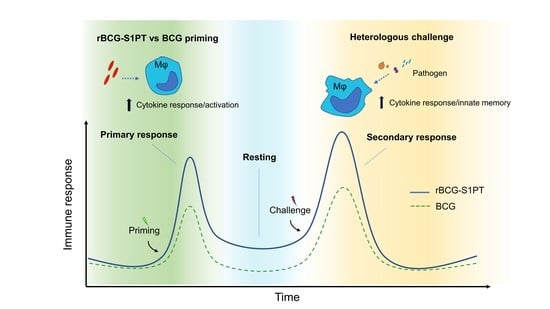
Recombinant BCG Expressing the Subunit 1 of Pertussis Toxin Induces Innate Immune Memory and Confers Protection against Non-Related Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Mice and Ethics Statement
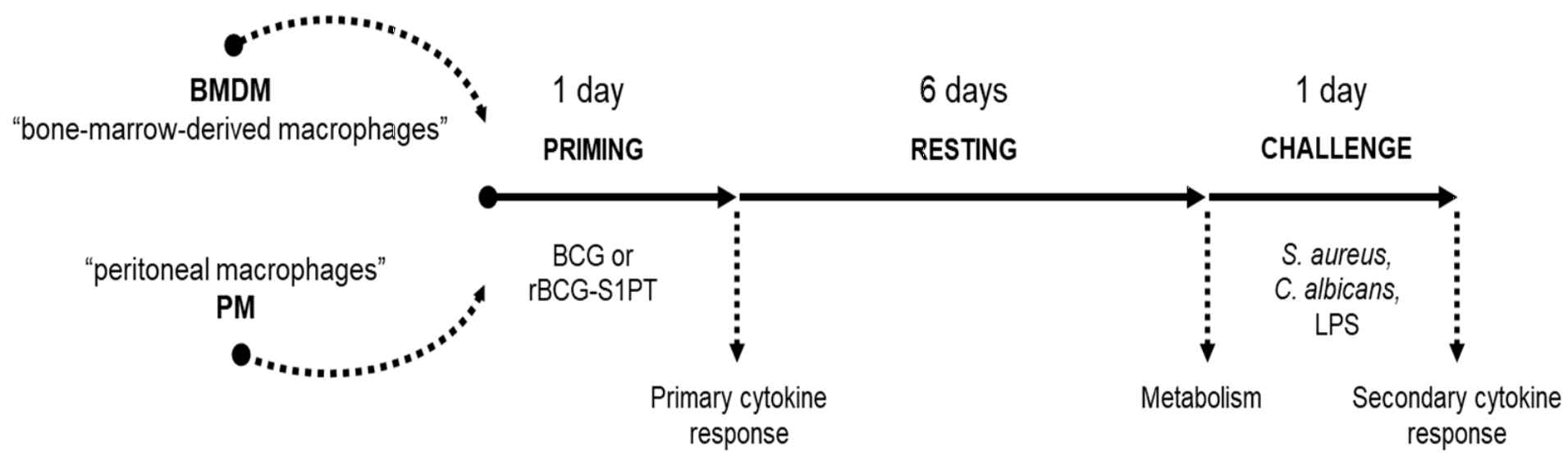
2.3. Bone Marrow Extraction and Macrophage Differentiation
2.4. Peritoneal Cells Collection
2.5. Priming and Challenge of Macrophages with Heterologous Pathogens
2.6. Analysis of Cell Viability
2.7. Metabolism Analysis
2.8. Cytokine Production
2.9. In Vivo Generation of Innate Memory in Immunocompetent Mice
2.10. In Vivo Challenge with C. albicans of Immunodeficient SCID Mice
2.11. Statistical Analysis
3. Results
3.1. In Vitro Model for Examining the Innate Memory Induced by rBCG-S1PT
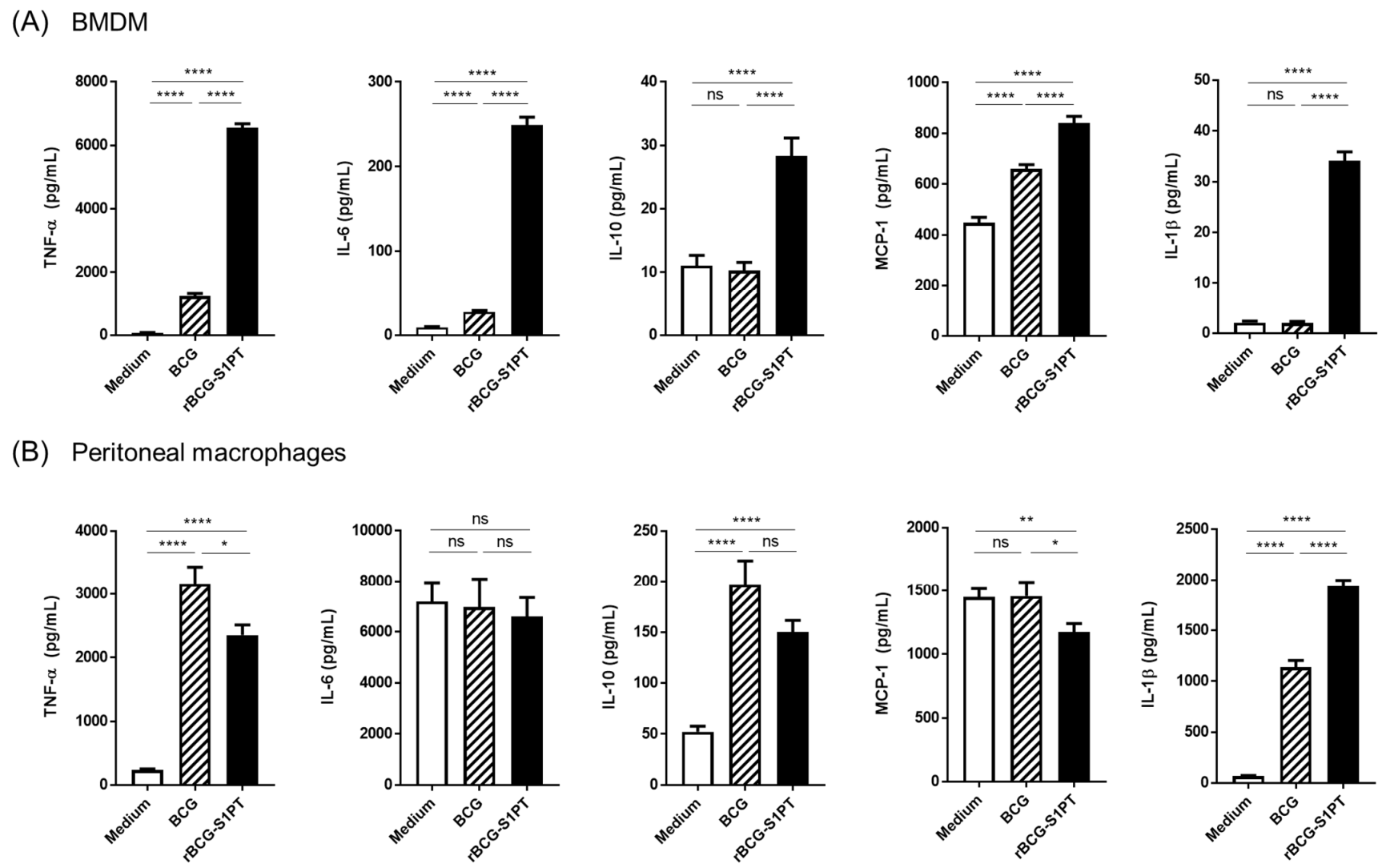
3.2. Primary Response of BMDM and PM to rBCG-S1PT
3.3. Priming with rBCG-S1PT Promotes a Higher Inflammatory Response to Challenge
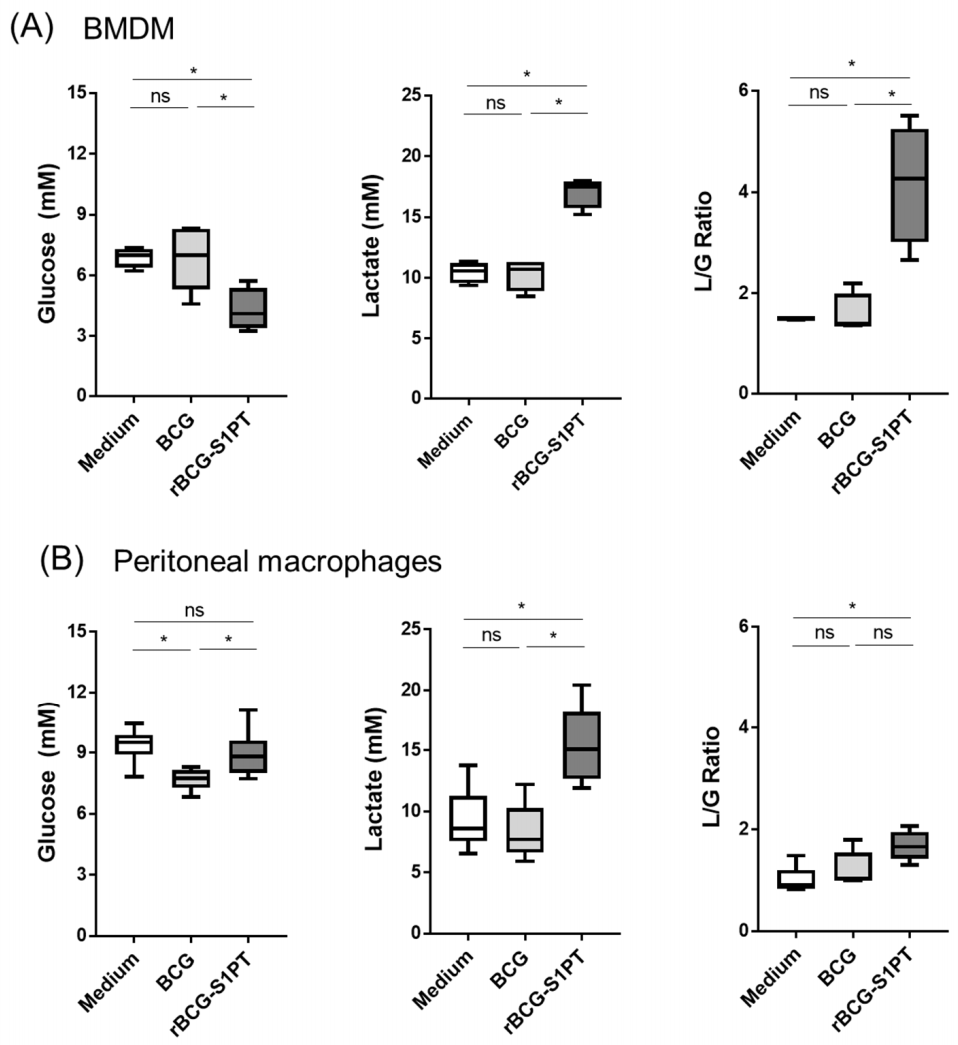
3.4. rBCG-S1PT Induces Metabolic Changes in Memory Macrophages
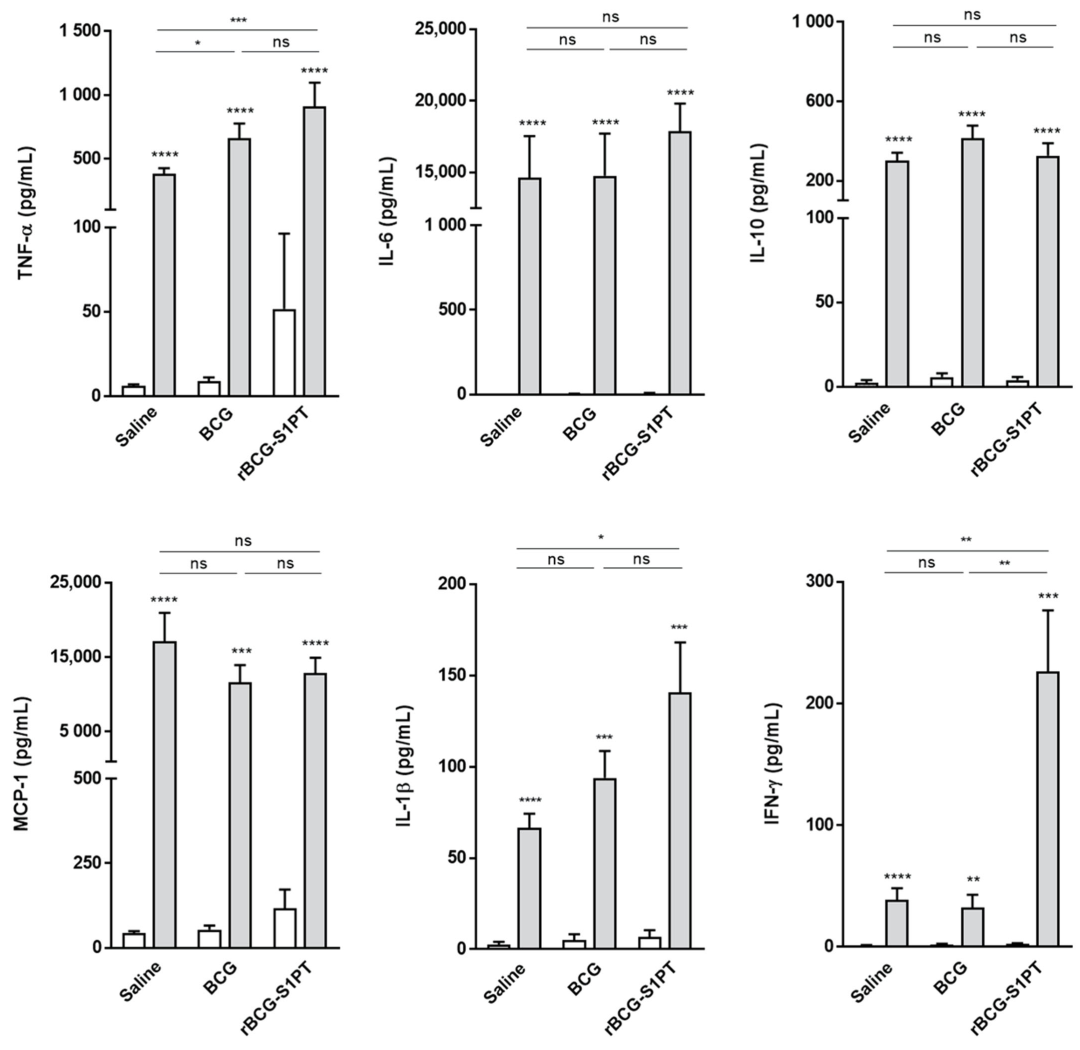
3.5. rBCG-S1PT Induces an Increased Cytokine Response upon LPS Challenge In Vivo
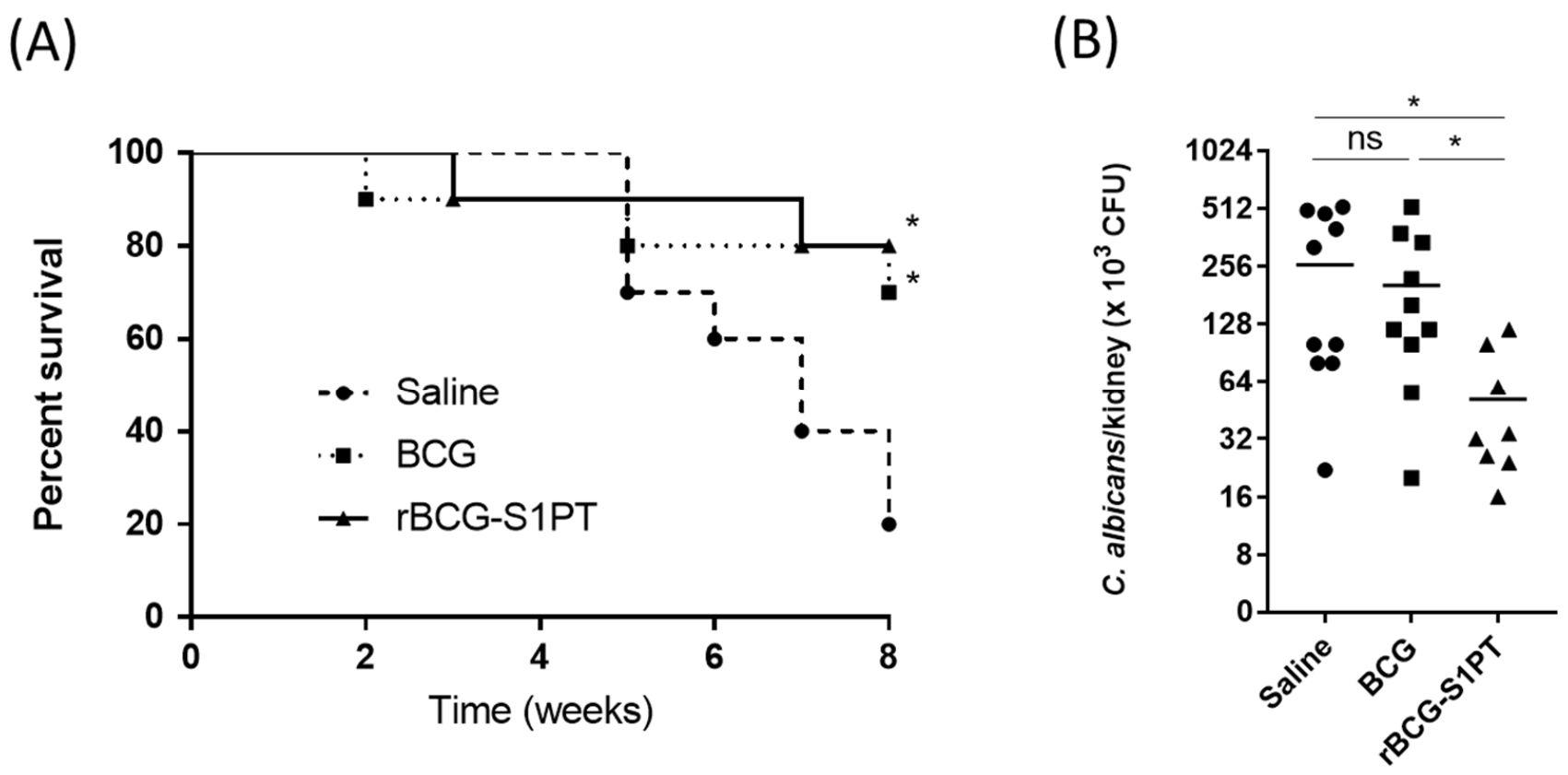
3.6. Previous Immunization with rBCG-S1PT Increases Protection against C. albicans Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.E.; Netea, M.G.; Mandalakas, A.M. 100 years of Mycobacterium bovis bacille Calmette-Guerin. Lancet Infect. Dis. 2021, 22, e2–e12. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Dias, W.O.; Mazzantini, R.P.; Miyaji, E.N.; Gamberini, M.; Quintilio, W.; Gebara, V.C.; Cardoso, D.F.; Ho, P.L.; Raw, I.; et al. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect. Immun. 2000, 68, 4877–4883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, I.P.; Dias, W.O.; Quintilio, W.; Christ, A.P.; Moraes, J.F.; Vancetto, M.D.; Ribeiro-Dos-Santos, G.; Raw, I.; Leite, L.C. Neonatal immunization with a single dose of recombinant BCG expressing subunit S1 from pertussis toxin induces complete protection against Bordetella pertussis intracerebral challenge. Microbes Infect. 2008, 10, 198–202. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Dias, W.O.; Quintilio, W.; Hsu, T.; Jacobs, W.R., Jr.; Leite, L.C. Construction of an unmarked recombinant BCG expressing a pertussis antigen by auxotrophic complementation: Protection against Bordetella pertussis challenge in neonates. Vaccine 2009, 27, 7346–7351. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.M.; Chade, D.C.; Borra, R.C.; Nascimento, I.P.; Villanova, F.E.; Leite, L.C.; Andrade, E.; Srougi, M. The therapeutic potential of recombinant BCG expressing the antigen S1PT in the intravesical treatment of bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2010, 28, 520–525. [Google Scholar] [CrossRef]
- Chade, D.C.; Borra, R.C.; Nascimento, I.P.; Villanova, F.E.; Leite, L.C.; Andrade, E.; Srougi, M.; Ramos, K.L.; Andrade, P.M. Immunomodulatory effects of recombinant BCG expressing pertussis toxin on TNF-alpha and IL-10 in a bladder cancer model. J. Exp. Clin. Cancer Res. 2008, 27, 78. [Google Scholar] [CrossRef] [Green Version]
- Christ, A.P.; Rodriguez, D.; Bortolatto, J.; Borducchi, E.; Keller, A.; Mucida, D.; Silva, J.S.; Leite, L.C.; Russo, M. Enhancement of Th1 lung immunity induced by recombinant Mycobacterium bovis Bacillus Calmette-Guerin attenuates airway allergic disease. Am. J. Respir. Cell Mol. Biol. 2010, 43, 243–252. [Google Scholar] [CrossRef]
- Rodriguez, D.; Goulart, C.; Pagliarone, A.C.; Silva, E.P.; Cunegundes, P.S.; Nascimento, I.P.; Borra, R.C.; Dias, W.O.; Tagliabue, A.; Boraschi, D.; et al. In vitro Evidence of Human Immune Responsiveness Shows the Improved Potential of a Recombinant BCG Strain for Bladder Cancer Treatment. Front. Immunol. 2019, 10, 1460. [Google Scholar] [CrossRef]
- Marques-Neto, L.M.; Piwowarska, Z.; Kanno, A.I.; Moraes, L.; Trentini, M.M.; Rodriguez, D.; Silva, J.; Leite, L.C.C. Thirty years of recombinant BCG: New trends for a centenary vaccine. Expert Rev. Vaccines 2021, 20, 1001–1011. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P. Innate Immune Memory: Time for Adopting a Correct Terminology. Front. Immunol. 2018, 9, 799. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarancon, R.; Dominguez-Andres, J.; Uranga, S.; Ferreira, A.V.; Groh, L.A.; Domenech, M.; Gonzalez-Camacho, F.; Riksen, N.P.; Aguilo, N.; Yuste, J.; et al. New live attenuated tuberculosis vaccine MTBVAC induces trained immunity and confers protection against experimental lethal pneumonia. PLoS Pathog. 2020, 16, e1008404. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.C.; Ganguly, R.; Waldman, R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guerin. J. Infect. Dis. 1977, 136, 171–175. [Google Scholar] [CrossRef]
- De Bree, L.C.J.; Marijnissen, R.J.; Kel, J.M.; Rosendahl Huber, S.K.; Aaby, P.; Benn, C.S.; Wijnands, M.V.W.; Diavatopoulos, D.A.; van Crevel, R.; Joosten, L.A.B.; et al. Bacillus Calmette-Guerin-Induced Trained Immunity Is Not Protective for Experimental Influenza A/Anhui/1/2013 (H7N9) Infection in Mice. Front. Immunol. 2018, 9, 869. [Google Scholar] [CrossRef]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Covian, C.; Fernandez-Fierro, A.; Retamal-Diaz, A.; Diaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; Gonzalez, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Barroso de Figueiredo, A.M.; Teodoro Silva, M.V.; Cirovic, B.; de Bree, L.C.J.; Damen, M.; Moorlag, S.; Gomes, R.S.; Helsen, M.M.; Oosting, M.; et al. beta-Glucan-Induced Trained Immunity Protects against Leishmania braziliensis Infection: A Crucial Role for IL-32. Cell Rep. 2019, 28, 2659–2672.e6. [Google Scholar] [CrossRef] [Green Version]
- Italiani, P.; Boraschi, D. Induction of Innate Immune Memory by Engineered Nanoparticles: A Hypothesis that May Become True. Front. Immunol. 2017, 8, 734. [Google Scholar] [CrossRef] [Green Version]
- Seeley, J.J.; Ghosh, S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef]
- WHO. SAGE Meeting of April 2014. Session: Non-Specific Effects of Vaccines on Mortality in Children under 5 Years of Age. 2014. Available online: https://www.who.int/immunization/sage/meetings/2014/april/presentations_background_docs/en/index1.html (accessed on 14 January 2021).
- Basu, J.; Shin, D.M.; Jo, E.K. Mycobacterial signaling through toll-like receptors. Front. Cell Infect. Microbiol. 2012, 2, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dockrell, H.M.; Smith, S.G. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front. Immunol. 2017, 8, 1134. [Google Scholar] [CrossRef] [PubMed]
- Bickett, T.E.; McLean, J.; Creissen, E.; Izzo, L.; Hagan, C.; Izzo, A.J.; Silva Angulo, F.; Izzo, A.A. Characterizing the BCG Induced Macrophage and Neutrophil Mechanisms for Defense Against Mycobacterium tuberculosis. Front. Immunol. 2020, 11, 1202. [Google Scholar] [CrossRef]
- Cooper, D.; Eleftherianos, I. Memory and Specificity in the Insect Immune System: Current Perspectives and Future Challenges. Front. Immunol. 2017, 8, 539. [Google Scholar] [CrossRef]
- Coustau, C.; Kurtz, J.; Moret, Y. A Novel Mechanism of Immune Memory Unveiled at the Invertebrate-Parasite Interface. Trends Parasitol. 2016, 32, 353–355. [Google Scholar] [CrossRef]
- Melillo, D.; Marino, R.; Italiani, P.; Boraschi, D. Innate Immune Memory in Invertebrate Metazoans: A Critical Appraisal. Front. Immunol. 2018, 9, 1915. [Google Scholar] [CrossRef]
- Milutinovic, B.; Kurtz, J. Immune memory in invertebrates. Semin. Immunol. 2016, 28, 328–342. [Google Scholar] [CrossRef]
- Pradeu, T.; Du Pasquier, L. Immunological memory: What’s in a name? Immunol. Rev. 2018, 283, 7–20. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Carvalho, A.; La Rocca, C.; Palma, C.; Rodrigues, F.; Silvestre, R.; Kleinnijenhuis, J.; Lachmandas, E.; Goncalves, L.G.; Belinha, A.; et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [Green Version]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Subramaniam, R.; Chen, H.; Smith, A.; Keshava, S.; Shams, H. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS ONE 2017, 12, e0180143. [Google Scholar] [CrossRef]
- Ifrim, D.C.; Quintin, J.; Joosten, L.A.; Jacobs, C.; Jansen, T.; Jacobs, L.; Gow, N.A.; Williams, D.L.; van der Meer, J.W.; Netea, M.G. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vaccine Immunol. 2014, 21, 534–545. [Google Scholar] [CrossRef] [Green Version]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef]
- Aguilo, N.; Uranga, S.; Marinova, D.; Monzon, M.; Badiola, J.; Martin, C. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis 2016, 96, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.D.; Sibley, L.; Sarfas, C.; Morrison, A.; Gullick, J.; Clark, S.; Gleeson, F.; McIntyre, A.; Arlehamn, C.L.; Sette, A.; et al. MTBVAC vaccination protects rhesus macaques against aerosol challenge with M. tuberculosis and induces immune signatures analogous to those observed in clinical studies. NPJ Vaccines 2021, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Broset, E.; Pardo-Seco, J.; Kanno, A.I.; Aguilo, N.; Dacosta, A.I.; Rivero-Calle, I.; Gonzalo-Asensio, J.; Locht, C.; Leite, L.C.C.; Martin, C.; et al. BCG vaccination improves DTaP immune responses in mice and is associated with lower pertussis incidence in ecological epidemiological studies. EBioMedicine 2021, 65, 103254. [Google Scholar] [CrossRef]
- Vierboom, M.P.M.; Dijkman, K.; Sombroek, C.C.; Hofman, S.O.; Boot, C.; Vervenne, R.A.W.; Haanstra, K.G.; van der Sande, M.; van Emst, L.; Dominguez-Andres, J.; et al. Stronger induction of trained immunity by mucosal BCG or MTBVAC vaccination compared to standard intradermal vaccination. Cell Rep. Med. 2021, 2, 100185. [Google Scholar] [CrossRef]
- Brooks, N.A.; Puri, A.; Garg, S.; Nag, S.; Corbo, J.; Turabi, A.E.; Kaka, N.; Zemmel, R.W.; Hegarty, P.K.; Kamat, A.M. The association of Coronavirus Disease-19 mortality and prior bacille Calmette-Guerin vaccination: A robust ecological analysis using unsupervised machine learning. Sci. Rep. 2021, 11, 774. [Google Scholar] [CrossRef]
- Levi, M.; Miglietta, A.; Romeo, G.; Bartolacci, S.; Ariani, F.; Cipriani, F.; De Filippo, C.; Cavalieri, D.; Balzi, D. Letter in response to article in journal of infection: Impact of routine infant BCG vaccination on COVID-19. J Infect. 2021, 82, e41–e43. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.N.; Ebinger, J.E.; Wu, M.; Sun, N.; Braun, J.; Sobhani, K.; Van Eyk, J.E.; Cheng, S.; Arditi, M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021, 131, e145157. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanno, A.I.; Boraschi, D.; Leite, L.C.C.; Rodriguez, D. Recombinant BCG Expressing the Subunit 1 of Pertussis Toxin Induces Innate Immune Memory and Confers Protection against Non-Related Pathogens. Vaccines 2022, 10, 234. https://doi.org/10.3390/vaccines10020234
Kanno AI, Boraschi D, Leite LCC, Rodriguez D. Recombinant BCG Expressing the Subunit 1 of Pertussis Toxin Induces Innate Immune Memory and Confers Protection against Non-Related Pathogens. Vaccines. 2022; 10(2):234. https://doi.org/10.3390/vaccines10020234
Chicago/Turabian StyleKanno, Alex I., Diana Boraschi, Luciana C. C. Leite, and Dunia Rodriguez. 2022. "Recombinant BCG Expressing the Subunit 1 of Pertussis Toxin Induces Innate Immune Memory and Confers Protection against Non-Related Pathogens" Vaccines 10, no. 2: 234. https://doi.org/10.3390/vaccines10020234
APA StyleKanno, A. I., Boraschi, D., Leite, L. C. C., & Rodriguez, D. (2022). Recombinant BCG Expressing the Subunit 1 of Pertussis Toxin Induces Innate Immune Memory and Confers Protection against Non-Related Pathogens. Vaccines, 10(2), 234. https://doi.org/10.3390/vaccines10020234