The Influenza Vaccine May Protect Pregnant and Postpartum Women against Severe COVID-19
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
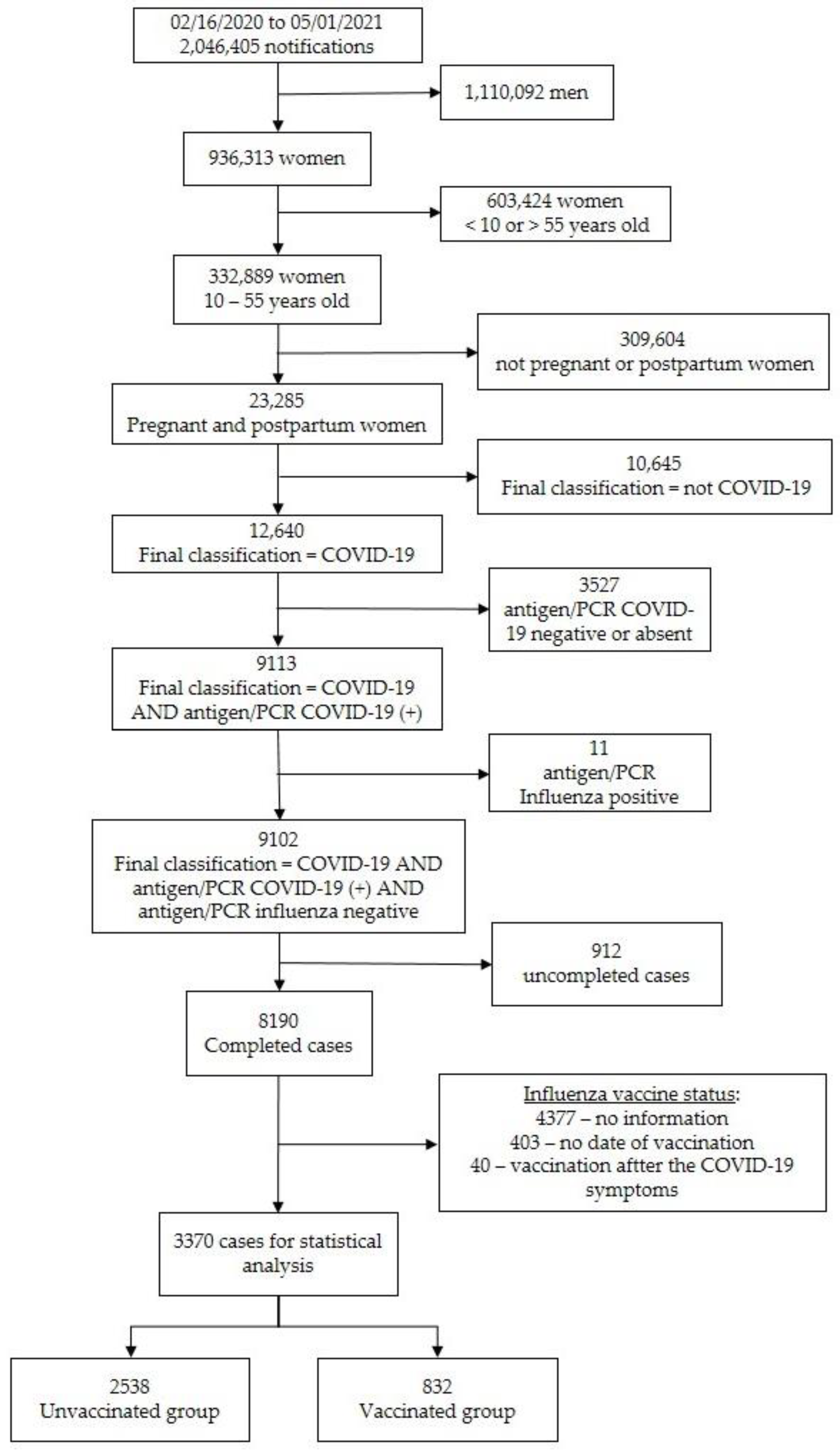
3.1. Study Population
3.2. Baseline Characteristics of the Subjects Enrolled
3.3. Clinical Manifestations of COVID-19
3.4. COVID-19 Adverse Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brasil, Ministério da Saúde, Secretaria de Atenção Primária à Saúde, Departamento de Ações Programáticas e Estratégicas. Manual de Recomendações para a Assistência à Gestante e Puérpera frente à Pandemia de Covid-19–Brasília: Ministério da Saúde. 2020. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/manual_assistencia_gestante_puerpera_covid-19_2ed.pdf (accessed on 1 October 2021).
- Thaddeus, S.; Maine, D. Too far to walk: Maternal mortality in context. Soc. Sci Med. 1994, 38, 1091–1110. [Google Scholar] [CrossRef]
- Rodrigues, A.; Lacerda, L.; Francisco, R.P. Brazilian Obstetric Observatory. arXiv 2021, arXiv:2105.06534. Available online: https://observatorioobstetrico.shinyapps.io/covid_gesta_puerp_br/ (accessed on 1 October 2021).
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde Departamento de Imunização e Doenças Transmissíveis, Coordenação-Geral do Programa Nacional de Imunizações. NOTA TÉCNICA No 651/2021. 2021. Available online: https://www.gov.br/saude/pt-br/media/pdf/2021/maio/19/nota-tecnica-651-2021-cgpni-deidt-svs-ms.pdf (accessed on 1 October 2021).
- Brasil, Ministério da Saúde, Secretaria Extraordinária de Enfrentamento à COVID-19, Gabinete. NOTA TÉCNICA No 2/2021. 2021. Available online: https://portaldeboaspraticas.iff.fiocruz.br/wp-content/uploads/2021/09/NotaTecnica_vacinacaocovid-19gestantespuerperas.pdf (accessed on 1 October 2021).
- Comitê Extraordinário de Monitoramento COVID-19 da Associação Médica Brasileira, Sociedade Brasileira de Pediatria, Sociedade Brasileira de Imunizações e Federação Brasileira de Ginecologia e Obstetrícia. ATUALIZAÇÃO DO BOLETIM 011/2021: CEM COVID_AMB. Gestantes e Puérperas Incluídas nos Grupos Prioritários Para Vacinas Contra COVID-19 no Plano Nacional de Imunização. 2021. Available online: https://amb.org.br/wp-content/uploads/2021/07/boletim11atualizado.pdf (accessed on 1 October 2021).
- Wu, P.; Lu, W.; He, L.; Meng, Y.; Ding, W.; Ma, K.; Liu, J. COVID-19 patients with recent influenza A/B infection: A retrospective study. J. Infect. 2021, 82, 159–198. [Google Scholar] [CrossRef] [PubMed]
- Jehi, L.; Ji, X.; Milinovich, A.; Erzurum, S.; Rubin, B.P.; Gordon, S.; Young, J.B.; Kattan, M.W. Individualizing risk prediction for positive coronavirus disease 2019 testing: Results from 11672 patients. Chest 2020, 158, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between influenza vaccination coverage rate and COVID-19 outbreak: An Italian ecological study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid. Based Med. 2021, 26, 192–193. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Islam, N.; Dambha-Miller, H. Association between influenza vaccination and hospitalization or all-cause mortality in people with COVID-19: A retrospective cohort study. BMJ Open. Respir. Res. 2021, 8, e000857. [Google Scholar] [CrossRef]
- Candelli, M.; Pignataro, G.; Torelli, E.; Gullì, A.; Nista, E.C.; Petrucci, M.; Saviano, A.; Marchesini, D.; Covino, M.; Ojetti, V.; et al. Effect of influenza vaccine on COVID-19 mortality: A retrospective study. Intern. Emerg. Med. 2021, 16, 1849–1855. [Google Scholar] [CrossRef]
- De La Cruz Conty, M.L.; Pardilla, M.B.E.; Sanchez, M.G.; Rodriguez, L.G.; Muner-Hernando, M.L.; Vicente, A.R.; Recarte, P.P.; Varea, A.M.; Diago, C.M.; Melguizo, S.C.; et al. Impact of recommended maternal vaccination programs on the clinical presentation of SARS-CoV-2 infection: A prospective observational study. Vaccines 2021, 9, 31. [Google Scholar] [CrossRef]
- Marín-Hernández, D.; Schwartz, R.E.; Nixon, D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2021, 93, 64–65. [Google Scholar] [CrossRef]
- Pawlowski, C.; Puranik, A.; Bandi, H.; Venkatakrishnan, A.J.; Agarwal, V.; Kennedy, R.; O´Horo, J.C.; Gores, G.J.; Williams, A.W.; Halamka, J.; et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci. Rep. 2021, 11, 4741. [Google Scholar] [CrossRef] [PubMed]
- Green, I.; Ashkenazi, S.; Merzon, E.; Vinker, S.; Golan-Cohen, A. The association of previous influenza vaccination and coronavirus disease-2019. Hum. Vaccines Immunother. 2021, 17, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Lin, S.W.; Sheng, W.H.; Wang, C.-C. Influenza vaccination and the risk of COVID-19 infection and severe illness in older adults in the United States. Sci. Rep. 2021, 11, 11025. [Google Scholar] [CrossRef] [PubMed]
- Noale, M.; Trevisan, C.; Maggi, S.; Incalzi, R.A.; Pedone, C.; Di Bari, M.; Adorni, F.; Jesuthasan, N.; Sojic, A.; Galli, M.; et al. The association between influenza and pneumococcal vaccinations and SARS-CoV-2 infection: Data from the EPICOVID19 web-based survey. Vaccines 2021, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, C.; Omar, M.; Dinalankara, W.; Imada, E.L.; Colantuoni, E.; Parmigiani, G.; Marchionni, L. Influenza vaccination and COVID-19 mortality in the USA: An ecological study. Vaccines 2021, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Arregui, I.; Navascués, A.; Adelantado, M.; Indurain, J.; Fresán, U.; Ezpeleta, C.; Castilla, J. Influenza vaccination and risk of SARS-CoV-2 infection in a cohort of health workers. Vaccines 2021, 8, 611. [Google Scholar] [CrossRef]
- Evidence-Based Medicine, Public Health and Environmental Toxicology (EBMPHET) Consortium. COVID-19 severity in Europe and the USA: Could the seasonal influenza vaccination play a role? Soc. Sci. Res. Netw. 2020. [Google Scholar] [CrossRef]
- Belingheri, M.; Paladino, M.E.; Latocca, R.; De Vito, G.; Riva, M.A. Association between seasonal flu vaccination and COVID-19 among healthcare workers. Occup. Med. 2020, 70, 665–671. [Google Scholar] [CrossRef]
- Saeed, Z.; Greer, O.; Shah, N.M. Is the host viral response and the immunogenicity of vaccines altered in pregnancy? Antibodies 2020, 9, 38. [Google Scholar] [CrossRef]
- Mackin, D.W.; Walker, S.P. The historical aspects of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 76, 13–22. [Google Scholar] [CrossRef]
- Vousden, N.; Knight, M. Lessons learned from the A (H1N1) influenza pandemic. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 76, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Quach, T.H.T.; Mallis, N.A.; Cordero, J.F. Influenza vaccine efficacy and effectiveness in pregnant women: Systematic review and meta-analysis. Matern Child. Health J. 2020, 24, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Sebghati, M.; Khalil, A. Uptake of vaccination in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 76, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde, Conselho Nacional de Saúde. Resolução no 510, de 7 de abril de 2016. Diário Oficial da União. Brasília, 24 May 2016. Available online: https://www.in.gov.br/materia/-%0A/asset_publisher/Kujrw0TZC2Mb/content/id/22917581 (accessed on 1 October 2021).
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R.-project.org/ (accessed on 1 October 2021).
- Ho, D.; Imai, K.; King, G.; Stuart, E.A. Match It: Nonparametric preprocessing for parametric causal inference. J. Stat. Softw. 2011, 42, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Brasil, Ministério da Economia. Painel de Preços-Análise de Materiais. 2021. Available online: https://paineldeprecos.planejamento.gov.br/graficos/detalhe?idSeq=220076&painel=MATERIAIS (accessed on 1 October 2021).
- Brasil, Ministério da Saúde, Secretaria de Atenção Especializada à Saúde. PORTARIA No 237, DE 18 DE MARÇO DE 2020 Tabela de Procedimentos, Medicamentos, Órteses, Próteses e Materiais Especiais do (SUS), para atendimento exclusivo de pacientes com diagnóstico clínico de COVID-19. Available online: https://www.in.gov.br/en/web/dou/-/portaria-n-237-de-18-de-marco-de-2020-*-261494685 (accessed on 1 October 2021).
- World Health Organization. Sustainable Development Goals (SDGs). 2021. Available online: https://www.who.int/health-topics/sustainable-development-goals#tab=tab_3 (accessed on 1 October 2021).
- Ragni, P.; Marino, M.; Formisano, D.; Bisaccia, E.; Scaltriti, S.; Bedeschi, E.; Grilli, R. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines 2020, 8, 675. [Google Scholar] [CrossRef]
- Wang, R.; Liu, M.; Liu, J. The association between influenza vaccination and COVID-19 and its outcomes: A systematic review and meta-analysis of observational studies. Vaccines 2021, 9, 529. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: http://orton.catie.ac.cr/cgi-bin/wxis.exe/?IsisScript=KARDEX.xis&method=post&formato=2&cantidad=1&expresion=mfn=003687 (accessed on 1 October 2021).
- Bridges, J.P.; Vladar, E.K.; Huang, H.; Mason, R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 2022, 77, 203–209. [Google Scholar] [CrossRef]
| Variables | Vaccinated (n = 832) | Unvaccinated (n = 2538) | p-Value |
|---|---|---|---|
| Age (years); median ± sd $ | 29.81 ± 6.78 | 30.44 ± 7.27 | 0.0234 1 |
| Ethnicity, n (%) White Non-white | 0.001 2 | ||
| 387/773 (50.1) | 997/2308 (43.2) | ||
| 386/773 (49.9) | 1311/2308 (56.8) | ||
| Comorbidities, n (%): | |||
| Cardiac | 61/371 (16.4) | 149/1143 (13.0) | 0.1181 2 |
| Diabetes mellitus | 59/371 (15.9) | 163/1158 (14.1) | 0.4327 2 |
| Hematologic | 6/359 (1.7) | 14/1122 (1.2) | 0.599 3 |
| Obesity | 48/366 (13.1) | 130/1145 (11.4) | 0.4141 2 |
| Asthma | 45/368 (12.2) | 83/1135 (7.3) | 0.0047 2 |
| Hepatic | 1/360 (0.3) | 11/1112 (1.0) | 0.3134 3 |
| Neurologic | 10/362 (2.8) | 19/1121 (1.7) | 0.2905 2 |
| Other lung diseases | 9/362 (2.5) | 21/1124 (1.9) | 0.6086 2 |
| Immunosuppression | 6/360 (1.7) | 37/1120 (3.3) | 0.1532 2 |
| Renal | 5/358 (1.4) | 13/1113 (1.2) | 0.7825 3 |
| Education, n (%) Up to 9 years From 9 to 12 years Over 12 years | <0.0012 | ||
| 103/577 (17.9) | 351/1406 (25.0) | ||
| 335/577 (58.1) | 806/1406 (57.3) | ||
| 139/577 (24.1) | 249/1406 (17.7) | ||
| Residence area, n (%) Urban Periurban Rural | 0.9147 3 | ||
| 749/790 (94.8) | 2270/2388 (95.1) | ||
| 2/790 (0.3) | 8/2388 (0.3) | ||
| 39/790 (4.9) | 110/2388 (4.6) |
| Variables | Vaccinated (n = 832) | Unvaccinated (n = 2538) | OR (95% CI) |
|---|---|---|---|
| Symptoms, n (%) | |||
| Fever | 509/744 (66.6) | 1529/2371 (64.5) | 1.10 (0.93–1.31) |
| Cough | 593/782 (75.8) | 1808/2402 (75.3) | 1.03 (0.85–1.24) |
| Sore Throat | 233/727 (32.0) | 664/2188 (30.3) | 1.08 (0.90–1.30) |
| Dyspnea | 418/759 (55.1) | 1477/2358 (62.6) | 0.73 (0.62–0.86) |
| Respiratory discomfort | 411/748 (54.9) | 1198/2269 (52.8) | 1.09 (0.92–1.29) |
| Desaturation | 243/734 (33.1) | 938/2254 (41.6) | 0.69 (0.58–0.83) |
| Diarrhea | 121/716 (16.9) | 329/2130 (15.4) | 1.11 (0.89–1.40) |
| Vomit | 109/718 (15.2) | 303/2136 (14.2) | 1.08 (0.85–1.37) |
| Abdominal pain | 56/440 (12.7) | 187/1597 (11.7) | 1.10 (0.80–1.51) |
| Fatigue | 160/450 (35.6) | 516/1633 (31.6) | 1.19 (0.96–1.49) |
| Loss of olfactory sense | 145/465 (31.2) | 340/1628 (20.9) | 1.72 (1.36–2.16) |
| Loss of taste | 137/461 (29.7) | 329/1631 (20.2) | 1.67 (1.32–2.11) |
| Any respiratory symptom | 570/783 (72.8) | 1865/2427 (76.8) | 0.81 (0.67–0.97) |
| Any symptom | 781/816 (95.7) | 2415/2517 (95.9) | 0.94 (0.64–1.40) |
| Variables n(%) | Vaccinated (n = 832) | Unvaccinated (n = 2.538) | OR (95% CI) | Vaccinated (n = 832) PSM | Unvaccinated (n = 832) PSM | OR (95% CI) PSM |
|---|---|---|---|---|---|---|
| ICU admission | 160/757 (21.1) | 629/2362 (26.6) | 0.74 (0.61–0.90) | 160/757 (21.1) | 237/782 (30.3) | 0.62 (0.49–0.78) |
| Intubation | 68/769 (8.8) | 336/2355 (14.3) | 0.58 (0.44–0.77) | 68/769 (8.8) | 122/786 (15.5) | 0.53 (0.39–0.72) |
| Final outcome, Cure Death | 788/832 (94.7) 44/832 (5.3) | 2213/2538 (87.2) 321/2538 (12.8) | 0.38 (0.27–0.53) | 788/832 (94.7) 44/832 (5.3) | 711/832 (85.5) 121/832 (14.5) | 0.33 (0.23–0.47) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganoti, C.d.F.; Rodrigues, A.S.; Francisco, R.P.V.; Costa, R.A.d. The Influenza Vaccine May Protect Pregnant and Postpartum Women against Severe COVID-19. Vaccines 2022, 10, 206. https://doi.org/10.3390/vaccines10020206
Paganoti CdF, Rodrigues AS, Francisco RPV, Costa RAd. The Influenza Vaccine May Protect Pregnant and Postpartum Women against Severe COVID-19. Vaccines. 2022; 10(2):206. https://doi.org/10.3390/vaccines10020206
Chicago/Turabian StylePaganoti, Cristiane de Freitas, Agatha Sacramento Rodrigues, Rossana Pulcineli Vieira Francisco, and Rafaela Alkmin da Costa. 2022. "The Influenza Vaccine May Protect Pregnant and Postpartum Women against Severe COVID-19" Vaccines 10, no. 2: 206. https://doi.org/10.3390/vaccines10020206
APA StylePaganoti, C. d. F., Rodrigues, A. S., Francisco, R. P. V., & Costa, R. A. d. (2022). The Influenza Vaccine May Protect Pregnant and Postpartum Women against Severe COVID-19. Vaccines, 10(2), 206. https://doi.org/10.3390/vaccines10020206







