8, 564. [Google Scholar] [CrossRef]
- Tu, T.D.; Van Phuc, K.; Dinh, N.T.K.; Quoc, D.N.; Spradbrow, P.B. Vietnamese trials with a thermostable Newcastle disease vaccine (strain I2) in experimental and village chickens. Prev. Vet. Med. 1998, 34, 205–214. [Google Scholar] [CrossRef]
- Verardi, P.H.; Aziz, F.H.; Ahmad, S.; Jones, L.A.; Beyene, B.; Ngotho, R.N.; Wamwayi, H.M.; Yesus, M.G.; Egziabher, B.G.; Yilma, T.D. Long-Term Sterilizing Immunity to Rinderpest in Cattle Vaccinated with a Recombinant Vaccinia Virus Expressing High Levels of the Fusion and Hemagglutinin Glycoproteins. J. Virol. 2002, 76, 484–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wambura, P.N.; Godfrey, S.K. Protective immune response of chickens to oral vaccination with thermostable live Fowlpox virus vaccine (strain TPV-1) coated on oiled rice. Trop. Anim. Health Prod. 2010, 42, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Wambura, P.N.; Kapaga, A.M.; Hyera, J.M.K. Experimental trials with a thermostable Newcastle disease virus (strain I2) in commercial and village chickens in Tanzania. Prev. Vet. Med. 2000, 43, 75–83. [Google Scholar] [CrossRef]
- Wambura, P.N.; Kataga, S. Putative protective antibody response following oral vaccination of multi-age free ranging helmeted guinea fowls (Numida meleagris) with Newcastle disease virus strain I-2 coated on oiled rice. Trop. Anim. Health Prod. 2011, 43, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Li, L.; Yu, Q.; Wang, H.; Luo, Q.; Zhang, T.; Zhang, R.; Zhang, W.; Shao, H. Evaluation of a thermostable Newcastle disease virus strain TS09-C as an in-ovo vaccine for chickens. PLoS ONE 2017, 12, e0172812. [Google Scholar] [CrossRef]
- Zuo, X.X.; Zhao, Y.H.; Zhou, M.X.; Deng, B.H.; Hu, L.G.; Lv, F.; Lu, Y.; Hou, J.B. Live vaccine preserved at room temperature: Preparation and characterization of a freeze-dried classical swine fever virus vaccine. Vaccine 2020, 38, 8371–8378. [Google Scholar] [CrossRef]
- Wen, G.; Hu, X.; Zhao, K.; Wang, H.; Zhang, Z.; Zhang, T.; Yang, J.; Luo, Q.; Zhang, R.; Pan, Z.; et al. Molecular basis for the thermostability of Newcastle disease virus. Sci. Rep. 2016, 6, 22492. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, S.; Soltani, M.; Behdani, M.; Evensen, Ø.; Alirahimi, E.; Soltani, E.; Hassanzadeh, R.; Ashrafi-Helan, J. VP2 (PTA motif) encoding DNA vaccine confers protection against lethal challenge with infectious pancreatic necrosis virus (IPNV) in trout. Mol. Immunol. 2018, 94, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bande, F.; Arshad, S.S.; Bejo, M.H.; Omar, A.R.; Moeini, H.; Khadkodaei, S.; Wei, T.S.; Keong, Y.S.; Abba, Y.; Anka, I.A. Development and immunogenic potentials of chitosan-saponin encapsulated DNA vaccine against avian infectious bronchitis coronavirus. Microb. Pathog. 2020, 149, 104560. [Google Scholar] [CrossRef] [PubMed]
- Bunning, M.L.; Fox, P.E.; Bowen, R.A.; Komar, N.; Chang, G.J.J.; Speaker, T.J.; Stephens, M.R.; Nemeth, N.; Panella, N.A.; Langevin, S.A.; et al. DNA vaccination of the American crow (Corvus brachyrhynchos) provides partial protection against lethal challenge with West Nile virus. Avian Dis. 2007, 51, 573–577. [Google Scholar] [CrossRef]
- Cai, S.H.; Lu, Y.S.; Jian, J.C.; Wang, B.; Huang, Y.C.; Tang, J.F.; Ding, Y.; Wu, Z.H. Protection against Vibrio alginolyticus in crimson snapper Lutjanus erythropterus immunized with a DNA vaccine containing the ompW gene. Dis. Aquat. Organ. 2013, 106, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Z.; Wang, W.; Hou, S.; Cai, J.; Xia, L.; Lu, Y. Development of DNA vaccines encoding ribosomal proteins (RplL and RpsA) against Nocardia seriolae infection in fish. Fish Shellfish Immunol. 2020, 96, 201–212. [Google Scholar] [CrossRef]
- Citarasu, T.; Lelin, C.; Thirumalaikumar, E.; Michael Babu, M.; Vakharia, V.N. Macrobrachium rosenbergii nodavirus (MrNV)-CP-RNA-2 DNA vaccine confers protective immunity in giant freshwater prawn Macrobrachium rosenbergii against MrNV infection. Fish Shellfish Immunol. 2019, 86, 319–326. [Google Scholar] [CrossRef]
- Clapp, B.; Walters, N.; Thornburg, T.; Hoyt, T.; Yang, X.; Pascual, D.W. DNA vaccination of bison to brucellar antigens elicits elevated antibody and IFN-γ responses. J. Wildl. Dis. 2011, 47, 501–510. [Google Scholar] [CrossRef]
- Cui, J.; O’connell, C.M.; Hagen, C.; Sawicki, K.; Smyth, J.A.; Verardi, P.H.; Van Kruiningen, H.J.; Garmendia, A.E. Broad protection of pigs against heterologous prrsv strains by a GP5-mosaic DNA vaccine prime/GP5-mosaic rvaccinia (VACV) vaccine boost. Vaccines 2020, 8, 106. [Google Scholar] [CrossRef]
- Dahiya, S.S.; Saini, M.; Kumar, P.; Gupta, P.K. Immunogenicity of a DNA-launched replicon-based canine parvovirus DNA vaccine expressing VP2 antigen in dogs. Res. Vet. Sci. 2012, 93, 1089–1097. [Google Scholar] [CrossRef]
- Davis, M.R.; Langan, J.N.; Johnson, Y.J.; Ritchie, B.W.; Van Bonn, W. West Nile virus seroconversion in penguins after vaccination with a killed virus vaccine or a DNA vaccine. J. Zoo Wildl. Med. 2008, 39, 582–589. [Google Scholar] [CrossRef]
- Eman, M.S.E.; Maha, A.N.G.; Zaki, F.F.; Saad, M.A.; Soliman, Y.A. A Novel DNA Vaccine Coding For H5 and N1 Genes of Highly Pathogenic Avian Influenza H5N1 Subtype. Indian J. Vet. Sci. Biotechnol. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, Z.; Li, C.; Liu, G. Protective immune responses in ducklings induced by a suicidal DNA vaccine of the VP1 gene of duck hepatitis virus type 1. Vet. Microbiol. 2012, 160, 314–318. [Google Scholar] [CrossRef]
- Garver, K.A.; LaPatra, S.E.; Kurath, G. Efficacy of an infectious hematopoietic necrosis (IHN) virus DNA vaccine in Chinook Oncorhynchus tshawytscha and sockeye O. nerka salmon. Dis. Aquat. Organ. 2005, 64, 13–22. [Google Scholar] [CrossRef]
- Gong, Q.; Kong, L.Y.; Niu, M.F.; Qin, C.L.; Yang, Y.; Li, X.; Ruan, M.D.; Tian, Y.; Li, Z.L. Construction of a ptfA chitosan nanoparticle DNA vaccine against Pasteurella multocida and the immune response in chickens. Vet. J. 2018, 231, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shen, H.; Jia, R.; Wang, M.; Chen, S.; Zhu, D.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Oral vaccination with a DNA vaccine encoding capsid protein of duck Tembusu virus induces protection immunity. Viruses 2018, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Kotla, S.; Vishanath, B.S.; H.J., D.; K., G.; V.V.S., S.; Reddy, G.R. DNA vaccine (P1-2A-3C-pCDNA) co-administered with Bovine IL-18 gives protective immune response against Foot and Mouth Disease in cattle. Vet. Microbiol. 2016, 193, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. Construction and evaluation of an Edwardsiella tarda DNA vaccine encoding outer membrane protein C. Microb. Pathog. 2017, 104, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Adams, L.J.; Zeng, X.; Lin, J. Evaluation of in ovo vaccination of DNA vaccines for Campylobacter control in broiler chickens. Vaccine 2019, 37, 3785–3792. [Google Scholar] [CrossRef]
- Pasnik, D.J.; Smith, S.A. Immunogenic and protective effects of a DNA vaccine for Mycobacterium marinum in fish. Vet. Immunol. Immunopathol. 2005, 103, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Sisteré-Oró, M.; López-Serrano, S.; Veljkovic, V.; Pina-Pedrero, S.; Vergara-Alert, J.; Córdoba, L.; Pérez-Maillo, M.; Pleguezuelos, P.; Vidal, E.; Segalés, J.; et al. DNA vaccine based on conserved HA-peptides induces strong immune response and rapidly clears influenza virus infection from vaccinated pigs. PLoS ONE 2019, 14, e0222201. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, C.S.; Sun, L. Construction and analysis of the immune effect of an Edwardsiella tarda DNA vaccine encoding a D15-like surface antigen. Fish Shellfish Immunol. 2011, 30, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tarradas, J.; Álvarez, B.; Fraile, L.; Rosell, R.; Muñoz, M.; Galindo-Cardiel, I.; Domingo, M.; Dominguez, J.; Ezquerra, A.; Sobrino, F.; et al. Immunomodulatory effect of swine CCL20 chemokine in DNA vaccination against CSFV. Vet. Immunol. Immunopathol. 2011, 142, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, K.; Del Crew, J.; Hermanson, G.; Wloch, M.K.; Riffenburgh, R.H.; Smith, C.R.; Van Bonn, W.G. A DNA vaccine against dolphin morbillivirus is immunogenic in bottlenose dolphins. Vet. Immunol. Immunopathol. 2007, 120, 260–266. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, F.; Huang, Y.; Ding, Y.; Jian, J.; Wu, Z. Construction of glutathione peroxidase (GPx) DNA vaccine and its protective efficiency on the orange-spotted grouper (Epinephelus coioides) challenged with Vibrio harveyi. Fish Shellfish Immunol. 2017, 60, 529–536. [Google Scholar] [CrossRef]
- Wium, M.; Jonker, H.I.; Olivier, A.J.; Bellstedt, D.U.; Botes, A. DNA vaccines against Mycoplasma elicit humoral immune responses in ostriches. Front. Immunol. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Xing, J.; Xu, H.; Tang, X.; Sheng, X.; Zhan, W. A DNA vaccine encoding the VAA gene of Vibrio anguillarum induces a protective immune response in flounder. Front. Immunol. 2019, 10, 499. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, J.; Liu, M.; Ren, G.; Jian, F.; Yin, J.; Feng, J.; Liu, H.; Lu, T. Bivalent DNA vaccine induces significant immune responses against infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout. Sci. Rep. 2017, 7, 5700. [Google Scholar] [CrossRef]
- Xu, H.; Xing, J.; Tang, X.; Sheng, X.; Zhan, W. Intramuscular administration of a DNA vaccine encoding OmpK antigen induces humoral and cellular immune responses in flounder (Paralichthys olivaceus) and improves protection against Vibrio anguillarum. Fish Shellfish Immunol. 2019, 86, 618–626. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.N.; Wang, X.; Tang, J.N.; Gao, R.; Li, J.; Guo, Z.C.; Li, Y.L. Multivalent DNA vaccine enhanced protection efficacy against infectious bronchitis virus in chickens. J. Vet. Med. Sci. 2009, 71, 1585–1590. [Google Scholar] [CrossRef]
- Yi, W.; Zhang, X.; Zeng, K.; Xie, D.F.; Song, C.; Tam, K.; Liu, Z.J.; Zhou, T.; Li, W. Construction of a DNA vaccine and its protective effect on largemouth bass (Micropterus salmoides) challenged with largemouth bass virus (LMBV). Fish Shellfish Immunol. 2020, 106, 103–109. [Google Scholar] [CrossRef]
- Yu, N.T.; Zheng, X.b.; Liu, Z.X. Protective immunity induced by DNA vaccine encoding viral membrane protein against SGIV infection in grouper. Fish Shellfish Immunol. 2019, 92, 649–654. [Google Scholar] [CrossRef]
- Yuan, D.; Qu, L.; Liu, J.; Guo, D.; Jiang, Q.; Lin, H.; Si, C. DNA vaccination with a gene encoding VP60 elicited protective immunity against rabbit hemorrhagic disease virus. Vet. Microbiol. 2013, 164, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, Z.; Liu, G.Y.; Li, J.; Wang, G.X.; Zhu, B. Immune response and protective effect against spring viremia of carp virus induced by intramuscular vaccination with a SWCNTs-DNA vaccine encoding matrix protein. Fish Shellfish Immunol. 2018, 79, 256–264. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Bi, Z.; Chen, Z.; Meng, C.; Wang, G.J.; Ding, C.; Liu, G. Protective immune responses in ducklings induced by a suicidal DNA vaccine of the sigma C gene of novel duck reovirus. Vet. Immunol. Immunopathol. 2015, 165, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, Q.; Huang, L.; Wang, K.; Wang, X.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; Lai, W. Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia. Dev. Comp. Immunol. 2017, 77, 77–87. [Google Scholar] [CrossRef]
- Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, A.P. and H.S. Improving Farmyard Poultry Production in Africa: Interventions and their Economic Assessment; Proceedings of a Final Research Coordination Meeting (IAEA-TECDOC-1489); International Atomic Energy Agency: Vienna, Austria, 2006. [Google Scholar]
- Joint FAO/IAEA Programme Newcastle. Disease Control of Chicken Improves the Welfare of Rural Households in Africa. Available online: http://www-naweb.iaea.org/nafa/news/2005-newcastle-vaccine.html (accessed on 25 November 2021).
- Mahmood, M.S.; Siddique, F.; Hussain, I.; Ahmad, S.I.; Rafique, A. Thermostable vaccines for Newcastle disease: A review. Worlds. Poult. Sci. J. 2014, 70, 829–838. [Google Scholar] [CrossRef]
- Zhao, H.; Njeumi, F.; Parida, S.; Benfield, C.T.O. Progress towards eradication of Peste des petits ruminants through vaccination. Viruses 2021, 13, 59. [Google Scholar] [CrossRef]
- USAID. Thermostable Peste des Petits Ruminants Vaccine Commercially Available for the First Time. Available online: https://agrilinks.org/post/thermostable-peste-des-petits-ruminants-vaccine-commercially-available-first-time (accessed on 25 November 2021).
- FAO. Lessons Learned from the Eradication of Rinderpest for Controlling other Transboundary Animal Diseases; FAO: Rome, Italy, 2012; ISBN 9789251073315. [Google Scholar]
- Kristensen, D.D.; Lorenson, T.; Bartholomew, K.; Villadiego, S. Can thermostable vaccines help address cold-chain challenges? Results from stakeholder interviews in six low- and middle-income countries. Vaccine 2016, 34, 899–904. [Google Scholar] [CrossRef]
- Lloyd, J.; Lydon, P.; Ouhichi, R.; Zaffran, M. Reducing the loss of vaccines from accidental freezing in the cold chain: The experience of continuous temperature monitoring in Tunisia. Vaccine 2015, 33, 902–907. [Google Scholar] [CrossRef]
- Fomsgaard, A.; Liu, M.A. The key role of nucleic acid vaccines for one health. Viruses 2021, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Mahendran, M.; Gupta, P.K.; Rai, A. DNA vaccines and their applications in veterinary practice: Current perspectives. Vet. Res. Commun. 2008, 32, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, S.D.; Sims, S.M.; Wang, S.-J.; Morrill, K.S. A Brief Answer: Why is China’s Aquaculture Industry so Successful? Environ. Manag. Sustain. Dev. 2017, 6, 234–241. [Google Scholar] [CrossRef]
- Thacker, S.B.; Stroup, D.F.; Carande-Kulis, V.; Marks, J.S.; Roy, K.; Gerberding, J.L. Measuring the public’s health. Public Health Rep. 2006, 121, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.D.; Edmond, K.; Gubbins, S.; Paton, D.J. Veterinary and human vaccine evaluation methods. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132839. [Google Scholar] [CrossRef]
- Bozorgi, A.; Pazour, J.; Nazzal, D. A new inventory model for cold items that considers costs and emissions. Int. J. Prod. Econ. 2014, 155, 114–125. [Google Scholar] [CrossRef]
- Liu, F.; Wu, X.; Li, L.; Ge, S.; Liu, Z.; Wang, Z. Virus-like particles: Promising platforms with characteristics of DIVA for veterinary vaccine design. Comp. Immunol. Microbiol. Infect. Dis. 2013, 36, 343–352. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed]
| Database | Strategy | No. of Publications |
|---|---|---|
| Thermostable Vaccines | ||
| PubMed | (“vaccin*”[Title/Abstract] AND (“thermostable”[Title/Abstract] OR “heat stable”[Title/Abstract] OR “freeze stable”[Title/Abstract] OR (“heat-freeze”[All Fields] AND “stable”[Title/Abstract]))) AND ((fft[Filter]) AND (1990:2021[pdat])) | 462 |
| CAB Abstracts | (title:(vaccin*) OR ab:(vaccin*))AND (title:(thermostable) OR ab:(thermostable) OR title:(heat stable) OR ab:(heat stable) OR title:(freeze stable) OR ab:(freeze stable) OR title:(heat-freeze stable) OR ab:(heat-freeze stable)) AND yr:[1990 TO 2021] | 500 |
| Web of Science | (TI = (vaccin*) OR AB = (vaccin*)) AND (TI = (thermostable) OR AB = (thermostable) OR TI = (heat stable) OR AB = (heat stable) OR TI = (freeze stable) OR AB = (freeze stable) OR TI = (heat-freeze stable) OR AB = (heat-freeze stable)) Timespan: 1 January 1990 to 5 September 2021 (Publication Date) Not: Document Types: Proceedings Papers or Editorial Materials or Meeting Abstracts or Book chapters or Notes or Early access | 693 |
| DNA Vaccines | ||
| PubMed | (“vaccines, dna”[MeSH Major Topic] AND “animals”[MeSH Major Topic]) AND ((fft[Filter]) AND (english[Filter])) | 417 First 100 sorted by best match |
| CAB Abstracts | title:(DNA vaccine) OR ab:(DNA vaccine) AND up:(Animals) AND yr:[1996 TO 2021] Refinements: Document type = Journal article AND Language = English | 6845 First 100 sorted by relevance |
| Web of Science | (TS = (“DNA vaccine”)) AND (DT == (“ARTICLE”) AND TASCA == (“VETERINARY SCIENCES”) AND LA == (“ENGLISH”)) | 557 First 100 sorted by relevance |
| Study | Target Agent | Type of Agent | Animal Species | Country | Product Name | Vaccine Type | Strain/Gene | Market Availability | Thermostability Characteristics | Route of Administration | Type of Study | Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abah et al. [42] | Newcastle disease virus | Virus | Chicken | Nigeria | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (feed) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Abdi et al. [43] | Newcastle disease virus | Virus | Chicken | Ethiopia | NDV vaccine (National Veterinary Institute of Bishoftu, Ethiopia) | Live-attenuated 2 | I-2 | Commercially available | N.A. | Oral (feed and water) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Acharya et al. [44] | Newcastle disease virus | Virus | Chicken | Nepal | N.A. | Live-attenuated | I-2 | Locally produced | 30 °C for 7 days | Intraocular | Field trial (all animals vaccinated) | Assessment of humoral immunity |
| Asl Najjari et al. [45] | Newcastle disease virus | Virus | Chicken | Iran | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Intraocular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Awa et al. [46] | Newcastle disease virus | Virus | Chicken | Cameroon | Multivax (LANAVET Garoua, Cameroon) | Live-attenuated | La Sota + Cholevax + Typhovax | Commercially available | 34 °C for 10 weeks | Intramuscolar | Clinical and field trials (all animals vaccinated) | Assessment of humoral immunity |
| Balamurugan et al. 2014 [47] | Peste des petits ruminants virus | Virus | Goats | India | N.A. | Live-attenuated 3 | Jhansi/2003 | Experimentally developed | 24–26 days at 25 °C 7–8 days at 37 °C 3–4 days at 40 °C (Riyesh et al. [48]) | Subcutaneous | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Daouam et al. [49] | Rift Valley Fever virus | Virus | Cattle, sheep, and goats | Morocco | N.A. | Live-attenuated 2 | Clone of CL13T | Experimentally developed | 37 °C for 4 days 20 months at 4 °C | Subcutaneous | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Daouam et al. [50] | Rift Valley Fever virus | Virus | Camels | Morocco | N.A. | Live-attenuated 2 | Clone of CL13T | Experimentally developed | (see Daouam et al. [49]) | Subcutaneous | Clinical trial (all animals vaccinated) | Assessment of humoral immunity |
| Dulal et al. [51] | Rift Valley Fever virus | Virus | Cattle | United Kingdom | ChAdOx1-GnGc | Recombinant vector 4 | MP-12 | Experimentally developed | 25°, 37°or 45 °C for 6 months | Intramuscolar | Clinical trial (all animals vaccinated) | Assessment of humoral immunity |
| Echeonwu et al. [52] | Newcastle disease virus | Virus | Chicken | Nigeria | N.A. | Live-attenuated 2 | I-2 | Locally produced | N.A. | Oral (feed) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Foster et al. [53] | Newcastle disease virus | Virus | Chicken | Tanzania | Websters HR V4 | Live-attenuated | V-4 | Locally produced | N.A. | Intraocular and oral (water) | Field trial (vaccinated vs. control) | Assessment of humoral immunity |
| Habibi et al. [54] | Newcastle disease virus | Virus | Chicken | Iran | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (feed and water) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Habibi et al. [55] | Newcastle disease virus | Virus | Chicken | Iran | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (feed) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Henning et al. [56] | Newcastle disease virus | Virus | Chicken | Myanmar | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Intraocular and intranasal | Field trial (vaccinated vs. control) | Assessment of humoral immunity |
| Illango et al. [57] | Newcastle disease virus | Virus | Chicken | Uganda | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (water) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Jeong et al. [58] | Newcastle disease virus | Virus | Chicken | Korea | N.A. | Live-attenuated | K148/08 | Experimentally developed | Thermostability test 13 | Cabinet sprayer and Intraocular | Clinical trial (vaccinated vs. control) | Assessment of Humoral immunity, histopathological lesions, and RPS post-challenge |
| Jones et al. [59] | Peste des petits ruminants virus | Virus | Goats | USA | vRVFH | Recombinant vector 5 | F and H (Rinderpest) | Experimentally developed | N.A. | Intramuscolar | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity and clinical signs post-challenge |
| Khandelwal et al. [60] | Peste des petits ruminants virus | Virus | Sheep | India | N.A. | Recombinant subunit 6 | HN | Experimentally developed | N.A. | Oral (feed) | Field trial (all animals vaccinated) | Assessment of humoral immunity |
| Lankester et al. [61] | Rabies | Virus | Dogs | Tanzania | Nobivac (rabies, MSD Animal Health, Boxmeer, The Netherlands) | Live-attenuated | Pasteur RIV | Commercially available | 25 °C for 6 months and 30 °C for 3 months | Subcutaneous | Field trial (all animals vaccinated) | Assessment of humoral immunity |
| Liu et al. [62] | Mycoplasma gallisepticum | Bacterium | Chicken | China | N.A. | Recombinant vector 7 | pmga1.2p | Experimentally developed | N.A. | Intra-gastric gavage | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Liu et al. [63] | Avian influenza virus | Virus | Chicken | China | N.A. | Recombinant vector 7 | NP of H9N2 | Experimentally developed | N.A. | Intra-gastric gavage | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Lv et al. [64] | Porcine reproductive and respiratory syndrome virus | Virus | Pigs | China | N.A. | Live-attenuated 8 | JXA1-R | Experimentally developed | 25 °C for 12 months and 37 °C for 4 months | Not specified | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Mariner et al. [65] | Rinderpest virus | Virus | Cattle | Nigeria | VRPV | Live-attenuated 3 | RBOK | Experimentally developed | 25.9 °C (17.1–37.8 °C) for 34 days | Subcutaneous | Field trial (vaccinated vs. control) | Assessment of humoral immunity |
| Mariner et al. [66] | Peste des petits ruminants virus | Virus | Goats | USA | TVRPV | Live-attenuated 3 | RBOK | Experimentally developed | 37 °C for up to 245 days | Subcutaneous | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Mehrabadi et al. [67] | Newcastle disease virus | Virus | Chicken | Iran | ND.TR.IR (Razi Institute, Iran) | Live-attenuated 2 | I-2 | Commercially available | N.A. | Oral (water) | Field trial (vaccinated vs. control) | Assessment of humoral immunity |
| Murr et al. [68] | Peste des petits ruminants virus | Virus | Goats | Germany | rNDV_HKur | Recombinant vector 9 | Kurdistan/11/H | Experimentally developed | −80 °C, −20 °C, 4 °C, 21 °C, and 37 °C for 7 days | Subcutaneous | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity and clinical signs post-challenge |
| Nega et al. [69] | Newcastle disease virus | Virus | Chicken | Ethiopia | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Intraocular | Field trial (all animals vaccinated) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Nwanta et al. [70] | Newcastle disease virus | Virus | Chicken | Nigeria | NDV4HR (Malaysian Vaccines and Pharmaceutical SNP BHD, Malaysia) | Live-attenuated 2 | V-4 | Commercially available | N.A. | Oral (feed) | Field trial (all animals vaccinated) | Assessment of humoral immunity |
| Omony et al. [71] | Newcastle disease virus | Virus | Chicken | Uganda | N.A. | Live-attenuated | NDV-133/UG/MU/2011, NDV-177/UG/MU/2011NDV-178/UG/MU/2011 and NDV-173/UG/MU/2011 | Experimentally developed | N.A. | Intraocular and Intranasal | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Rahman et al. [72] | Peste des petits ruminants virus | Virus | Goats | Bangladesh | N.A. | Live-attenuated | N.A. | Experimentally developed | Percent inhibition values decreased by 8–20% at 180 DPV, when the vaccine is kept 25°, 30°, 35°, and 40 °C for 7 and 14 days | Subcutaneous | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Ruan et al. [73] | Newcastle disease virus | Virus | Chicken | China | N.A. | Live-attenuated 10 | rHR09 | Experimentally developed | Thermostability test 13 | Intramuscolar | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Shendy et al. [74] | Bovine ephemeral fever virus | Virus | Cattle | Egypt | N.A. | Live-attenuated 2 | BEF/AVS/2000 | Experimentally developed | 25 °C for 6 months 37 °C for 3 months 45 °C for 20 days | Subcutaneous | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Siddique et al. [75] | Newcastle disease virus | Virus | Chicken | Pakistan | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (water) | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Siddique et al. [76] | Newcastle disease virus | Virus | Ring-necked pheasants | Pakistan | N.A. | Live-attenuated 2 | I-2 | Locally produced | 28 °C for 6–8 weeks and 4–8 °C for 1 year | Oral (feed) | Field trial (vaccinated vs. control) | Assessment of humoral immunity |
| Smith et al. [77] | Rabies virus | Virus | Gray foxes | USA | N.A. | Live-attenuated 8 | ERA | Experimentally developed | 22° ± 4 °C for up to 65 days | Intestinal endoscopy | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Tan et al. [78] | Newcastle disease virus and infectious bronchitis virus | Virus | Chicken | China | rLS-T-HN-T/B | Recombinant bivalent live 12 | HN and S1 | Experimentally developed | 25 °C for 16 days | Intraocular and intranasal | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Tu et al. [79] | Newcastle disease virus | Virus | Chicken | Vietnam | N.A. | Live-attenuated 2 | I-2 | Locally produced | 30 °C for 3 weeks | Intraocular and oral (water) | Clinical and field trials (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Verardi et al. [80] | Rinderpest virus | Virus | Cattle | Ethiopia and Kenya | N.A. | Recombinant vector 11 | v2RVFH | Experimentally developed | N.A. | Intramuscolar | Field trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Wambura et al. [81] | Flow pox virus | Virus | Chicken | Tanzania | N.A. | Live-attenuated | TPV-1 | Locally produced | 25–34 °C for 6 months | Wing web stab | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Wambura et al. [82] | Newcastle disease virus | Virus | Chicken | Tanzania | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (feed and water) and ocular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Wambura et al. [83] | Newcastle disease virus | Virus | Helmeted guinea fowls | Tanzania | N.A. | Live-attenuated | I-2 | Locally produced | N.A. | Oral (feed) | Field trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and RPS post-challenge |
| Wen et al. [84] | Newcastle disease virus | Virus | Chicken 1 | China | N.A. | Live-attenuated | TS09-C | Experimentally developed | N.A. | In ovo | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, histopathological lesions, and RPS post-challenge |
| Zuo et al. [85] | Classical swine fever virus | Virus | Pigs | China | ST16 | Live-attenuated 2 | C | Experimentally developed | 25 °C for 6 months | Intramuscolar | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Study | Target Agent | Type of Agent | Animal Species | Country | Product Name | Encoding Gene | Market Availability | Route of Administration | Type of Study | Assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadivand et al. [87] | Infectious pancreatic necrosis virus | Virus | Fish (rainbow trout) | Iran | pcDNA3.1-VP2 | VP2 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Bande et al. [88] | Avian infectious bronchitis coronavirus | Virus | Chicken | Malaysia | pBudCR88-S1/M41-S1 | S1 glycoprotein | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, and histopathological lesions post-challenge |
| Bunning et al. [89] | West Nile virus | Virus | American crows | USA | N.A. | prM and E | Experimentally developed | Oral and intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and survival rate post-challenge |
| Cai et al. [90] | Vibrio alginolyticus | Bacterium | Fish (crimson snapper) | China | pcDNA-ompW | ompW | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and survival rate post-challenge |
| Chen et al. [91] | Nocardia seriolae | Bacterium | Fish(hybrid snakehead) | China | pcDNA-RplL and pcDNA-RpsA | RpsA and RplL | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Citarasu et al. [92] | Macrobrachium rosenbergii nodavirus | Virus | Fish(giant freshwater) | India | MrNV-CP-RNA-2-pVAX1 | MrNV-CP-RNA-2 | Experimentally developed | Oral (feed) | Clinical trial (vaccinated vs. control) | Assessment of immunological and hematological parameters, and survival rate post-challenge |
| Clapp et al. [93] | Brucella abortus | Bacterium | Bison | USA | pCMVbp26 + pCMVTF | bp26 + TF | Experimentally developed | N.A. | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity |
| Cui et al. [94] | Porcine reproductive and respiratory syndrome virus | Virus | Pigs | USA | DNA GP5-Mosaic/VACV GP5-Mosaic | ATCC VR-2332 and MN184C | Experimentally developed | Intradermal and intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Dahiya et al. [95] | Canine parvovirus | Virus | Dogs | India | pAlpha-CPV-VP2 | VP2 | Experimentally developed | Intradermal | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity |
| Davis et al. [96] | West Nile virus | Virus | Penguins | USA | WNDV Vaccine (Aldevron Llc, Fargo, North Dakota, USA). | prM/M and E | Commercially available | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Eman et al. [97] | Avian influenza(H5N1) | Virus | Chicken | India | pDEST 40/H5 and pDEST 40/N1 | H5 and N1 | Experimentally developed | Ocular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Fu et al. [98] | Duck hepatitis virus type 1 | Virus | Ducks | China | pSCA/VP1 | VP1 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Garver et al. [99] | Infectious hematopoietic necrosis virus | Virus | Fish(spring chinook, sockeye and kokanee salmon fry) | USA | pIHNw-G | G | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity |
| Gong et al. [100] | Pasteurella multocida | Bacterium | Chicken | China | N.A. | ptfA | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Huang et al. [101] | Duck Tembusu Virus | Virus | Ducks | China | pVAX1-C | Capsid gene (GenBank: JX196334.1) | Experimentally developed | Oral | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and survival rate post-challenge |
| Kotla et al. [102] | Foot-and-mouth disease virus | Virus | Cattle | India | P1-2A-3CpCDNA + bIL-18pCDNA | P1-2A-3C + bovine IL-18 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity |
| Liu et al. [103] | Edwardsiella tarda | Bacterium | Fish (olive flounder) | China | pCG-OmpC | OmpC | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Liu et al. [104] | Campylobacter spp. | Bacterium | Chicken | USA | pCAGGS_CfrA and pCAGGS_CmeC | cfrA and cmeC | Experimentally developed | In ovo | Clinical trial (all animals vaccinated) | Assessment of humoral and intestinal colonization post-challenge |
| Pasnik and Smith [105] | Mycobacterium marinum | Bacterium | Fish(hybrid striped bass) | USA | pCMV-85A | Ag85A | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Sisteré-Oró et al. [106] | Swine influenza virus | Virus | Pigs | Spain | VC4-flagellin DNA | VC-4-flagel-lin (constructed multipeptide) | Experimentally developed | Intradermal (IDAL1 device, MSD Animal Health) | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, histopathological lesions, and survival rate post-challenge |
| Sun et al. [107] | Edwardsiella tarda | Bacterium | Fish (olive flounder) | China | pCEsa1 | Esa1 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, histopathological lesions, and survival rate post-challenge |
| Tarradas et al. [108] | Classical swine fever virus | Virus | Pigs | Spain | pE2 and pCCL20 | E2 and swine CCL20 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, and clinical signs, post-challenge |
| Vaughan et al. [109] | Dolphin morbillivirus | Virus | Atlantic bottlenose dolphins | USA | pVR-DMV-F and pVR-DMV-H (vaccinated) | Fusion (F) and hemagglutinin (H) | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity |
| Wang et al. [110] | Vibrio harvey | Bacterium | Fish(orange-spotted grouper) | China | pcDNA-GPx | GPx | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral immunity, clinical signs, and survival rate post-challenge |
| Wium et al. [111] | Mycoplasma spp. | Bacterium | Ostriches | South Africa | pCI-neo_oppA and VR1020_oppA | oppA | Experimentally developed | Intramuscular | Field trial (vaccinated vs. control) | Assessment of humoral immunity |
| Xing et al. [112] | Vibrio anguillarum | Bacterium | Fish (olive flounder) | China | pcDNA3.1-VAA (pVAA) | VAA | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, histopathological lesions, and survival rate post-challenge |
| Xu et al. [113] | Infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus | Virus | Fish (rainbow trout) | China | pCh-IHN/IPN | G gene of IHNV Sn1203 and VP2 and VP3 genes of IPNV ChRtm213 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, histopathological lesions, and survival rate post-challenge |
| Xu et al. [114] | Vibrio anguillarum | Bacterium | Fish (olive flounder) | China | pcDNA3.1-OmpK (pOmpK) | OmpK | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Yang et al. [115] | Infectious bronchitis virus | Virus | Chicken | China | pVAX1-S1/M/N | S1,N,M | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, and clinical signs, post-challenge |
| Yi et al. [116] | Largemouth bass virus | Virus | Fish (largemouth bass) | China | pCDNA3.1(+)-MCP-Flag | MCP | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Yu et al. [117] | Singapore grouper iridovirus | Virus | Fish(grouper) | China | pcDNA3.1–19R | SGIV-19R | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Yuan et al. [118] | Rabbit hemorrhagic disease virus | Virus | Rabbits | China | pcDNA-VP60 | VP60 | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity |
| Zhang et al. [119] | Spring viremia of carp virus | Virus | Fish(common carp) | China | pcDNA-M and SWCNTs-pcDNA-M | M | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
| Zhu et al. [120] | Novel duck reovirus | Virus | Ducks | China | pSCA/sigma C | Sigma C | Experimentally developed | Intramuscular | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, histopathological lesions, and survival rate post-challenge |
| Zhu et al. [121] | Streptococcus agalactiae | Bacterium | Fish (Nile tilapia) | China | SL7207-pVAX1-sip | Sip | Experimentally developed | Oral (gavage and mixed fodder) | Clinical trial (vaccinated vs. control) | Assessment of humoral and cell-mediated immunity, clinical signs, and survival rate post-challenge |
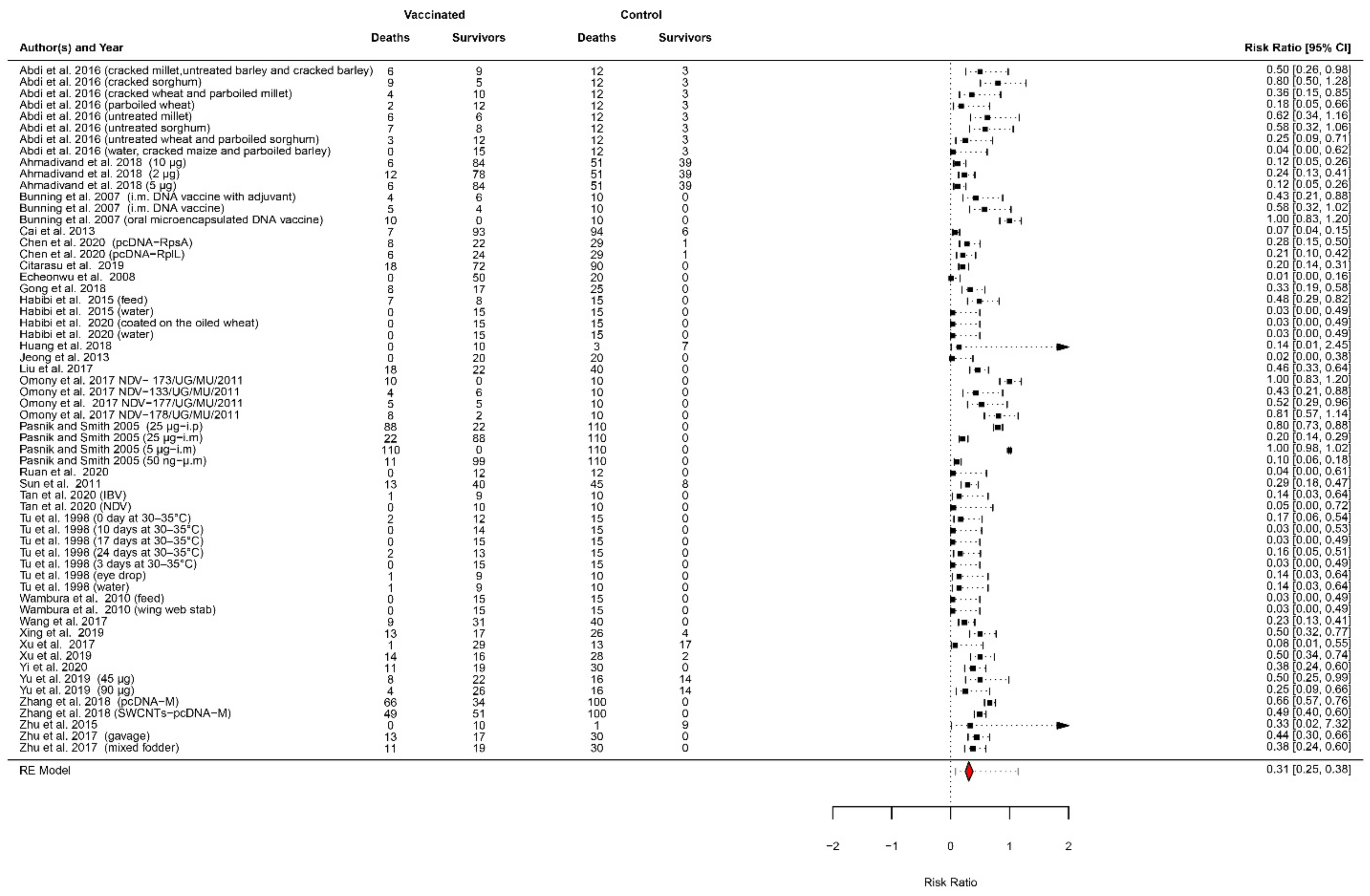
| Study | Vaccinated Group Deaths | Vaccinated Group Survivals | Control Group Deaths | Control Group Survivals | Challenge Time (dpv) | Relative Percent of Survival (RPS)-Days Post-Challenge |
|---|---|---|---|---|---|---|
| Abdi et al. [43] (cracked millet, untreated barley, and cracked barley) | 6 | 9 | 12 | 3 | 21 | 50%-28 days |
| Abdi et al. [43] (cracked sorghum) | 9 | 5 | 12 | 3 | 21 | 20%-28 days |
| Abdi et al. [43] (cracked wheat and parboiled millet) | 4 | 10 | 12 | 3 | 21 | 64%-28 days |
| Abdi et al. [43] (parboiled wheat) | 2 | 12 | 12 | 3 | 21 | 82%-28 days |
| Abdi et al. [43] (untreated millet) | 6 | 6 | 12 | 3 | 21 | 38%-28 days |
| Abdi et al. [43] (untreated sorghum) | 7 | 8 | 12 | 3 | 21 | 42%-28 days |
| Abdi et al. [43] (untreated wheat and parboiled sorghum) | 3 | 12 | 12 | 3 | 21 | 75%-28 days |
| Abdi et al. [43] (water, cracked maize, and parboiled barley) | 0 | 15 | 12 | 3 | 21 | 100%-28 days |
| Ahmadivand et al. [87] (10 ng) | 6 | 84 | 51 | 39 | 30 | 88%-30 days |
| Ahmadivand et al. [87] (2 ng) | 12 | 78 | 51 | 39 | 30 | 76%-30 days |
| Ahmadivand et al. [87] (5 ng) | 6 | 84 | 51 | 39 | 30 | 88%-30 days |
| Bunning et al. [89] (i.m. DNA vaccine with adjuvant) | 4 | 6 | 10 | 0 | 70 | 60%-14 days |
| Bunning et al. [89] (i.m. DNA vaccine) | 5 | 4 | 10 | 0 | 70 | 44%-14 days |
| Bunning et al. [89] (oral microencapsulated DNA vaccine) | 10 | 0 | 10 | 0 | 70 | 0%-14 days |
| Cai et al. [90] | 7 | 93 | 94 | 6 | 49 | 92%-14 days |
| Chen et al. [91] (pcDNA-RpsA) | 8 | 22 | 29 | 1 | 35 | 71%-14 days |
| Chen et al. [91] (pcDNA-RplL) | 6 | 24 | 29 | 1 | 35 | 78%-14 days |
| Citarasu et al. [92] | 18 | 72 | 90 | 0 | 40 | 80%-10 days |
| Echeonwu et al. [52] | 0 | 50 | 20 | 0 | 14 | 100%-10 days |
| Gong et al. [100] | 8 | 17 | 25 | 0 | 14 | 68%-15 days |
| Habibi et al. [54] (feed) | 7 | 8 | 15 | 0 | 14 | 53%-10 days |
| Habibi et al. [54] (water) | 0 | 15 | 15 | 0 | 14 | 100%-10 days |
| Habibi et al. [55] (coated on the oiled wheat) | 0 | 15 | 15 | 0 | 14 | 100%-17 days |
| Habibi et al. [55] (water) | 0 | 15 | 15 | 0 | 14 | 100%-17 days |
| Huang et al. [101] | 0 | 10 | 3 | 7 | 16 | 100%-10 days |
| Jeong et al. [58] | 0 | 20 | 20 | 0 | 14 | 100%-7 days |
| Liu et al. [103] | 18 | 22 | 40 | 0 | 42 | 55%-15 days |
| Omony et al. [71] NDV-173/UG/MU/2011 | 10 | 0 | 10 | 0 | 21 | 0%-14 days |
| Omony et al. [71] NDV-133/UG/MU/2011 | 4 | 6 | 10 | 0 | 21 | 60%-14 days |
| Omony et al. [71] 2014 NDV-177/UG/MU/2011 | 5 | 5 | 10 | 0 | 21 | 50%-14 days |
| Omony et al. [71] NDV-178/UG/MU/2011 | 8 | 2 | 10 | 0 | 21 | 20%-14 days |
| Pasnik and Smith [105] (25 ng-i.p) | 88 | 22 | 110 | 0 | 90 | 20%-36 days |
| Pasnik and Smith [105] (25 ng-i.m) | 22 | 88 | 110 | 0 | 90 | 80%-36 days |
| Pasnik and Smith [105] (5 ng-i.m) | 110 | 0 | 110 | 0 | 90 | 0%-36 days |
| Pasnik and Smith [105] (50 ng-i.m) | 11 | 99 | 110 | 0 | 90 | 90%-36 days |
| Ruan et al. 2020 [73] | 0 | 12 | 12 | 0 | 21 | 100%-14 days |
| Sun et al. 2011 [107] | 13 | 40 | 45 | 8 | 60 | 71%-20 days |
| Tan et al. [78] (IBV) | 1 | 9 | 10 | 0 | 21 | 90%-14 days |
| Tan et al. [78] (NDV) | 0 | 10 | 10 | 0 | 21 | 100%-14 days |
| Tu et al. [79] (0 days at 30–35 °C) | 2 | 12 | 15 | 0 | 12 | 86%-14 days |
| Tu et al. [79] (10 days at 30–35 °C) | 0 | 14 | 15 | 0 | 12 | 100%-14 days |
| Tu et al. [79] (17 days at 30–35 °C) | 0 | 15 | 15 | 0 | 12 | 100%-14 days |
| Tu et al. [79] (24 days at 30–35 °C) | 2 | 13 | 15 | 0 | 12 | 87%-14 days |
| Tu et al. [79] (3 days at 30–35 °C) | 0 | 15 | 15 | 0 | 12 | 100%-14 days |
| Tu et al. [79] (eye drop) | 1 | 9 | 10 | 0 | 14 | 90%-14 days |
| Tu et al. [79] (water) | 1 | 9 | 10 | 0 | 14 | 90%-14 days |
| Wambura et al. [81] (feed) | 0 | 15 | 15 | 0 | 35 | 100%-7 days |
| Wambura et al. [81] (wing web stab) | 0 | 15 | 15 | 0 | 35 | 100%-7 days |
| Wang et al. [110] | 9 | 31 | 40 | 0 | 35 | 77%-14 days |
| Xing et al. [112] | 13 | 17 | 26 | 4 | 42 | 50%-15 days |
| Xu et al. [113] | 1 | 29 | 13 | 17 | 60 | 92%-21 days |
| Xu et al. [114] | 14 | 16 | 28 | 2 | 42 | 50%-15 days |
| Yi et al. [116] | 11 | 19 | 30 | 0 | 30 | 63%-20 days |
| Yu et al. [117] (45 ng) | 8 | 22 | 16 | 14 | 15 | 50%-21 days |
| Yu et al. [117] (90 ng) | 4 | 26 | 16 | 14 | 15 | 75%-21 days |
| Zhang et al. [119] (pcDNA-M) | 66 | 34 | 100 | 0 | 28 | 34%-20 days |
| Zhang et al. [119] (SWCNTs-pcDNA-M) | 49 | 51 | 100 | 0 | 28 | 51%-20 days |
| Zhu et al. [120] | 0 | 10 | 1 | 9 | 14 | 100%-10 days |
| Zhu et al. [121] (gavage) | 13 | 17 | 30 | 0 | 21 | 57%-30 days |
| Zhu et al. [121] (mixed fodder) | 11 | 19 | 30 | 0 | 21 | 63%-30 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanelli, A.; Mantegazza, L.; Hendrickx, S.; Capua, I. Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized. Vaccines 2022, 10, 245. https://doi.org/10.3390/vaccines10020245
Fanelli A, Mantegazza L, Hendrickx S, Capua I. Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized. Vaccines. 2022; 10(2):245. https://doi.org/10.3390/vaccines10020245
Chicago/Turabian StyleFanelli, Angela, Luca Mantegazza, Saskia Hendrickx, and Ilaria Capua. 2022. "Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized" Vaccines 10, no. 2: 245. https://doi.org/10.3390/vaccines10020245
APA StyleFanelli, A., Mantegazza, L., Hendrickx, S., & Capua, I. (2022). Thermostable Vaccines in Veterinary Medicine: State of the Art and Opportunities to Be Seized. Vaccines, 10(2), 245. https://doi.org/10.3390/vaccines10020245







