Vaccine Candidate Double Mutant Variants of Enterotoxigenic Escherichia coli Heat-Stable Toxin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction, Expression, and Purification of STh Mutant Peptides
2.2. Mass Spectrometry
2.3. STh Mutant Peptide Antigenicity by Competitive ELISA
2.4. STh Mutant Peptide Toxicity by T84 Cell Assay
2.5. Construction of STh Mutant Peptide-mi3 Nanoparticle Immunogens
2.6. Construction of STh Mutant Peptide BSA Conjugate Immunogens
2.7. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Immunoblots
2.8. Transmission Electron Microscopy (TEM) of mi3-SpyC:SpyT-STh-L9A/A14T Nanoparticles
2.9. Estimation of Hapten-Carrier Ratio by Amino Acid Analysis
2.10. Mouse Immunizations
2.11. Estimation of Serum Antibody Titers
2.12. Neutralization of Native STh in T84 Cell Assay
2.13. Immunological Cross-Reactivity by Competitive ELISA
3. Results and Discussion
3.1. Purification and Characterization of Double Mutant STh Peptides
3.2. Construction and Characterization of STh Mutant Immunogens
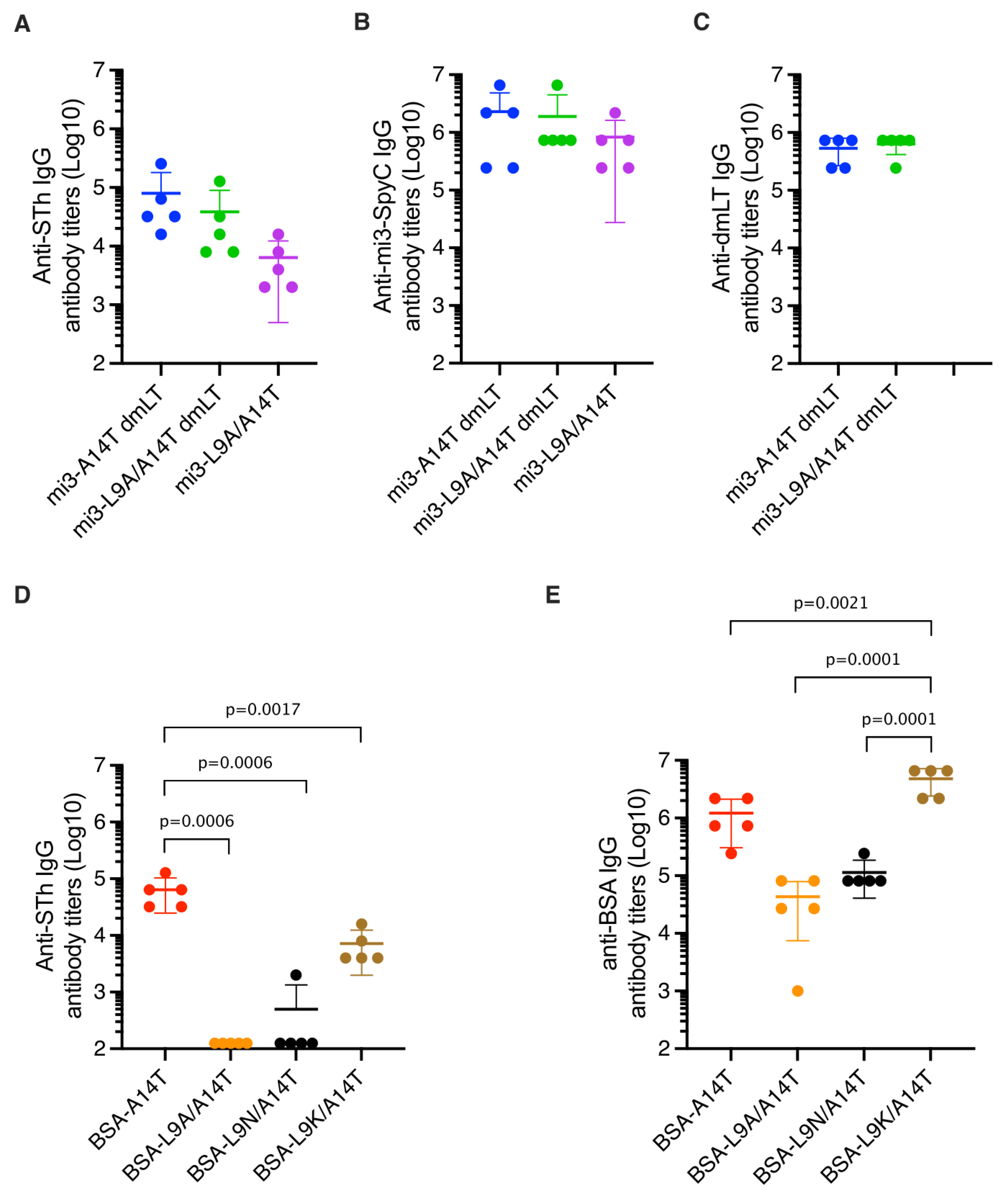
3.3. Immunization of Mice and Estimation of Anti-STh and Anti-Carrier Titers
3.4. Mutant-Specific Antibodies Suggest That dmSTh May Contain Neoepitopes
3.5. Single and Double Mutant STh Immunogens Elicit Toxin-Neutralizing Antibodies
3.6. Immunological Cross-Reactivity to STp, Uroguanylin, and Guanylin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.; Panchalingham, S.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob. Health 2019, 7, e568–e584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.M.; Nasrin, D.; Acácio, S.; Bassat, Q.; Powell, H.; Tennant, S.M.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: Analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet. Glob. Health 2020, 8, e204–e214. [Google Scholar] [CrossRef] [Green Version]
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet. Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef] [Green Version]
- Guerrant, R.L.; DeBoer, M.D.; Moore, S.R.; Scharf, R.J.; Lima, A.A.M. The impoverished gut—A triple burden of diarrhoea, stunting and chronic disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Read, L.T.; Hahn, R.W.; Thompson, C.C.; Bauer, D.L.; Norton, E.B.; Clements, J.D. Simultaneous exposure to Escherichia coli heat-labile and heat-stable enterotoxins increases fluid secretion and alters cyclic nucleotide and cytokine production by intestinal epithelial cells. Infect. Immun. 2014, 82, 5308–5316. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, T.P.V.; Sakellaris, H. Colonization factors of enterotoxigenic Escherichia coli. Adv. Appl. Microbiol. 2015, 90, 155–197. [Google Scholar] [CrossRef]
- Arshad, N.; Visweswariah, S.S. The multiple and enigmatic roles of guanylyl cyclase C in intestinal homeostasis. FEBS Lett. 2012, 586, 2835–2840. [Google Scholar] [CrossRef] [Green Version]
- Hamra, F.K.; Forte, L.R.; Eber, S.L.; Pidhorodeckyj, N.V.; Krause, W.J.; Freeman, R.H.; Chin, D.T.; Tompkins, J.A.; Fok, K.F.; Smith, C.E. Uroguanylin: Structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA 1993, 90, 10464–10468. [Google Scholar] [CrossRef] [Green Version]
- Zegeye, E.D.; Govasli, M.L.; Sommerfelt, H.; Puntervoll, P. Development of an enterotoxigenic Escherichia coli vaccine based on the heat-stable toxin. Hum. Vaccin. Immunother. 2019, 15, 1379–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinsland, H.; Valentiner-Branth, P.; Perch, M.; Dias, F.; Fischer, T.K.; Aaby, P.; Mølbak, K.; Sommerfelt, H. Enterotoxigenic Escherichia coli Infections and Diarrhea in a Cohort of Young Children in Guinea-Bissau. J. Infect. Dis. 2002, 186, 1740–1747. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, H.; Sato, T.; Kubota, H.; Hata, Y.; Katsube, Y.; Shimonishi, Y. Molecular structure of the toxin domain of heat-stable enterotoxin produced by a pathogenic strain of Escherichia coli. A putative binding site for a binding protein on rat intestinal epithelial cell membranes. J. Biol. Chem. 1991, 266, 5934–5941. [Google Scholar] [CrossRef]
- Bourgeois, A.L.; Wierzba, T.F.; Walker, R.I. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 2016, 34, 2880–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harro, C.; Louis Bourgeois, A.; Sack, D.; Walker, R.; DeNearing, B.; Brubaker, J.; Maier, N.; Fix, A.; Dally, L.; Chakraborty, S.; et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 2019, 37, 1978–1986. [Google Scholar] [CrossRef]
- Leach, S.; Lundgren, A.; Carlin, N.; Löfstrand, M.; Svennerholm, A.-M. Cross-reactivity and avidity of antibody responses induced in humans by the oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine ETVAX. Vaccine 2017, 35, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Sahl, J.W.; Sistrunk, J.R.; Baby, N.I.; Begum, Y.; Luo, Q.; Sheikh, A.; Qadri, F.; Fleckenstein, J.M.; Rasko, D.A. Insights into enterotoxigenic Escherichia coli diversity in Bangladesh utilizing genomic epidemiology. Sci. Rep. 2017, 7, 3402. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.D.; Leneghan, D.B.; Brian, I.J.; Ishizuka, A.S.; Bachmann, M.F.; Draper, S.J.; Biswas, S.; Howarth, M. Plug-and-Display: Decoration of Virus-Like Particles via isopeptide bonds for modular immunization. Sci. Rep. 2016, 6, 19234. [Google Scholar] [CrossRef] [Green Version]
- Govasli, M.L.; Diaz, Y.; Puntervoll, P. Virus-like particle-display of the enterotoxigenic Escherichia coli heat-stable toxoid STh-A14T elicits neutralizing antibodies in mice. Vaccine 2019, 37, 6405–6414. [Google Scholar] [CrossRef]
- Bruun, T.U.J.; Andersson, A.-M.C.; Draper, S.J.; Howarth, M. Engineering a Rugged Nanoscaffold To Enhance Plug-and-Display Vaccination. ACS Nano 2018, 12, 8855–8866. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.K.; Rijal, P.; Rahikainen, R.; Keeble, A.H.; Schimanski, L.; Hussain, S.; Harvey, R.; Hayes, J.W.P.; Edwards, J.C.; McLean, R.K.; et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. Nat. Commun. 2021, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Taxt, A.M.; Diaz, Y.; Aasland, R.; Clements, J.D.; Nataro, J.P.; Sommerfelt, H.; Puntervoll, P. Towards Rational Design of a Toxoid Vaccine against the Heat-Stable Toxin of Escherichia coli. Infect. Immun. 2016, 84, 1239–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandre, R.; Ruan, X.; Duan, Q.; Zhang, W. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTa N12S -dmLT induces neutralizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol. Lett. 2016, 363, fnw246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandre, R.M.; Duan, Q.; Wang, Y.; Zhang, W. Passive antibodies derived from intramuscularly immunized toxoid fusion 3xSTaN12S-dmLT protect against STa+ enterotoxigenic Escherichia coli (ETEC) diarrhea in a pig model. Vaccine 2017, 35, 552–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govasli, M.L.; Diaz, Y.; Zegeye, E.D.; Darbakk, C.; Taxt, A.M.; Puntervoll, P. Purification and Characterization of Native and Vaccine Candidate Mutant Enterotoxigenic Escherichia coli Heat-Stable Toxins. Toxins 2018, 10, 274. [Google Scholar] [CrossRef] [Green Version]
- Taxt, A.M.; Diaz, Y.; Bacle, A.; Grauffel, C.; Reuter, N.; Aasland, R.; Sommerfelt, H.; Puntervoll, P. Characterization of immunological cross-reactivity between enterotoxigenic Escherichia coli heat-stable toxin and human guanylin and uroguanylin. Infect. Immun. 2014, 82, 2913–2922. [Google Scholar] [CrossRef] [Green Version]
- Diaz, Y.; Govasli, M.L.; Zegeye, E.D.; Sommerfelt, H.; Steinsland, H.; Puntervoll, P. Immunizations with Enterotoxigenic Escherichia coli Heat-Stable Toxin Conjugates Engender Toxin-Neutralizing Antibodies in Mice That Also Cross-React with Guanylin and Uroguanylin. Infect. Immun. 2019, 87, e00099-19. [Google Scholar] [CrossRef] [Green Version]
- Bogomolovas, J.; Simon, B.; Sattler, M.; Stier, G. Screening of fusion partners for high yield expression and purification of bioactive viscotoxins. Protein Expr. Purif. 2009, 64, 16–23. [Google Scholar] [CrossRef]
- Aida, Y.; Pabst, M.J. Removal of endotoxin from protein solutions by phase separation using Triton X. J. Immunol. Methods 1990, 132, 191–195. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Chromatographic determination of amino acids by the use of automatic recording equipment. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 1963; pp. 819–831. [Google Scholar]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.; Garcia, C.; Ruan, X.; Duan, Q.; Sack, D.A.; Zhang, W. Preclinical Characterization of Immunogenicity and Efficacy against Diarrhea from MecVax, a Multivalent Enterotoxigenic E. coli Vaccine Candidate. Infect. Immun. 2021, 89, e00106-21. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Huang, J.; Xiao, N.; Seo, H.; Zhang, W. Neutralizing Anti-Heat-Stable Toxin (STa) Antibodies Derived from Enterotoxigenic Escherichia coli Toxoid Fusions with STa Proteins Containing N12S, L9A/N12S, or N12S/A14T Mutations Show Little Cross-Reactivity with Guanylin or Uroguanylin. Appl. Environ. Microbiol. 2018, 84, e01737-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Name | Sequence (5’-3’) |
|---|---|
| L9A/A14T-f | tgtaatcctacatgtaccgggtgctattaaGGCGCCATGGGCAAAGTG |
| L9A/A14T-r | acatgcttcacagcagtaattgctactattCTGAAAATAAAGATTCTCGCTACCCG |
| L9N/A14T-f | tgtaatcctacatgtaccgggtgctattaaGGCGCCATGGGCAAAGTG |
| L9N/A14T-r | acagttttcacagcagtaattgctactattCTGAAAATAAAGATTCTCGCTACCCG |
| L9K/A14T-f | tgtaatcctacctgtaccgggtgctattaaGGCGCCATGGGCAAAGTG |
| L9K/A14T-r | acatttttcacagcagtaattgctactattCTGAAAATAAAGATTCTCGCTACCCG |
| SpyL9A/A14T-f | ctgctgtgaagcgtgttgtaatcc |
| SpyL9A/A14T-r | taattgctactattACCGG |
| Plasmid | Parent Plasmid | Forward Primer | Reverse Primer | Tag | STh Variant | Ref |
|---|---|---|---|---|---|---|
| pETDsbCin_1b | - | - | - | - | - | [28] |
| pET-DsbC-STh-A14T | pETDsbCin_1b | - | - | - | STh-A14T | [25] |
| pET-DsbC-STh-L9A/A14T | pETDsbCin_1b | L9A/A14T-f | L9A/A14T-r | - | STh-L9A/A14T | - |
| pET-DsbC-STh-L9N/A14T | pETDsbCin_1b | L9N/A14T-f | L9N/A14T-r | - | STh-L9N/A14T | - |
| pET-DsbC-STh-L9K/A14T | pETDsbCin_1b | L9K/A14T-f | L9K/A14T-r | - | STh-L9K/A14T | - |
| pET-DsbC-SpyT-A14T | pET-DsbC-STh-A14T | - | - | SpyTag | SpyT-STh-A14T | [19] |
| pET-DsbC-SpyT-L9A/A14T | pET-DsbC-SpyT-A14T | SpyL9A/A14T-f | SpyL9A/A14T-r | SpyTag | SpyT-STh-L9A/A14T | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zegeye, E.D.; Diaz, Y.; Puntervoll, P. Vaccine Candidate Double Mutant Variants of Enterotoxigenic Escherichia coli Heat-Stable Toxin. Vaccines 2022, 10, 241. https://doi.org/10.3390/vaccines10020241
Zegeye ED, Diaz Y, Puntervoll P. Vaccine Candidate Double Mutant Variants of Enterotoxigenic Escherichia coli Heat-Stable Toxin. Vaccines. 2022; 10(2):241. https://doi.org/10.3390/vaccines10020241
Chicago/Turabian StyleZegeye, Ephrem Debebe, Yuleima Diaz, and Pål Puntervoll. 2022. "Vaccine Candidate Double Mutant Variants of Enterotoxigenic Escherichia coli Heat-Stable Toxin" Vaccines 10, no. 2: 241. https://doi.org/10.3390/vaccines10020241
APA StyleZegeye, E. D., Diaz, Y., & Puntervoll, P. (2022). Vaccine Candidate Double Mutant Variants of Enterotoxigenic Escherichia coli Heat-Stable Toxin. Vaccines, 10(2), 241. https://doi.org/10.3390/vaccines10020241






