Immunogenicity of Multi-Target Chimeric RHDV Virus-Like Particles Delivering Foreign B-Cell Epitopes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptides, Virus, Cells, and Mice
2.2. Generation of Recombinant Baculovirus Transfer Vectors
2.3. Construction of Recombinant Baculoviruses
2.4. Expression and Purification of Chimeric RHDV VLPs
2.5. Western Blot Analyses
2.6. Transmission Electron Microscopy
2.7. Mice Immunization
2.8. Detection of Specific Antibodies by ELISA
2.9. Indirect Immunofluorescence Assays
2.10. Statistical Analysis
2.11. Ethics Statement
3. Results
3.1. Design, Generation, and Characterization of Chimeric RHDV VLPs Displaying FCV and CPV Target Epitopes
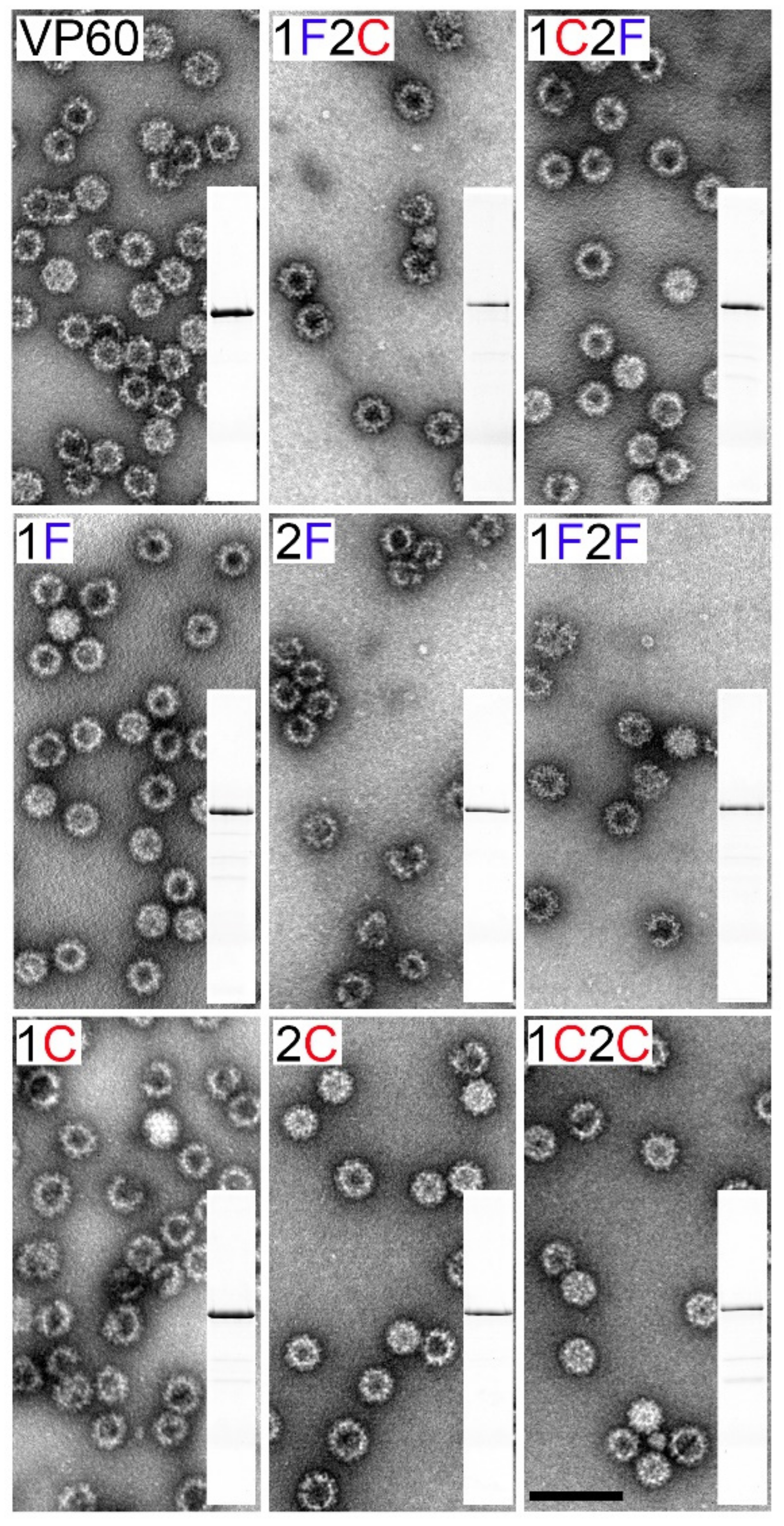
3.2. Electron Microscopy Analysis of the Chimeric RHDV VLPs
3.3. Humoral Immune Responses Elicited by Chimeric VLPs Displaying FCV and CPV B-Cell Epitopes in Mice
3.4. Evaluation of Sera Reactivity with FCV and CPV Viruses by Immunofluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chackerian, B.; Peabody, D.S. Factors That Govern the Induction of Long-Lived Antibody Responses. Viruses 2020, 12, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsen, M.O.; Augusto, G.; Bachmann, M.F. The 3Ds in Virus-Like Particle Based-Vaccines: Design, Delivery and Dynamics. Immunol. Rev. 2020, 296, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 1100. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Crisci, E.; Bárcena, J.; Montoya, M. Virus-Like Particles: The New Frontier of Vaccines for Animal Viral Infections. Veter.-Immunol. Immunopathol. 2012, 148, 211–225. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major Findings and Recent Advances in Virus–Like Particle (VLP)-Based Vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Qian, C.; Liu, X.; Xu, Q.; Wang, Z.; Chen, J.; Li, T.; Zheng, Q.; Yu, H.; Gu, Y.; Li, S.; et al. Recent Progress on the Versatility of Virus-Like Particles. Vaccines 2020, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Charlton Hume, H.K.; Vidigal, J.; Carrondo, M.J.T.; Middelberg, A.P.J.; Roldão, A.; Lua, L.H.L. Synthetic Biology for Bioengineering Virus-Like Particle Vaccines. Biotechnol. Bioeng. 2019, 116, 919–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Jiang, X. Subviral Particle as Vaccine and Vaccine Platform. Curr. Opin. Virol. 2014, 6, 24–33. [Google Scholar] [CrossRef]
- Wen, A.M.; Steinmetz, N.F. Design of Virus-Based Nanomaterials for Medicine, Biotechnology, and Energy. Chem. Soc. Rev. 2016, 45, 4074–4126. [Google Scholar] [CrossRef] [Green Version]
- Crisci, E.; Almanza, H.; Mena, I.; Córdoba, L.; Gomez-Casado, E.; Castón, J.; Fraile, L.; Barcena, J.; Montoya, M. Chimeric CaliciVirus-Like Particles Elicit Protective Anti-Viral Cytotoxic Responses without Adjuvant. Virology 2009, 387, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, N.; Mena, I.; Angulo, I.; Gómez, Y.; Crisci, E.; Montoya, M.; Caston, J.; Blanco, E.; Bárcena, J. Rabbit Hemorrhagic Disease Virus Capsid, a Versatile Platform for Foreign B-Cell Epitope Display Inducing Protective Humoral Immune Responses. Sci. Rep. 2016, 6, 31844. [Google Scholar] [CrossRef] [PubMed]
- Rangel, G.; Bárcena, J.; Moreno, N.; Mata, C.; Castón, J.; Alejo, A.; Blanco, E. Chimeric RHDV Virus-Like Particles Displaying Foot-and-Mouth Disease Virus Epitopes Elicit Neutralizing Antibodies and Confer Partial Protection in Pigs. Vaccines 2021, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Young, V.; Donaldson, B.; Woodall, M.; Shields, N.; Walker, G.; Ward, V.; Young, S. Delivering Two Tumour Antigens Survivin and Mucin-1 on Virus-Like Particles Enhances Anti-Tumour Immune Responses. Vaccines 2021, 9, 463. [Google Scholar] [CrossRef]
- Donaldson, B.; Al-Barwani, F.; Pelham, S.J.; Young, K.; Ward, V.K.; Young, S.L. Multi-Target Chimaeric VLP as a Therapeutic Vaccine in a Model of Colorectal Cancer. J. Immunother. Cancer 2017, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Peacey, M.; Wilson, S.; Baird, M.A.; Ward, V.K. Versatile RHDV Virus-Like Particles: Incorporation of Antigens by Genetic Modification and Chemical Conjugation. Biotechnol. Bioeng. 2007, 98, 968–977. [Google Scholar] [CrossRef]
- Henning, J.; Meers, J.; Davies, P.R.; Morris, R.S. Survival of Rabbit Haemorrhagic Disease Virus (RHDV) in the Environment. Epidemiol. Infect. 2005, 133, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Dalton, K.P.; Alvarado, C.; Reytor, E.; Nuñez, M.D.C.; Podadera, A.; Martínez-Alonso, D.; Alonso, J.M.M.; Nicieza, I.; Gómez-Sebastián, S.; Dalton, R.M.; et al. Chimeric VLPs Bearing VP60 from Two Serotypes of Rabbit Haemorrhagic Disease Virus Are Protective against Both Viruses. Vaccines 2021, 9, 1005. [Google Scholar] [CrossRef]
- Da Silva, D.M.; Pastrana, D.V.; Schiller, J.T.; Kast, W. Effect of Preexisting Neutralizing Antibodies on the Anti-Tumor Immune Response Induced by Chimeric Human Papillomavirus Virus-Like Particle Vaccines. Virology 2001, 290, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Jegerlehner, A.; Wiesel, M.; Dietmeier, K.; Zabel, F.; Gatto, D.; Saudan, P.; Bachmann, M.F. Carrier Induced Epitopic Suppression of Antibody Responses Induced by Virus-Like Particles Is a Dynamic Phenomenon Caused by Carrier-Specific Antibodies. Vaccine 2010, 28, 5503–5512. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Liu, J.; Gao, B.; Liu, Y.; Zhai, Y.; Ma, J.; Zhang, K.; Baker, T.S.; Schulten, K.; et al. Atomic Model of Rabbit Hemorrhagic Disease Virus by Cryo-Electron Microscopy and Crystallography. PLoS Pathog. 2013, 9, e1003132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuthold, M.M.; Dalton, K.P.; Hansman, G.S. Structural Analysis of a Rabbit Hemorrhagic Disease Virus Binding to Histo-Blood Group Antigens. J. Virol. 2014, 89, 2378–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouco, C.; Abrantes, J.; Serronha, A.; Lopes, A.; Maio, E.; Magalhães, M.J.; Blanco, E.; Barcena, J.; Esteves, P.; Santos, N.; et al. Epidemiology of RHDV2 (Lagovirus europaeus/GI.2) in Free-Living Wild European Rabbits in Portugal. Transbound. Emerg. Dis. 2017, 65, e373–e382. [Google Scholar] [CrossRef]
- Bárcena, J.; Guerra, B.; Angulo, I.; González, J.; Valcárcel, F.; Mata, C.P.; Castón, J.R.; Blanco, E.; Alejo, A. Comparative Analysis of Rabbit Hemorrhagic Disease Virus (RHDV) and New RHDV2 Virus Antigenicity, Using Specific Virus-Like Particles. Veter. Res. 2015, 46, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque, D.; González, J.M.; Gómez-Blanco, J.; Marabini, R.; Chichón, J.; Mena, I.; Angulo, I.; Carrascosa, J.L.; Verdaguer, N.; Trus, B.L.; et al. Epitope Insertion at the N-Terminal Molecular Switch of the Rabbit Hemorrhagic Disease Virus T=3 Capsid Protein Leads to Larger T=4 Capsids. J. Virol. 2012, 86, 6470–6480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcena, J.; Verdaguer, N.; Roca, R.; Morales, M.; Angulo, I.; Risco, C.; Carrascosa, J.L.; Torres, J.M.; Castón, J.R. The Coat Protein of Rabbit Hemorrhagic Disease Virus Contains a Molecular Switch at the N-Terminal Region Facing the Inner Surface of the Capsid. Virology 2004, 322, 118–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubillos-Zapata, C.; Angulo, I.; Almanza, H.; Borrego, B.; Zamora-Ceballos, M.; Castón, J.R.; Mena, I.; Blanco, E.; Bárcena, J. Precise Location of Linear Epitopes on the Capsid Surface of Feline Calicivirus Recognized by Neutralizing and Non-Neutralizing Monoclonal Antibodies. Veter. Res. 2020, 51, 1–8. [Google Scholar] [CrossRef]
- Turiso, J.A.L.D.; Cortes, E.; Ranz, A.; Garcia, J.; Sanz, A.; Vela, C.; Casal, J.I. Fine Mapping of Canine Parvovirus B Cell Epitopes. J. Gen. Virol. 1991, 72, 2445–2456. [Google Scholar] [CrossRef]
- Casal, J.I.; Langeveld, J.P.; Cortés, E.; Schaaper, W.W.; van Dijk, E.; Vela, C.; Kamstrup, S.; Meloen, R.H. Peptide Vaccine against Canine Parvovirus: Identification of Two Neutralization Subsites in the N Terminus of VP2 and Optimization of the Amino Acid Sequence. J. Virol. 1995, 69, 7274–7277. [Google Scholar] [CrossRef] [Green Version]
- Dalsgaard, K.; Uttenthal, Å.; Jones, T.D.; Xu, F.; Merryweather, A.; Hamilton, W.D.; Langeveld, J.P.; Boshuizen, R.S.; Kamstrup, S.; Lomonossoff, G.P.; et al. Plant–Derived Vaccine Protects Target Animals against a Viral Disease. Nat. Biotechnol. 1997, 15, 248–252. [Google Scholar] [CrossRef]
- Koudelka, K.J.; Pitek, A.S.; Manchester, M.; Steinmetz, N.F. Virus-Based Nanoparticles as Versatile Nanomachines. Annu. Rev. Virol. 2015, 2, 379–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, B.; Douglas, T. Development of Virus-Like Particles for Diagnostic and Prophylactic Biomedical Applications. WIREs Nanomed. Nanobiotechnol. 2015, 7, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of Nanotechnology for Immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Mateu, M.G. Assembly, Engineering and Applications of Virus-Based Protein Nanoparticles. Adv. Exp. Med. Biol. 2016, 940, 83–120. [Google Scholar] [CrossRef]
- Gomes, A.C.; Mohsen, M.; Bachmann, M.F. Harnessing Nanoparticles for Immunomodulation and Vaccines. Vaccines 2017, 5, 6. [Google Scholar] [CrossRef]
- Plummer, E.M.; Manchester, M. Viral Nanoparticles and Virus-Like Particles: Platforms for Contemporary Vaccine Design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 174–196. [Google Scholar] [CrossRef]
- Frietze, K.M.; Peabody, D.S.; Chackerian, B. Engineering Virus-Like Particles as Vaccine Platforms. Curr. Opin. Virol. 2016, 18, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Liu, X.S.; Zhao, K.N.; Leggatt, G.; Frazer, I. Papillomavirus Virus-Like Particles for the Delivery of Multiple Cytotoxic T Cell Epitopes. Virology 2000, 273, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Sominskaya, I.; Skrastina, D.; Dislers, A.; Vasiljev, D.; Mihailova, M.; Ose, V.; Dreilina, D.; Pumpens, P. Construction and Immunological Evaluation of Multivalent Hepatitis B Virus (HBV) Core Virus-Like Particles Carrying HBV and HCV Epitopes. Clin. Vaccine Immunol. 2010, 17, 1027–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Xu, Y.; Qiao, L.; Wang, Y.; Wu, X.; Fan, D.; Peng, Q.; Xu, X. Trivalent Human Papillomavirus (HPV) VLP Vaccine Covering HPV Type 58 can Elicit High Level of Humoral Immunity but also Induce Immune Interference among Component Types. Vaccine 2010, 28, 3479–3487. [Google Scholar] [CrossRef] [PubMed]
- Nieto, K.; Weghofer, M.; Sehr, P.; Ritter, M.; Sedlmeier, S.; Karanam, B.; Seitz, H.; Müller, M.; Kellner, M.; Hörer, M.; et al. Development of AAVLP(HPV16/31L2) Particles as Broadly Protective HPV Vaccine Candidate. PLoS ONE 2012, 7, e39741. [Google Scholar] [CrossRef] [PubMed]
- Debbink, K.; Lindesmith, L.C.; Donaldson, E.F.; Swanstrom, J.; Baric, R.S. Chimeric GII.4 Norovirus Virus-Like-Particle-Based Vaccines Induce Broadly Blocking Immune Responses. J. Virol. 2014, 88, 7256–7266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, M.; Tumban, E.; Peabody, D.S.; Chackerian, B. The Use of Hybrid Virus-Like Particles to Enhance the Immunogenicity of a Broadly Protective HPV Vaccine. Biotechnol. Bioeng. 2014, 111, 2398–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhu, R.; Xu, L.; Li, Y.; Li, S.; Yu, H.; Li, S.; Zhu, H.; Cheng, T.; Xia, N. A Novel Combined Vaccine Based on Monochimeric VLP Co-Displaying Multiple Conserved Epitopes against Enterovirus 71 and Varicella-Zoster Virus. Vaccine 2017, 35, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Atcheson, E.; Cabral-Miranda, G.; Salman, A.M.; Reyes-Sandoval, A. Discovery of Four New B-Cell Protective Epitopes for Malaria Using Q Beta Virus-Like Particle as Platform. NPJ Vaccines 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Hunt, J.D.; Jackson, D.; Brown, L.; Wood, P.R.; Stewart, D.J. Antigenic Competition in a Multivalent Foot Rot Vaccine. Vaccine 1994, 12, 457–464. [Google Scholar] [CrossRef]
- Klenerman, P.; Zinkernagel, R.M. Original Antigenic Sin Impairs Cytotoxic T Lymphocyte Responses to Viruses Bearing Variant Epitopes. Nature 1998, 394, 482–485. [Google Scholar] [CrossRef] [Green Version]
- Frey, S.; Dagan, R.; Ashur, Y.; Chen, X.Q.; Ibarra, J.; Kollaritsch, H.; Mazur, M.H.; Poland, G.A.; Reisinger, K.; Walter, E.; et al. Interference of Antibody Production to Hepatitis B Surface Antigen in a Combination Hepatitis A/Hepatitis B Vaccine. J. Infect. Dis. 1999, 180, 2018–2022. [Google Scholar] [CrossRef]
- Sedegah, M.; Charoenvit, Y.; Minh, L.; Belmonte, M.; Majam, V.F.; Abot, S.; Ganeshan, H.; Kumar, S.; Bacon, D.J.; Stowers, A.; et al. Reduced Immunogenicity of DNA Vaccine Plasmids in Mixtures. Gene Ther. 2004, 11, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Larke, N.; Im, E.-J.; Wagner, R.; Williamson, C.; Williamson, A.-L.; McMichael, A.J.; Hanke, T. Combined Single-Clade Candidate HIV-1 Vaccines Induce T Cell Responses Limited by Multiple Forms of in Vivo Immune Interference. Eur. J. Immunol. 2007, 37, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, M.R.; Martinez-Torrecuadrada, J.L.; Casal, J.I.; Garcia, J.A. Development of an Antigen Presentation System Based on Plum Pox Potyvirus. FEBS Lett. 1998, 427, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Crisci, E.; Fraile, L.; Moreno, N.; Blanco, E.; Cabezón, R.; Costa, C.; Mussá, T.; Baratelli, M.; Martinez-Orellana, P.; Ganges, L.; et al. Chimeric Calicivirus-Like Particles Elicit Specific Immune Responses in Pigs. Vaccine 2012, 30, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Chuan, Y.P.; Rivera-Hernandez, T.; Wibowo, N.; Connors, N.K.; Wu, Y.; Hughes, F.K.; Lua, L.H.; Middelberg, A.P. Effects of Pre-Existing Anti-Carrier Immunity and Antigenic Element Multiplicity on Efficacy of a Modular Virus-Like Particle Vaccine. Biotechnol. Bioeng. 2013, 110, 2343–2351. [Google Scholar] [CrossRef]
- Pascual, E.; Mata, C.P.; Gomez-Blanco, J.; Moreno, N.; Barcena, J.; Blanco, E.; Rodríguez-Frandsen, A.; Nieto, A.; Carrascosa, J.L.; Castón, J.R. Structural Basis for the Development of Avian Virus Capsids That Display Influenza Virus Proteins and Induce Protective Immunity. J. Virol. 2014, 89, 2563–2574. [Google Scholar] [CrossRef] [Green Version]
- De Filette, M.; Martens, W.; Smet, A.; Schotsaert, M.; Birkett, A.; Londoño-Arcila, P.; Fiers, W.; Saelens, X. Universal Influenza A M2e-HBc Vaccine Protects against Disease Even in the Presence of Pre-Existing Anti-HBc Antibodies. Vaccine 2008, 26, 6503–6507. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Ceballos, M.; Moreno, N.; Gil-Cantero, D.; Castón, J.R.; Blanco, E.; Bárcena, J. Immunogenicity of Multi-Target Chimeric RHDV Virus-Like Particles Delivering Foreign B-Cell Epitopes. Vaccines 2022, 10, 229. https://doi.org/10.3390/vaccines10020229
Zamora-Ceballos M, Moreno N, Gil-Cantero D, Castón JR, Blanco E, Bárcena J. Immunogenicity of Multi-Target Chimeric RHDV Virus-Like Particles Delivering Foreign B-Cell Epitopes. Vaccines. 2022; 10(2):229. https://doi.org/10.3390/vaccines10020229
Chicago/Turabian StyleZamora-Ceballos, María, Noelia Moreno, David Gil-Cantero, José R. Castón, Esther Blanco, and Juan Bárcena. 2022. "Immunogenicity of Multi-Target Chimeric RHDV Virus-Like Particles Delivering Foreign B-Cell Epitopes" Vaccines 10, no. 2: 229. https://doi.org/10.3390/vaccines10020229
APA StyleZamora-Ceballos, M., Moreno, N., Gil-Cantero, D., Castón, J. R., Blanco, E., & Bárcena, J. (2022). Immunogenicity of Multi-Target Chimeric RHDV Virus-Like Particles Delivering Foreign B-Cell Epitopes. Vaccines, 10(2), 229. https://doi.org/10.3390/vaccines10020229







